Abstract
Claudin tight junction proteins have been identified to primarily determine intestinal epithelial barrier properties. While functional contribution of single claudins has been characterized in detail, information on the interplay with secretory mechanisms in native intestinal epithelium is scarce. Therefore, effects of cholera toxin and theophylline on rat colon were analyzed, including detection of sealing claudins. Tissue specimens were stripped off submucosal tissue layers and mounted in Ussing chambers, and short-circuit current (ISC) and transepithelial resistance (TER) were recorded. In parallel, expression and localization of claudins was analyzed and histological studies were performed employing hematoxylin-eosin staining and light and electron microscopy. Theophylline induced a strong increase of ISC in colon tissue specimens. In parallel, a decrease of TER was observed. In contrast, cholera toxin did not induce a significant increase of ISC, whereas an increase of TER was detected after 120 min. Western blots of membrane fractions revealed an increase of claudin-3 and -4 after incubation with cholera toxin, and theophylline induced an increase of claudin-4. In accordance, confocal laser-scanning microscopy exhibited increased signals of claudin-3 and -4 after incubation with cholera toxin, and increased signals of claudin-4 after incubation with theophylline, within tight junction complexes. Morphological analyses revealed no general changes of tight junction complexes, but intercellular spaces were markedly widened after incubation with cholera toxin and theophylline. We conclude that cholera toxin and theophylline have different effects on sealing tight junction proteins in native colon preparations, which may synergistically contribute to transport functions, in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Theophylline and cholera toxin have been described as inducers of electrogenic chloride secretion in epithelial cells [1, 11, 16, 17]. In the signaling cascades of both theophylline and cholera toxin, adenylate cyclase is involved. Whereas induction of chloride secretion by the agents has been analyzed in detail, information regarding effects on paracellular permeability is scarce.
The structural correlate of paracellular permeability is represented by tight junctions. Tight junctions (TJs) comprise a mosaic of different transmembrane proteins which differently contribute to barrier properties. Two main families of tight junction proteins have been reported as follows: the MARVEL family, including occludin, tricellulin, and marvelD3; and the claudin family [15]. The latter comprises of 27 members and several splice variants, which differentially contribute to paracellular barrier properties, ranging from sealing function [13, 23] to distinct channel formation [2] (for review, see [18]). Thus, single claudins have been identified to primarily and specifically determine the paracellular barrier for ions. Among the TJ proteins primarily contributing to paracellular sealing, claudin-1, -3, and -4 have been shown to play a major role in the intestine [5, 24]. Therefore, these claudins are determinants for regulation of adaptive barrier mechanisms in intestinal tissues. Clusters of different claudins interact in a homophilic and heterophilic way and are organized in strands in the apicolateral membrane of epithelial cells.
The majority of TJ protein analyses in vitro focused on single proteins in epithelial cell culture monolayers. Regarding the complexity of TJ regulation and in order to evaluate the functional relation of transport and barrier mechanisms, it is important to investigate the interplay of claudins in native epithelial tissue specimens.
Rat distal colonic epithelium specimens can be investigated in Ussing chambers and thus are widely used for the study of intestinal Cl− secretion [16]. Studies focusing on this approach revealed that segmental expression of tight junction proteins is in accordance with epithelial barrier properties along the longitudinal axis of rat intestinal epithelium [19]. Moreover, in parallel studies on human tissue, synergistic regulation of transport and barrier function has been demonstrated [4].
Regarding effects of theophylline and cholera toxin, cell lines and preparations of rat colon have been analyzed recently [1, 25]. Although effects on barrier function might strongly functionally interact with transcellular secretory processes, possible effects on tight junctions have not been analyzed, though. Thus, to elucidate mechanisms of theophylline and cholera toxin on both colonic epithelial transport and barrier function, native tissue preparations of rat colon specimens were analyzed in vitro.
Material and methods
Methods
Chemicals
All chemicals were provided by Sigma-Aldrich, when not otherwise noted. Cholera toxin was obtained from Vibrio cholerae, Sigma-Aldrich Catalog Number C8052. The stock solution was 1 mg/ml water, respectively. Experiments were performed in parallel to a study focusing on a cell model, HT-29/B6. Therefore, full functionality of the compound was monitored throughout the experiments [25].
Preparation of tissue specimens
Tissue specimens were obtained from male Wistar rats (250–300 g). Animals were anesthetized and sacrificed by inhalation of CO2. Preparations were performed as described in detail recently, according to the segment’s mapping of rat intestine [19]. Briefly, distal colon was stripped off submucosal tissue layers leaving only mucosa, lamina propria, and the outer layer of the muscularis mucosae, and mounted on plastic rings resulting in a surface of 0.49 cm2.
Electrophysiological measurements
After preparation, the colonic epithelial tissues were mounted in Ussing chambers as described before [12] and 10 ml circulating Ringer’s solution was added on each side. The solution contained (in mM) Na+ (140.5), K+ (5.4), Ca2+ (1.2), Mg2+ (1.2), Cl− (123.8), HCO3 − (21), HPO4 2− (2.4), H2PO4 − (0.6), d(+)-glucose (10), and d(+)-mannose (10). During all experiments, the solution was gassed with 95 % O2 and 5 % CO2 at 37 °C, resulting in a pH of 7.4. For measurement of transepithelial resistance (TER) in Ussing chambers, a preamplifier (Model EVC-3, World Precision Instruments, USA), and a voltage clamp device (EVC-4000, World Precision Instruments, USA) were employed. Specimens of rat intestine were incubated with theophylline or cholera toxin for 2 h. Short-circuit current (ISC) and TER was reported, and self-same tissues were prepared for detection of tight junction proteins.
Western blotting
Immunoblots and immunostaining were performed as described in detail previously [19]. Tissues were homogenized in Tris buffer containing 20 mM Tris, 5 mM MgCl2, 1 mM EDTA, 0.3 mM EGTA, and protease inhibitors (Complete, Boehringer, Mannheim, Germany).
Membrane fractions were obtained by two centrifugation steps (5 min at 200×g, 30 min at 43,000×g, 4 °C). Pellets were resuspended in Tris buffer. Protein contents were determined using BCA protein assay reagent (Pierce, Rockford, IL, USA) quantified with a plate reader (Tecan, Grodig, Austria). Samples were mixed with SDS buffer (Laemmli), loaded on a 12.5 % SDS polyacrylamide gel and electrophoresed.
Proteins were assessed by immunoblotting employing rabbit anti-claudin-1, -3, and mouse anti-claudin-4 primary antibodies used in a concentration of 1:100 according to the manufacturer’s protocols (Invitrogen, San Francisco, CA, USA). β-actin signals were detected as loading controls (Sigma-Aldrich, Taufkirchen, Germany).
To detect bound antibodies peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG antibodies and the chemiluminescence detection system Lumi-LightPLUS Western blotting kit (Roche, Mannheim, Germany) were used. Signals were visualized by luminescence imaging (LAS-1000, Fujifilm, Japan). For comparison of Western blot signals, densitometry analysis was performed using AIDA Raytest 2.5 software (Straubenhardt, Germany).
Confocal laser-scanning microscopy
Tissues were fixed in 2 % paraformaldehyde for 2 h at room temperature and embedded in paraffin. For immunostaining, paraffin was removed from cross sections (8 μm) by a xylol–ethanol gradient. For antigen retrieval, sections were boiled in 1 mM EDTA buffer solution. To block non-specific binding sites, tissues were bathed in PBS containing 6 % (v/v) goat serum and 1 % BSA (blocking solution) for 60 min at room temperature. All subsequent washing procedures were performed with this blocking solution. Combinations of mouse (monoclonal) and rabbit (polyclonal) anti-occludin, and rabbit (polyclonal) anti-claudin-1 and -3 and mouse (monoclonal) anti-claudin-4 antibodies were employed, respectively (Invitrogen, San Francisco, CA, USA). Antibodies were diluted 1:100 in blocking solution according to the manufacturer’s recommendations, respectively. Tissues were incubated for 60 min and, after two washes, were incubated with Alexa Fluor goat anti-mouse IgG and Alexa Fluor goat anti-rabbit IgG diluted 1:500 in blocking solution for 45 min (Molecular Probes, USA). Furthermore, nuclei were stained by DAPI (Roche, 1:5,000). Sections were mounted with ProTags MountFluor (Biocyc, Luckenwalde, Germany). Fluorescence images were obtained with a confocal laser-scanning microscope (LSM 510 Meta, Zeiss, Jena, Germany).
Histological analysis
Histological analysis was performed as reported before [3]. After 1 h fixation of tissues in 3 % formalin for 1 h, samples were paraffined employing increasing ethanol concentrations according to a standard protocol. Sections were mounted on sample glasses and deparaffined. Hematoxylin- and eosin-stained sections were analyzed by optical microscopy.
Electron microscopy
Fixation of tissue samples was performed in 2.5 % glutaraldehyde and Hanks’ balanced salt solution, pH = 7.0, at 4 °C for 2 h as reported recently [19]. Subsequently, tissues were postfixed in osmium tetroxide solution (1 % OsO4 in Hanks’ solution) at 4 °C for 2 h, and block-stained in 2 % uranyl acetate buffer at 40 °C for 1 h, dehydrated in ethanol and acetone, and embedded in Spurr. Ultra-thin sections were obtained employing the ultramicrotome LKB-8800 (LKB, Sweden), and were stained in uranyl acetate and lead citrate. A JEM-100C microscope (JEOL, Japan) was used for examination of the sections.
Statistical analysis
Data are expressed as means ± SEM. Statistical analysis was performed using Mann–Whitney U test and p < 0.05 was considered as significant.
Results
Ussing chamber experiments
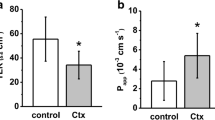
Specimens of rat colon were mounted in Ussing chambers, and theophylline (10 mM) or cholera toxin (1 μg/ml) were added, respectively. Theophylline markedly induced ISC in the colon from 40.0 ± 9.5 to 177.0 ± 20.4 μA/cm2, after 20 min, and to 201.1 ± 23.9 μA/cm2 after 2 h (n = 11, p < 0.001), whereas no significant increase of this parameter was detected in controls (30.2 ± 7.2 to 29.9 ± 7.3 μA/cm2 after 20 min, and 32.3 ± 9.3 μA/cm2 after 2 h, n = 11, not significant (n.s.)) and cholera toxin experiments (31.5 ± 4.4 to 30.7 ± 4.1 μA/cm2 after 20 min, and 35.3 ± 4.4 μA/cm2 after 2 h (n = 18, n.s.)); Fig. 1a.
Effects of cholera toxin and theophylline on a Short-circuit current (ISC) and (b) transepithelial resistance (TER). a Theophylline markedly induced ISC in the colon from, whereas no significant increase of was detected in controls and cholera toxin experiments. b TER measurements revealed no changes of barrier properties in controls, whereas cholera toxin induced an increase and theophylline induced a decrease in TER (*p < 0.05, **p < 0.01, ***p < 0.001, respectively n = 11, 18, and 11, respectively)
TER measurements revealed no changes of barrier properties in controls, whereas both cholera toxin and theophylline induced changes of this parameter. Tissues incubated with cholera toxin induced an increase in TER from 122.1 ± 8.3 to 126.2 ± 9.0 Ω · cm2 after 20 min, and to 137.9 ± 8.3 after 2 h (n = 18, p < 0.01), and theophylline induced a decrease 139.1 ± 12.2 to 99.9 ± 9.2 Ω · cm2 after 20 min, and to 91.9 ± 6.4 after 2 h (n = 11, p < 0.05 and p < 0.01, respectively), Fig. 1b.
Western blotting
Protein preparations were performed for Western blot analyses to detect major sealing TJ proteins in colon tissues. Western blots revealed specific signals for claudins and beta-actin in control tissues and after incubation with theophylline and cholera toxin (Fig. 2a). Densitometric analysis of Western blots revealed marked changes of single claudins after incubation with cholera toxin and theophylline (Fig. 2b). Whereas claudin-1 exhibited no change compared to controls after incubation with theophylline and cholera toxin, claudin-3 and -4 showed a significant increase in colonic epithelium after incubation with cholera toxin (321 ± 98 % of controls set to 100 %, n = 6, and 313 ± 79 % of controls set to 100 %, n = 7; p < 0.05), respectively. Moreover claudin-4 was increased after incubation with theophylline (303 ± 129 % of controls set to 100 %, n = 6, p < 0.05).
Confocal laser-scanning immunofluorescent microscopy
Confocal laser-scanning immunofluorescent microscopy revealed specific signals for occludin and claudin-1, -3, and -4 in all tissue specimens (Fig. 3). Whereas no increased signals of claudin-1 after theophylline and cholera toxin incubation were detected, increased signals of claudin-3 and -4 after incubation with cholera toxin, and increased signals of claudin-4 after incubation with theophylline were observed within TJ complexes in colocalization with occludin, identified by an increase of merged signals in the surface epithelium. Although the LSM images of stained tissues may provide limited information regarding quantitative changes, results were in accordance with Western blots.
Confocal laser-scanning immunofluorescence microscopy. Confocal laser-scanning immunofluorescence microscopy detecting tight junction proteins revealed specific signals for occludin (green), and claudin-1, -3, and -4 (red). Increased signals of claudin-4 after theophylline incubation, and increased signals of claudin-3 and -4 after incubation with cholera toxin were consistently localized within TJ complexes in colocalization with occludin, resulting in an increase of merged yellow signals in epithelium
Histological analyses
Morphology of colon epithelium was analyzed by light microscopy on eosin-hematoxylin-stained sections of formalin-fixed tissue samples. In this set of experiments, no change of epithelial morphology was detected (Fig. 4a–c).
Morphology and cell structure by cholera toxin and theophylline treatment. a–c Light microscopy (bar 100 μm); d–f Electron micrographs (bar 1 μm); images show of colon epithelium from controls (a, d), and after 2-h incubation with cholera toxin (b, e), and theophylline (c, f). Intercellular spaces (ICS) below tight junctions were widened after incubation with the agents. Luminal membranes are orientated to the top of the figures, respectively. Representative images of tissues from four animals
Ultrastructural analysis of cell structure by transmission electron microscopy revealed typical features of secretory epithelial cells in control tissue specimens (Fig. 4d). Microvilli were detected in the apical membrane and tight junctions were found in the apical parts of the cells. Numerous mitochondria and nuclei in the basal area of secretory epithelial cells were detected. In contrast, both cholera toxin and theophylline groups showed markedly increased intercellular spaces (Fig. 4e, f), whereas cells remained interconnected by dense tight junction complexes.
Discussion
To elucidate the effects of cholera toxin and theophylline on transport and barrier function in intestinal epithelium, a comparative study of these agents on both chloride secretion and barrier properties was performed. Therefore, effects were analyzed on functional and molecular level focusing on self-same intestinal tissue preparations.
Theophylline induces a chloride secretion in colonic epithelia via cAMP, which can be measured as ISC [16]. In our study employing rat colon, simultaneously with an increase of ISC, a decrease of TER was observed, which has not been described so far. Cholera toxin was expected to have comparable effects, as both theophylline and cholera toxin has been reported to be inducers of electrogenic chloride secretion in epithelial cells [1, 16, 17]. However, in our experiments, no significant change of ISC was detected by incubation with cholera toxin. This could be due to different model systems, as a previous detection of a cholera toxin-induced ISC was observed under different experimental conditions employing nystatin for permeabilization of cell membranes [1]. The latter study might provide an explanation of this tentative contradiction though, as a preincubation of the basolateral membrane with nystatin omits possible compensatory mechanisms provided by basolateral transport systems. In order to present the most physiologically conditions, we have focused on apical incubation with cholera toxin, though. An interaction with carrier proteins, along with tight junctions as shown for CFTR might be a possible explanation [8]. However, application of further agents perturbing membrane properties might be an interesting field for subsequent studies.
Cholera toxin effects on transepithelial resistance have not been reported in rat colon [1] and in confluent monolayers of the colonic intestinal epithelial cell model HT-29/B6 cells [25]. However, in our experiments, a significant increase of TER was detectable in Ussing chambers, and an increase of sealing tight junction proteins within tight junction complexes was detected.
This mechanism could add to the general strategy of bacterial colonization and infection, which benefits from a perturbation of tight junctions [7]. In culture supernatants of Vibrio cholerae, a toxin was identified (Zonula occludens toxin, ZOT), that increases the permeability of the small intestinal mucosa by affecting the TJ structure [9]. ZOT effects on TJ modulation were shown to be mediated by a cascade of intracellular events that lead to a PKCα-dependent polymerization of actin microfilaments which are involved in regulation of the paracellular pathway [10].
Another cytotoxin of V. cholerae, namely hemagglutinin/protease (HA/P), has also been reported to have effects on the tight junction [28]. HA/P was demonstrated to perturb the barrier function of Madin-Darby canine kidney epithelial cell line I by affecting TJs and the F-actin cytoskeleton. Western blot analyses revealed that occludin was digested by HA/P to two predominant bands of around 50 and 35 kDa in Western blots. In contrast, ZO-1 was not degraded by HA/P in parallel experiments, suggesting the selectivity of HA/P-associated protein degradation. This interplay of different toxins affecting the tight junction may also become a target for development of novel therapeutic or preventive strategies though [7].
In our study, for the first time, an effect of cholera toxin on the TJ has been identified, showing a simultaneous and functionally synergistic induction of claudin-3 and -4. A synergistic co-regulation of these TJ proteins has been also reported in a variety of different models, including CaCo-2 [26], colorectal carcinoma [21], and colitis [22].
Claudin-3 is expressed in many epithelia, such as intestine, kidney, endothelia, and mammary glands [20, 24]. The functional characterization of claudin-3 has been performed in detail previously, revealing a general barrier-forming role of the protein, sealing the paracellular pathway against the passage of small ions of either charge [23]. Increased expression of claudin-3 also explains a physiological barrier regulation towards a sealing of the TJ in mammary glands [20].
Claudin-4 is markedly expressed in intestine and kidney [19, 24]. Specific effects on general barrier function and paracellular Na+ permeability without effects on Cl− permeability was reported [27]. In the colon, there is indirect evidence that claudin-4 tightens the paracellular pathway, as claudin-4 is down-regulated under various conditions that cause increased permeability (for review, see [15]). Accordingly, an increase of claudin-4 may indicate a sealing of the epithelial barrier against the paracellular passage of Na+. However, in our study, the theophylline induced chloride secretion might have concealed an increase of TER expected by the increase of claudin-4, as TER can also decrease due to membrane channels and not necessarily due to an increase of paracellular permeability. This furthermore highlights the physiological interaction of transport and barrier mechanisms affecting trans- and paracellular resistance.
Claudin-1 is regarded as a classical sealing tight junction protein, as it is strongly expressed in tight epithelia [19] and a knockout has demonstrated that it is crucial for epithelial barrier function of the epidermis [14]. In our study, however, no significant changes of this major sealing tight junction protein were observed. However, the stable expression of this important determinant of barrier function in our current study underlines the differential physiological regulation of intestinal tight junctions.
In our study, both cholera toxin and theophylline induced a marked widening of intercellular spaces between colon epithelial cells, which was most obviously visible in EM images, but also even indicated in HE stainings. This widening might be attributed to different processes, e.g., an activity of the Na+/K+ pump and synergistic action of membrane transporters may induce local osmotic pressure, and an increase of tightening tight junction proteins might at the same time promote an increase of fluid volume in lateral intercellular spaces. Moreover, cell shrinkage under secretion stimulus might also be a cause of the observation, as shown in different epithelial model systems [6, 14]
Our present study reveals that classic inducers of chloride secretion, namely cholera toxin and theophylline, have differential effects on the regulation of sealing tight junction proteins in native colon preparations, as opposite changes concerning barrier properties of colonic epithelium, and different effects on TJ proteins were observed. The effects of theophylline and cholera toxin on TER and ISC indicate different mechanisms on molecular level, which might be elucidated further in future studies.
References
Alzamora R, O’Mahony F, Harvey BJ (2011) Estrogen inhibits chloride secretion caused by cholera and Escherichia coli enterotoxins in female rat distal colon. Steroids 76:867–876
Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115:4969–4976
Amasheh S, Dullat S, Fromm M, Schulzke JD, Buhr HJ, Kroesen AJ (2009) Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int J Color Dis 24:1149–1156
Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke JD, Fromm M (2009) Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem Biophys Res Commun 378:45–50
Amasheh S, Fromm M, Günzel D (2011) Claudins of intestine and nephron—a correlation of molecular tight junction structure and barrier function. Acta Physiol (Oxf) 201:133–140
Bachmann O, Heinzmann A, Mack A, Manns MP, Seidler U (2007) Mechanisms of secretion-associated shrinkage and volume recovery in cultured rabbit parietal cells. Am J Physiol Gastrointest Liver Physiol 292:G711–G717
Bücker R, Schumann M, Amasheh S, Schulzke JD (2010) Claudins in intestinal function and disease. Curr Top Membr 65:195–229
Castellani S, Guerra L, Favia M, Di Gioia S, Casavola V, Conese M (2012) NHERF1 and CFTR restore tight junction organisation and function in cystic fibrosis airway epithelial cells: role of ezrin and the RhoA/ROCK pathway. Lab Investig 92:1527–1540
Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD, Ketley JM, Kaper JB (1991) Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci U S A 88:5242–5246
Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper JB, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum SE (1995) Zonula occludens toxin modulates tight junctions through protein kinase C dependent actin reorganization, in vitro. J Clin Invest 96:710–720
Field M (2003) Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111:931–943
Fromm M, Schulzke JD, Hegel U (1985) Epithelial and subepithelial contributions to transmural electrical resistance of intact rat jejunum, in vitro. Pflugers Arch 405:400–402
Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156:1099–1111
Greger R, Heitzmann D, Hug MJ, Hoffmann EK, Bleich M (1999) The Na+2Cl−K+ cotransporter in the rectal gland of Squalus acanthias is activated by cell shrinkage. Pflugers Arch 438:165–176
Günzel D, Fromm M (2012) Claudins and other tight junction proteins. Compr Physiol 2:1819–1852
Köckerling A, Fromm M (1993) Origin of cAMP-dependent Cl- secretion from both crypts and surface epithelia of rat intestine. Am J Physiol Cell Physiol 264:C1294–C1301
Kroesen AJ, Stockmann M, Ransco C, Schulzke JD, Fromm M, Buhr HJ (2002) Impairment of epithelial transport but not of barrier function in idiopathic pouchitis after ulcerative colitis. Gut 50:821–826
Krug SM, Günzel D, Conrad MP, Lee IM, Amasheh S, Fromm M, Yu ASL (2012) Charge-selective claudin channels. Ann N Y Acad Sci 1257:20–28
Markov AG, Veshnyakova A, Fromm M, Amasheh M, Amasheh S (2010) Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J Comp Physiol B 180:591–598
Markov AG, Kruglova NM, Fomina YA, Fromm M, Amasheh S (2012) Altered expression of tight junction proteins in mammary epithelium after discontinued suckling in mice. Pflugers Arch 463:391–398
Mees ST, Mennigen R, Spieker T, Rijcken E, Senninger N, Haier J, Bruewer M (2009) Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: upregulation of claudin-1, claudin-3, claudin-4, and beta-catenin. Int J Color Dis 24:361–368
Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M (2009) Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 296:G1140–G1149
Milatz S, Krug SM, Rosenthal R, Günzel D, Müller D, Schulzke JD, Amasheh S, Fromm M (2010) Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta Biomembr 1798:2048–2057
Rosenthal R, Heydt MS, Amasheh M, Stein C, Fromm M, Amasheh S (2012) Analysis of absorption enhancers in epithelial cell models. Ann N Y Acad Sci 1258:86–92
Schulzke JD, Andres S, Amasheh M, Fromm A, Günzel D (2011) Anti-diarrheal mechanism of the traditional remedy uzara via reduction of active chloride secretion. PLoS ONE 6:1–9
Suzuki T, Tanabe S, Hara H (2011) Kaempferol enhances intestinal barrier function through the cytoskeletal association and expression of tight junction proteins in Caco-2 cells. J Nutr 141:87–94
Van Itallie C, Rahner C, Anderson JM (2001) Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107:1319–1327
Wu Z, Nybom P, Magnusson KE (2000) Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol 2:11–17
Acknowledgments
The study has been supported by the Deutsche Forschungsgemeinschaft (DFG FOR 721, SFB 852), the Sonnenfeld-Stiftung Berlin, the Partnership Program FU Berlin–University St. Petersburg, by Saint Petersburg University Research Grant no. 1.37.118.2011, and grant RFBR 10-04-01575.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markov, A.G., Falchuk, E.L., Kruglova, N.M. et al. Comparative analysis of theophylline and cholera toxin in rat colon reveals an induction of sealing tight junction proteins. Pflugers Arch - Eur J Physiol 466, 2059–2065 (2014). https://doi.org/10.1007/s00424-014-1460-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1460-z