Abstract
The melastatin (M) transient receptor potential (TRP) channel TRPM4 is selective for monovalent cations and is activated by high levels of intracellular Ca2+. TRPM4 is broadly distributed and may be involved in numerous functions, including electrical conduction in the heart, respiratory rhythm, immune response, and secretion of insulin by pancreatic β-cells. The significance of TRPM4 in smooth muscle cell function is reviewed here. Several studies indicate that TRPM4 channels are critically important for pressure-induced cerebral arterial myocyte depolarization and myogenic vasoconstriction as well as autoregulation of cerebral blood flow. Regulation of TRPM4 activity in arterial smooth muscle cells is complex and involves release of Ca2+ from the sarcoplasmic reticulum through inositol 1,4,5-trisphosphate receptors and translocation of TRPM4 channels to the plasma membrane in response to protein kinase Cδ. TRPM4 is also present in colonic, urinary bladder, aortic, interlobar pulmonary and renal artery, airway, and corpus cavernosum smooth muscle cells, but its significance and regulation in these tissues is less well characterized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transient receptor potential (TRP) superfamily of cation channels was unexpectedly discovered during studies of Drosophila phototransduction mutants that behaved as if blind in bright light and displayed abnormalities in electroretinograms [5]. Subsequent cloning of the responsible gene, trp [42], and sequence analysis revealed the presence of homologous ion channels in many other organisms [65]. It is now known that the human genome encodes 27 distinct TRP genes and that an additional one is present in rats and mice [66]. Mammalian TRP genes are assigned to six subfamilies designated canonical (C), vanilloid (V), melastatin (M), ankyrin (A), polycystin (P), and mucoliptin (ML). This classification is based on sequence homology rather than functional similarity [4]. All TRP channels are permeable to cations with varying selectivities. Many are nonselective, two (TRPV5 and TRPV6) are highly selective for Ca2+ ions, and two (TRPM4 and TRPM5) are selective for monovalent cations. TRP channels are expressed as six-transmembrane domain (S1–S6) subunits with a pore-forming structure between the S5 and S6 domains. Four of these subunits assemble to form functional ion channels. Heteromultimeric channels composed of two or three different subunits can form with properties distinct from homomeric channels [67]. This situation is well characterized for TRPC channels [10, 27, 35], TRPM6/M7 [3], and TRPV5/V6 [25], but other combinations are also possible. Multiple TRP channels are present in most, if not all, cells where they are involved in astonishingly diverse physiological functions. In general, TRP channels serve as fundamental sensors of environmental conditions at both macroscopic and cellular levels. To perform these functions, TRP channels are activated by stimuli such as chemical agonists, temperature, pH, osmolarity, light, and pressure. The reader is directed to several recent review articles for more comprehensive information about TRP channel structure [41], function [64, 66], and pathophysiology [48]. The current review is narrowly focused on the involvement of TRPM4 in the regulation of smooth muscle cell function.
Biophysical properties of TRPM4
Two splice variants of TRPM4 have been described. The first, a short form designated as TRPM4a, was initially cloned by Xu et al. [68]. TRPM4a displays little activity, and its significance is unknown. A second, longer variant was reported by Launay et al. [33] and was initially designated as TRPM4b. TRPM4b is now generally accepted to be the commonly expressed and functional isoform of the channel and will be referred to hereafter simply as TRPM4. The unitary conductance of TRPM4 is ∼24 pS [33]. TRPM4 and the closely related channel TRPM5 [26] display two defining biophysical properties: Ca2+-dependent activation and selectivity for monovalent cations [33]. The relative ionic selectivity of TRPM4, as determined by substitution experiments employing HEK 293 cells expressing the recombinant human gene, is Na+ ≈ K+ > Cs+ > Li+. Ionic selectivity is conveyed by negatively charged amino acid residues between E981 and V985 of the human TRPM4 subunit [50].
TRPM4 channel activity is dependent upon high levels of intracellular Ca2+ [33]. Human TRPM4 channels expressed in HEK 293 cells have an EC50 for Ca2+-dependent activation of approximately 400 nM [33] or 15 μM [49] under whole-cell patch clamp conditions. It is not clear why these two laboratories find differences in the channel’s sensitivity to intracellular Ca2+, but higher levels of Ca2+ (EC50 = 10 μM) are required to activate TRPM4 channels in native vascular smooth muscle cells under whole-cell patch-clamp conditions [11]. TRPM4 currents recorded from inside-out membrane patches are much less sensitive to Ca2+, with an EC50 for activation of 370 μM [50]. Diminished Ca2+ sensitivity under inside-out vs. whole-cell patch-clamp conditions suggests that cytosolic factors lost when membrane patches are excised influence the Ca2+-dependent activation of TRPM4. Consistent with this possibility, deletions of calmodulin-binding sites on the C-terminus diminished Ca2+-dependent TRPM4 current activation, indicating a role for calmodulin in this response [52]. Ca2+-dependent activation of TRPM4 is influenced by the activity of protein kinase C (PKC). Stimulation of PKC with the phorbol compound phorbol-12-myristate-13-acetate (PMA) increases TRPM4 activity by enhancing the sensitivity of the channel to intracellular Ca2+. This response is blocked by point mutations at S1145 and S1152, suggesting that PKC-dependent phosphorylation of these residues is responsible for increased Ca2+-dependent channel activation [52].
Ca2+-dependent activation of TRPM4 is transient under the conditions typically used for conventional whole-cell or inside-out patch-clamp experiments. For example, when intracellular Ca2+ is maintained at 10 μM, TRPM4 currents exhibit a time-dependent decay, with currents completely inactivated in most cells within 120 s in HEK and A7r5 cell expression systems [16, 52] and naive cerebral artery smooth muscle cells [11]. The inhibition of phospholipase C (PLC) or inclusion of the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PI(4,5)P(2)) in the intracellular solution prevents the time-dependent decrease of TRPM4 currents [47, 71]. Sustained TRPM4 currents can be recorded from native smooth muscle cells patch-clamped using the amphotericin B perforated patch configuration [15], a technique which does not disrupt physiological intracellular Ca2+ signaling mechanisms. These data suggest that time-dependent inactivation may be a consequence of the high levels of intracellular Ca2+ used during conventional whole-cell and inside-out patch-clamp experiments rather than an inherent property of the channel.
TRPM4 pharmacology
Investigations into the functional significance of TRPM4 have been impeded by a lack of selective pharmacological activators and inhibitors. Decavanadate [51] and the pyrazole derivative N-[4-3,5-bis(trifluromethyl)pyrazol-1-yl]-4-methyl-1,2,3-thiadiazole-5-carboxamide (BTP2) [62] potentiate Ca2+-dependent TRPM4 activity, but these agents lack specificity. Decavanadate has effects on inositol 1,4,5-trisphosphate (IP3) receptors and purinoreceptors (P2X) [39], whereas BTP2 inhibits TRPC3 and TRPC5 channels [24]. Flufenamic acid and clotrimazole act as gating inhibitors, and spermine and Gd3+ as pore blockers [8, 49, 53, 63], but these agents are not selective for TRPM4 and influence many other channels. The hydroxytricyclic derivative 9-phenanthrol may be a more useful TRPM4 inhibitory compound. In heterologous expression systems, 9-phenanthrol blocks TRPM4 currents with little effect on the closely related channel TRPM5 or the cystic fibrosis transmembrane conductance regulator chloride channel [19]. Furthermore, at a concentration (30 μM, ∼3 × IC50) that almost completely blocks TRPM4 activity, 9-phenanthrol does not alter TRPC3, TRPC6, voltage-gated K+ channels (Kv), large-conductance Ca2+-activated K+ channels (BKCa), inwardly rectifying K+ channels (KIR), or L-type Ca2+ channel currents [18]. 9-Phenanthrol also has little effect on TRPM7 currents in mouse interstitial cells of Cajal or human gastric cancer cells [29, 30]. At higher concentrations (∼100 μM, 10 × IC50), 9-phenanthrol can block K+ and voltage-dependent Ca2+ channels [58]. Although additional studies are necessary to fully evaluate specificity, these data suggest that 9-phenanthrol is a useful compound for studying the functional significance of TRPM4.
TRPM4 tissue distribution and function
TRPM4 is broadly distributed and mRNA is expressed at high levels in the heart, pancreas, placenta, and prostate and at lower levels in the kidney, skeletal muscle, liver, intestines, thymus, testis, and spleen [33]. TRPM4 is present in smooth muscle cells found in rat cerebral arteries [12], the urinary bladder of rats and guinea pigs [60], monkey and human colon [9], rat aorta [70], rat pulmonary [70] and renal (Earley et al., unpublished observations) intralobar arteries, rat airway (Earley et al., unpublished observations), and mouse corpus cavernosum (Werner and Earley, unpublished observations; Table 1). TRPM4 is detected by immunolabeling in the central nervous system, including the substantia nigra pars compacta [44], the cerebral cortex, hippocampal neurons, and spinal cord motor neurons [56]. TRPM4 channels are involved in numerous physiological processes [22]. TRPM4 regulates cytokine secretion in lymphocytes and insulin secretion in pancreatic β-cells [57]. TRPM4 is involved in controlling respiratory rhythmogenesis in neurons of the pre-Bötzinger complex [40]. TRPM4 channels were recently implicated in axonal and neuronal degeneration in experimental autoimmune encephalomyelitis, a model of multiple sclerosis [56]. In the heart [21, 23], TRPM4 channels are involved in early afterdepolarizations associated with re-oxygenation following hypoxia [58]. In addition, gain-of-function point mutations within the TRPM4 gene are associated with an inherited form of conduction block in the heart, indicating that the channel is involved in the function of cardiac Purkinje fibers [32, 34].
Function of TRPM4 in arterial smooth muscle
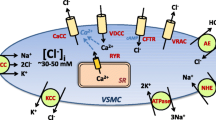
The resting membrane potential of vascular smooth muscle cells in the walls of arteries under physiological intraluminal pressure (60–80 mmHg) is significantly depolarized (approx. −45 mV) compared with the K+ equilibrium potential (−80 mV) [31]. This is functionally significant because vascular smooth muscle cell contractility is largely regulated by Ca2+ influx via L-type voltage-dependent Ca2+ channels that are sharply activated at membrane potentials between −30 and −50 mV. Thus, resting membrane potential is a major determinate of myocyte contractility, arterial diameter, and blood flow and pressure regulation.
Soon after discovery [33], TRPM4 was shown to be a critical regulator of cerebral artery smooth muscle cell membrane potential and contractility [12]. Earley et al. [12] reported that TRPM4 mRNA was present in native cerebral artery smooth muscle cells. Patch-clamp experiments using the inside-out configuration identified a novel non-rectifying current (in symmetrical cationic solutions) in these cells with a unitary conductance of approximately 24 pS [12]. Like TRPM4, this 24-pS channel exhibited Ca2+-dependent activation and rapid time-dependent inactivation. Ion substitution experiments demonstrated that the channel was selective for monovalent cations vs. Ca2+ ions [12]. The 24-pS channel was observed much more frequently in membrane patches obtained from cells acutely treated with PMA compared with controls, suggesting sensitivity to PKC activity [12]. The properties of this channel, including Ca2+ dependence, monovalent cation selectivity, time-dependent inactivation, and sensitivity to PKC activity, are reminiscent of those reported for recombinant TRPM4 channels in HEK expression systems. To firmly establish the channel’s molecular identity, antisense oligonucleotides were used to decrease the expression of TRPM4 currents in native cerebral artery smooth muscle cells. The frequency of observation of the 24-pS channel in cells treated with TRPM4 antisense was much lower compared with controls, clearly establishing its identity [12].
Due to selectivity for monovalent cations, TRPM4 channels are predicted to act as Na+ influx channels in vascular smooth muscle cells under physiological conditions as the resting membrane potential (approx. −45 mV) is hyperpolarized compared to the Na+ equilibrium potential (+60 mV). Consistent with this expectation, antisense-mediated downregulation of TRPM4 expression impaired pressure-induced smooth muscle cell depolarization and vasoconstriction in isolated rat cerebral arteries [12]. Membrane depolarization in response to the purinergic receptor agonist uridine triphosphate was not altered by the downregulation of TRPM4, suggesting that the channel is specifically involved in pressure-dependent depolarization [12]. Small interfering RNA (siRNA)-mediated downregulation of TRPM4 expression has similar effects (Earley et al., unpublished observations). In addition, the administration of 9-phenanthrol to isolated rat cerebral arteries with pre-developed myogenic tone resulted in the hyperpolarization of the plasma membrane and relaxation to a nearly passive diameter [18]. More interestingly, the membrane potential hyperpolarized to −70 mV in response to 9-phenanthrol, approaching the K+ equilibrium potential, suggesting that TRPM4 channels are a dominant depolarizing influence in cerebral arterial myocytes [18]. Preliminary data from our laboratory indicate that 9-phenanthrol prevents the development of myogenic tone in renal intralobar arteries (unpublished observations), suggesting that TRPM4 channels are also important in vasomotor responsiveness in this vascular bed.
The physiological significance of the TRPM4-dependent regulation of arterial myocyte membrane potential was elegantly revealed by Reading and Brayden [54], who found that the administration of TRPM4 antisense oligonucleotides into the cerebral spinal fluid of the third cerebral ventricle of rats caused TRPM4 downregulation in pial artery smooth muscle cells in vivo. Cerebral blood flow was measured over a range of mean arterial pressures (MAP) using fluorescent microsphere methods and was found to be significantly greater at elevated MAP in animals treated with TRPM4 antisense vs. controls, indicating that the cerebral arteries in these rats failed to appropriately respond to changes in perfusion pressure. These data provide compelling evidence that TRPM4 is crucially important for the autoregulation of cerebral blood flow in response to pressure changes in vivo.
TRPM4 knockout (TRPM4−/−) mice have an unexpected cardiovascular phenotype. TRPM4−/− mice are hypertensive compared to controls, likely due to elevated circulating catecholamine levels [36]. Phenylephrine-induced contraction of the aortic ring segments and pressure-induced increases in vascular resistance in isolated hind limbs did not differ between TRPM4−/− and wild-type mice, suggesting that agonist-induced and myogenic vasomotor responses were not affected by TRPM4 knockout [36]. The reasons for the difference between the effects of TRPM4 knockout and more acute suppression of TRPM4 expression or activity are not apparent. Possible explanations include differences in species (rats vs. mice), differences in experimental preparation (perfused hind limb preparation vs. pressure myography), and differences in arterial bed (skeletal muscle vs. cerebral). Alternatively, unintended phenotypic changes associated with TRPM4 gene knockout could be compensating for the loss of TRPM4 activity during development. Further experiments, including the use of inducible and/or tissue specific knockout animals, are needed to conclusively resolve these issues.
Ca2+-dependent regulation of TRPM4 in smooth muscle
The regulation of TRPM4 activity in native, contractile cerebral artery myocytes has been extensively characterized. In both heterologous expression systems and freshly isolated cerebral artery smooth muscle cells, TRPM4 channels require high levels of intracellular Ca2+ for activation. Ca2+ levels of 10–100 μM are typically used to record channel activity from cells patch-clamped in the conventional whole-cell and inside-out configurations. This level of Ca2+ is much greater than global Ca2+ levels in vascular smooth muscle cells under normal conditions and results in rapid Ca2+-dependent inactivation of TRPM4. However, native arterial myocytes transiently generate subcellular regions where localized Ca2+ levels are much higher than global levels. Ca2+ micro (or nano) domains created by Ca2+ influx or by Ca2+ are released from the sarcoplasmic reticulum (SR) and are critical for numerous cellular functions. The best-characterized Ca2+ microdomains in arterial smooth muscle cells are Ca2+ sparks, regions of intracellular Ca2+ in the 1- to 100-μM range generated by the release of SR Ca2+ via ryanodine receptors [46]. Ca2+ sparks are functionally coupled to BKCa channels and are important regulators of cerebral artery tone [46]. To record currents generated by Ca2+ microdomains, cells are patch-clamped in the amphotericin B perforated patch configuration to avoid the disruption of intracellular Ca2+ signaling pathways. Using this method, sustained inward cation currents can be recorded from native cerebral artery smooth muscle cells voltage-clamped at negative holding potentials for as long as seal viability is maintained (>30 min). These currents are referred to as “transient inward cation currents” (TICCs) [15]. TICCs have an apparent single-channel conductance of ∼24 pS, reverse near 0 mV in symmetrical cation solution, and are abolished when extracellular Na+ is substituted with the impermeant cation N-methyl-d-glucamine (NMDG) [15]. These properties are similar to those of recombinant TRPM4 channels. In addition, TICC activity is inhibited by the TRPM4 blocking compounds flufenamic acid and 9-phenanthrol and attenuated by the siRNA-mediated downregulation of TRPM4 expression in cerebral artery myocytes [15]. These findings suggest that Na+ influx via TRPM4 is responsible for TICC activity in arterial smooth muscle cells.
The ability to record sustained TRPM4 current activity as TICCs allowed detailed analysis of the Ca2+-dependent activation mechanisms in arterial myocytes. Removal of extracellular Ca2+ did not acutely affect TRPM4 activity recorded from native cerebral artery smooth muscle cells, suggesting that Ca2+ influx is not directly involved [15, 17]. However, blocking the sarco(endo)plasmic reticulum Ca2+-ATPase rapidly inhibited activity, indicating that Ca2+ released from the SR is necessary for TRPM4 activity in these cells [15, 17]. The inhibition of IP3 receptors with Xestospongin C attenuated TICC activity, whereas blocking of ryanodine receptors had no effect on these currents [15]. In addition, TICC activity was stimulated by the membrane permeable IP3 analog Bt-IP3 [17]. These data suggest that TRPM4 channels are activated by Ca2+ released from SR via IP3 receptors [15, 17]. Further evidence of functional coupling between TRPM4 channels and IP3 receptors was provided by conventional whole-cell patch-clamp experiments utilizing the Ca2+ buffers ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) or bis-ethane-N,N,N′,N′-tetraacetic acid (BAPTA) in the pipette solution. BAPTA and EGTA have identical steady-state kinetics and Ca2+-binding affinities, but significantly differ in their binding rate constants [1]. BAPTA binds free Ca2+ at a rate that is two orders of magnitude faster than EGTA [1]. As such, when present at equimolar concentrations, BAPTA will bind Ca2+ released from a point source much more rapidly than EGTA, limiting diffusion. Accordingly, Ca2+ microdomains, with a Ca2+ source-to-sensor distance of >50 nm, are defined by Ca2+-dependent responses that are equally disrupted by identical concentrations of BAPTA and EGTA [45], whereas Ca2+ nanodomains, with a Ca2+ source-to-sensor distance <50 nm, are defined by Ca2+-dependent responses that are significantly interfered with by BAPTA, but not by equimolar concentrations of EGTA [45]. In whole-cell patch-clamp experiments, the activation of TRPM4 was observed when 10 mM EGTA, but not 10 mM BAPTA, was included in the recording pipette [17]. This differential effect on channel activity by equal concentrations of BAPTA or EGTA indicates that IP3 receptor-mediated activation of TRPM4 occurs within spatially restricted domains, suggesting that TRPM4 channels and IP3 receptors are located no more than 50 nm apart (Fig. 1). Furthermore, loss of TRPM4 channel activity in the presence of BAPTA provides electrophysiological evidence that the IP3 receptor-mediated activation of TRPM4 channels depends on Ca2+ release rather than the physical coupling between TRPM4 and IP3 receptors. The regulation of TRPM4 by this mechanism may not be unique to arterial smooth muscle cells as functional coupling between TRPM4 and the IP3 receptor has been reported for the neonatal mouse preBötzinger complex, where the channels contribute to neuronal control of respiratory rhythms [7].
Stimulation of TRPM4 activity by Ca2+ nanodomains created by Ca2+ released from the sarcoplasmic reticulum via IP3 receptors. In freshly isolated smooth muscles, TRPM4 activation requires Ca2+ released from the SR through proximal IP3 receptors. The Ca2+ concentration profile comparing the buffering capacity of the slow and fast Ca2+ chelators EGTA and BAPTA on [Ca2+]i as a function of distance from a single IP3R Ca2+ release site. Simulation data using CalC software v. 6.0 [37] was re-plotted from Gonzales and Earley [17]. From Gonzales and Earley [16]
PKC-dependent regulation of TRPM4 in smooth muscle
TRPM4 activity is stimulated by PKC [11, 12, 20, 52]. PKC is also involved in the development of myogenic tone in cerebral arteries [11, 28, 55], suggesting that PKC may influence myocyte contractility via TRPM4. Consistent with this hypothesis, the PKC activator PMA elicits TRPM4-dependent smooth muscle cell membrane depolarization and cerebral artery constriction [11]. The effects of PKC stimulation on the subcellular distribution of TRPM4 protein were investigated. Using cell surface biotinylation and total internal reflection fluorescence microscopy, Crnich and colleagues [6] showed that the stimulation of PKC activity with PMA caused TRPM4 channel protein to rapidly traffic to the plasma membrane in A7r5 cells, primary cerebral artery myocytes, and intact cerebral arteries. PMA-induced translocation of TRPM4 to the plasma membrane was blocked by selective inhibition of PKCδ, but was insensitive to the inhibition of PKCα and PKCβ [6]. In addition, siRNA-mediated downregulation and pharmacological inhibition of PKCδ caused TRPM4 channels to rapidly translocate from the plasma membrane into the cytosol [13], suggesting that basal PKCδ activity is required to maintain TRPM4 channels at the plasma membrane. Predictably, TRPM4 activity was attenuated by blocking of PKCδ activity [13], and suppression of PKCδ expression hyperpolarized smooth muscle cells and impaired vessel constriction in response to both PMA and increases in intraluminal pressure in intact cerebral arteries [6]. These findings suggest that PKC-induced increases in Ca2+-dependent activation of TRPM4 activity may result from elevated levels of channel protein at the cell surface. Furthermore, these data suggest that smooth muscle cell excitability can be regulated by dynamic trafficking of TRPM4 channels to and from the plasma membrane.
Hypothetical unified scheme for the regulation of TRPM4 activity in cerebral artery smooth muscle cells
Evidence discussed above suggests that TRPM4 channel activity is an important determinant of cerebral artery smooth muscle cell resting membrane potential and contributes to pressure-induced vasoconstriction and autoregulation of blood flow. The data also indicate that in cerebral arterial myocytes, TRPM4 channels are activated by a combination of Ca2+ nanodomains created by Ca2+ released from the SR through IP3 receptors and by PKCδ-mediated translocation of TRPM4 channels to the plasma membrane. The activity of PLC could potentially unify these two pathways. PLC cleaves PtdIns(4,5)P2 into the second messengers diacylglycerol (DAG) and IP3. IP3 elevates Ca2+ release from IP3 receptors and DAG stimulates PKC activity. Thus, it is possible that PLC activity accelerates the generation of TRPM4-activating Ca2+ nanodomains and promotes the translocation of TRPM4 channels to the plasma membrane (Fig. 2). A preliminary report from my laboratory is consistent with this possibility [14], but further verification is necessary. If this scheme is correct, the upstream activators of PLC in smooth muscle cells are of considerable interest. G-protein-coupled receptors that are stimulated by vasoconstrictor agonists such as endothelin-1, purinergic nucleotides, and angiotensin II are well-characterized activators of PLC. In addition, the concept that G-protein-coupled receptors are involved in pressure-induced vasoconstriction has been put forth [38], but this idea remains controversial [2]. Nonetheless, it remains possible that G-protein-mediated activation of PLC could influence smooth muscle cell membrane potential and contractility in response to vasoconstrictor agonists as well as intraluminal pressure by activating TRPM4, but significant additional work is required to test this hypothesis.
Hypothetical unified scheme for the regulation of TRPM4 activity in cerebral artery smooth muscle cells. PM plasma membrane, GPC G-protein-coupled receptor, PLC phospholipase C, PtdIns(4,5) 2 phosphatidylinositol 4,5-bisphosphate, DAG diacylglycerol, IP 3 inositol 1,4,5-triphosphate, IP 3 R inositol 1,4,5-triphosphate receptors, TRPM4 transient receptor potential melastatin 4, ΔV M , change in membrane potential, VDCC voltage-dependent Ca2+ channel
Function of TRPM4 in other types of smooth muscle
TRPM4 channels are present (Table 1) and functional in several types of smooth muscle outside of the vasculature. Dwyer et al. [9] recorded basally active non-selective cation currents in freshly isolated colonic smooth muscle cells from humans and monkeys that are reminiscent of TICCs recorded from native cerebral artery myocytes. These currents were partially inhibited when Na+ was replaced with the impermeant ion NMDG and attenuated with the TRPM4 blocker 9-phenanthrol [9]. In addition, NMDG substitution significantly hyperpolarized the resting membrane potential of these cells [9]. These findings suggest that Na+ influx via TRPM4 channels contributes to the maintenance of resting membrane potential in colonic smooth muscle cells. Two reports by Smith et al. [59, 61] indicate that TRPM4 channels are present in rat and guinea pig detrusor smooth muscle cells that make up the wall of the urinary bladder. TICC-like currents that were sensitive to 9-phenanthrol were recorded from these cells. In addition, 9-phenanthrol reduced the amplitude and frequency of spontaneous and pharmacologically induced contractions and the amplitude of electrical field stimulation-induced contractions of isolated bladder strips, suggesting an important role for TRPM4 in urinary bladder function. TRPM4 mRNA was also detected by RT-PCR in rat pulmonary intralobar artery and aortic smooth muscle, but the channel’s functional significance in these tissues has not been reported [70]. Preliminary findings also suggest that TRPM4 is present in the corpus cavernosum of mice. Precontracted corpus cavernosum strips are relaxed by 9-phenanthrol, indicating that TRPM4 contributes to the maintenance of tonic contraction of this tissue (Werner and Earley, unpublished observations). Collectively, these studies suggest that TRPM4 may be important for establishing the resting membrane potential of many types of smooth muscle cells.
Conclusions
Pharmacological and molecular evidence demonstrate an important role for TRPM4 channels in the regulation of rat cerebral artery smooth muscle cell resting membrane potential and contractility, which critically influences the autoregulation of cerebral blood flow in response to elevations in intraluminal pressure. The regulation of TRPM4 activity in arterial myocytes is complex and involves the release of Ca2+ from the SR through IP3 receptors and translocation of TRPM4 channels to the plasma membrane mediated by the activity of PKCδ. Although the channel appears to be important for myogenic regulation, it remains unresolved how increases in intraluminal pressure are translated into elevated TRPM4-dependent cation current activity. Conceptually, increases in pressure and stretch of arterial myocytes could directly increase TRPM4 activity if the channel is inherently mechanosensitive. In support of this possibility, one report demonstrates increased activity of TRPM4 channels expressed in HEK cells in response to the application of negative pressure to the recording electrode, implying direct mechanosensitivity [43]. However, a preliminary study from my laboratory was unable to replicate these findings, but provides evidence that TRPM4 channels are indirectly activated by stretch through a phospholipase C-dependent pathway [69]. Resolution of this issue and achieving a better understanding of mechosensing in the vascular in general are important areas for future studies. It is also important to consider TRPM4 channels in the context of diseases affecting smooth muscle cells and determine whether the channel or its regulatory pathways are viable targets for pharmaceutical development.
References
Allbritton NL, Meyer T, Stryer L (1992) Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258:1812–1815
Anfinogenova Y, Brett SE, Walsh MP, Harraz OF, Welsh DG (2011) Do TRPC-like currents and G protein-coupled receptors interact to facilitate myogenic tone development? Am J Physiol Heart Circ Physiol 301:H1378–H1388. doi:10.1152/ajpheart.00460.2011
Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T (2004) Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A 101:2894–2899. doi:10.1073/pnas.0305252101
Clapham DE, Julius D, Montell C, Schultz G (2005) International Union of Pharmacology. XLIX. Nomenclature and structure–function relationships of transient receptor potential channels. Pharmacol Rev 57:427–450. doi:10.1124/pr.57.4.6
Cosens DJ, Manning A (1969) Abnormal electroretinogram from a Drosophila mutant. Nature 224:285–287
Crnich R, Amberg GC, Leo MD, Gonzales AL, Tamkun MM, Jaggar JH, Earley S (2010) Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells. Am J Physiol Cell Physiol 299:C682–C694. doi:10.1152/ajpcell.00101.2010
Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA (2007) Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBotzinger complex. J Physiol 582:1047–1058. doi:10.1113/jphysiol.2007.134577
Demion M, Bois P, Launay P, Guinamard R (2007) TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res 73:531–538. doi:10.1016/j.cardiores.2006.11.023
Dwyer L, Rhee PL, Lowe V, Zheng H, Peri L, Ro S, Sanders KM, Koh SD (2011) Basally activated nonselective cation currents regulate the resting membrane potential in human and monkey colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol 301:G287–G296. doi:10.1152/ajpgi.00415.2010
Earley S (2006) Molecular diversity of receptor operated channels in vascular smooth muscle: a role for heteromultimeric TRP channels? Circ Res 98:1462–1464. doi:10.1161/01.RES.0000231255.32630.df
Earley S, Straub SV, Brayden JE (2007) Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292:H2613–H2622. doi:10.1152/ajpheart.01286.2006
Earley S, Waldron BJ, Brayden JE (2004) Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95:922–929. doi:10.1161/01.RES.0000147311.54833.03
Garcia ZI, Bruhl A, Gonzales AL, Earley S (2011) Basal protein kinase Cdelta activity is required for membrane localization and activity of TRPM4 channels in cerebral artery smooth muscle cells. Channels (Austin) 5:210–214
Garcia ZI, Earley S (2011) PLCγ1 is required for IP3-mediated activation of TRPM4 and pressure-induced depolarization and vasoconstriction in cerebral arteries. FASEB J 25 (Abstract)
Gonzales AL, Amberg GC, Earley S (2010) Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 299:C279–C288. doi:10.1152/ajpcell.00550.2009
Gonzales AL, Earley S (2013) Regulation of cerebral artery smooth muscle membrane potential by Ca(2+)-activated cation channels. Microcirculation. doi:10.1111/micc.12023
Gonzales AL, Earley S (2012) Endogenous cytosolic Ca(2+) buffering is necessary for TRPM4 activity in cerebral artery smooth muscle cells. Cell Calcium 51:82–93. doi:10.1016/j.ceca.2011.11.004
Gonzales AL, Garcia ZI, Amberg GC, Earley S (2010) Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol 299:C1195–C1202. doi:10.1152/ajpcell.00269.2010
Grand T, Demion M, Norez C, Mettey Y, Launay P, Becq F, Bois P, Guinamard R (2008) 9-Phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br J Pharmacol 153:1697–1705. doi:10.1038/bjp.2008.38
Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, Bois P (2004) Functional characterization of a Ca(2+)-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol 558:75–83. doi:10.1113/jphysiol.2004.063974
Guinamard R, Chatelier A, Lenfant J, Bois P (2004) Activation of the Ca(2+)-activated nonselective cation channel by diacylglycerol analogues in rat cardiomyocytes. J Cardiovasc Electrophysiol 15:342–348. doi:10.1046/j.1540-8167.2004.03477.x
Guinamard R, Demion M, Launay P (2010) Physiological roles of the TRPM4 channel extracted from background currents. Physiology (Bethesda) 25:155–164. doi:10.1152/physiol.00004.2010
Guinamard R, Demion M, Magaud C, Potreau D, Bois P (2006) Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension 48:587–594. doi:10.1161/01.HYP.0000237864.65019.a5
He LP, Hewavitharana T, Soboloff J, Spassova MA, Gill DL (2005) A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J Biol Chem 280:10997–11006. doi:10.1074/jbc.M411797200
Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ (2003) Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 22:776–785. doi:10.1093/emboj/cdg080
Hofmann T, Chubanov V, Gudermann T, Montell C (2003) TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol 13:1153–1158
Hofmann T, Schaefer M, Schultz G, Gudermann T (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A 99:7461–7466. doi:10.1073/pnas.102596199
Jarajapu YP, Knot HJ (2005) Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am J Physiol Heart Circ Physiol 289:H1917–H1922. doi:10.1152/ajpheart.01012.2004
Kim BJ, Kim SY, Lee S, Jeon JH, Matsui H, Kwon YK, Kim SJ, So I (2012) The role of transient receptor potential channel blockers in human gastric cancer cell viability. Can J Physiol Pharmacol 90:175–186. doi:10.1139/y11-114
Kim BJ, Nam JH, Kim SJ (2011) Effects of transient receptor potential channel blockers on pacemaker activity in interstitial cells of Cajal from mouse small intestine. Mol Cells 32:153–160. doi:10.1007/s10059-011-1019-1
Knot HJ, Nelson MT (1998) Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508(Pt 1):199–209
Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Brink P, Pongs O (2009) Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest 119:2737–2744. doi:10.1172/JCI38292
Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP (2002) TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109:397–407
Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, Kurtbay G, Megarbane A, Ohmert I, Blaysat G, Villain E, Pongs O, Bouvagnet P (2010) Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 3:374–385. doi:10.1161/CIRCGENETICS.109.930867
Maruyama Y, Nakanishi Y, Walsh EJ, Wilson DP, Welsh DG, Cole WC (2006) Heteromultimeric TRPC6-TRPC7 channels contribute to arginine vasopressin-induced cation current of A7r5 vascular smooth muscle cells. Circ Res 98:1520–1527. doi:10.1161/01.RES.0000226495.34949.28
Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londono JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M (2010) Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest 120:3267–3279. doi:10.1172/JCI41348
Matveev V, Zucker RS, Sherman A (2004) Facilitation through buffer saturation: constraints on endogenous buffering properties. Biophys J 86:2691–2709. doi:10.1016/S0006-3495(04)74324-6
Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T (2008) Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27:3092–3103. doi:10.1038/emboj.2008.233
Michel AD, Xing M, Thompson KM, Jones CA, Humphrey PP (2006) Decavanadate, a P2X receptor antagonist, and its use to study ligand interactions with P2X7 receptors. Eur J Pharmacol 534:19–29. doi:10.1016/j.ejphar.2006.01.009
Mironov SL (2013) Calmodulin and CaMKII mediate emergent bursting activity in the brainstem respiratory network (preBotC). J Physiol. doi:10.1113/jphysiol.2012.237362
Moiseenkova-Bell VY, Wensel TG (2009) Hot on the trail of TRP channel structure. J Gen Physiol 133:239–244. doi:10.1085/jgp.200810123
Montell C, Rubin GM (1989) Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2:1313–1323
Morita H, Honda A, Inoue R, Ito Y, Abe K, Nelson MT, Brayden JE (2007) Membrane stretch-induced activation of a TRPM4-like nonselective cation channel in cerebral artery myocytes. J Pharmacol Sci 103:417–426
Mrejeru A, Wei A, Ramirez JM (2011) Calcium-activated non-selective cation currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J Physiol 589:2497–2514. doi:10.1113/jphysiol.2011.206631
Naraghi M, Neher E (1997) Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci 17:6961–6973
Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ (1995) Relaxation of arterial smooth muscle by calcium sparks. Science 270:633–637
Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T (2006) The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 25:467–478. doi:10.1038/sj.emboj.7600963
Nilius B, Owsianik G (2010) Transient receptor potential channelopathies. Pflugers Arch 460:437–450. doi:10.1007/s00424-010-0788-2
Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V (2003) Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem 278:30813–30820. doi:10.1074/jbc.M305127200
Nilius B, Prenen J, Janssens A, Owsianik G, Wang C, Zhu MX, Voets T (2005) The selectivity filter of the cation channel TRPM4. J Biol Chem 280:22899–22906. doi:10.1074/jbc.M501686200
Nilius B, Prenen J, Janssens A, Voets T, Droogmans G (2004) Decavanadate modulates gating of TRPM4 cation channels. J Physiol 560:753–765. doi:10.1113/jphysiol.2004.070839
Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX (2005) Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem 280:6423–6433. doi:10.1074/jbc.M411089200
Nilius B, Prenen J, Voets T, Droogmans G (2004) Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflugers Arch 448:70–75. doi:10.1007/s00424-003-1221-x
Reading SA, Brayden JE (2007) Central role of TRPM4 channels in cerebral blood flow regulation. Stroke 38:2322–2328. doi:10.1161/STROKEAHA.107.483404
Robertson BE, Schubert R, Hescheler J, Nelson MT (1993) cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol 265:C299–C303
Schattling B, Steinbach K, Thies E, Kruse M, Menigoz A, Ufer F, Flockerzi V, Bruck W, Pongs O, Vennekens R, Kneussel M, Freichel M, Merkler D, Friese MA (2012) TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 18:1805–1811. doi:10.1038/nm.3015
Schwarz EC, Wolfs MJ, Tonner S, Wenning AS, Quintana A, Griesemer D, Hoth M (2007) TRP channels in lymphocytes. Handb Exp Pharmacol 179:445–456. doi:10.1007/978-3-540-34891-7_26
Simard C, Salle L, Rouet R, Guinamard R (2012) Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br J Pharmacol 165:2354–2364. doi:10.1111/j.1476-5381.2011.01715.x
Smith AC, Hristov KL, Cheng Q, Xin W, Parajuli SP, Earley S, Malysz J, Petkov GV (2013) Novel role for the transient potential receptor melastatin 4 channel in guinea pig detrusor smooth muscle physiology. Am J Physiol Cell Physiol. doi:10.1152/ajpcell.00169.2012
Smith AC, Hristov KL, Parajuli SP, Cheng Q, Xin W, Malysz J, Petkov GV (2012) Role of TRPM4 channel in urinary bladder function. Fourth International Congress on Cell Membranes and Oxidative Stress: Focus on Calcium Signaling and TRP channels 4, pp 18–19
Smith AC, Parajuli SP, Hristov KL, Cheng Q, Soder RP, Afeli SA, Earley S, Xin W, Malysz J, Petkov GV (2013) TRPM4 channel: a new player in urinary bladder smooth muscle function in rats. Am J Physiol Renal Physiol. doi:10.1152/ajprenal.00417.2012
Takezawa R, Cheng H, Beck A, Ishikawa J, Launay P, Kubota H, Kinet JP, Fleig A, Yamada T, Penner R (2006) A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol Pharmacol 69:1413–1420. doi:10.1124/mol.105.021154
Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B (2005) Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium 37:267–278. doi:10.1016/j.ceca.2004.11.001
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417. doi:10.1146/annurev.biochem.75.103004.142819
Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C (1995) TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A 92:9652–9656
Wu LJ, Sweet TB, Clapham DE (2010) International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62:381–404. doi:10.1124/pr.110.002725
Xu XZ, Li HS, Guggino WB, Montell C (1997) Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell 89:1155–1164
Xu XZ, Moebius F, Gill DL, Montell C (2001) Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci U S A 98:10692–10697. doi:10.1073/pnas.191360198
Yang Y, Gonzales AL, Sanders L, Earley S (2012) Membrane stretch-induced activation of TRPM4 in cerebral artery smooth muscle cells. FASEB J 26 (Abstract)
Yang XR, Lin MJ, McIntosh LS, Sham JS (2006) Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290:L1267–L1276. doi:10.1152/ajplung.00515.2005
Zhang Z, Okawa H, Wang Y, Liman ER (2005) Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem 280:39185–39192. doi:10.1074/jbc.M506965200
Acknowledgments
I thank Michelle N. Sullivan and Dr. Albert L. Gonzales for critical comments on the manuscript. This work was supported by R01HL091905 from NHLBI and a Monfort Excellence Award from the Monfort Family Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Earley, S. TRPM4 channels in smooth muscle function. Pflugers Arch - Eur J Physiol 465, 1223–1231 (2013). https://doi.org/10.1007/s00424-013-1250-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1250-z