Abstract
The purpose of this study was to gain further insight into passive force enhancement by testing whether passive force enhancement occurs in single myofibrils. Myofibrils (n = 6) isolated from rabbit psoas muscle were fixed at a sarcomere length of 2.4 μm, and then stretched passively and actively to a sarcomere length of 3.4 μm. Passive force after deactivation of the myofibrils was increased after active compared to passive stretching. Therefore, passive force enhancement, previously observed in muscle and fiber preparations, also occurs in single myofibrils. Passive force enhancement in myofibrils ranged from 86 to 145% of the steady-state force observed after passive stretch. Because titin is the main source of passive force in myofibrils, we propose that titin might be responsible for passive force enhancement observed in myofibrils. We propose that this might occur through an increase in stiffness when calcium concentration increases upon activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well accepted that the steady-state force produced by a muscle after active stretch is greater than the corresponding purely isometric force [1, 4, 10, 23, 28]. This stretch-induced force enhancement is often accompanied by an increase in passive force after deactivation. This phenomenon known as “passive force enhancement” (PFE) is observed in whole cat soleus muscle [10], in intact human muscles activated voluntarily and through electrical nerve stimulation [20], and in single frog muscle fibers [19, 28, 29].
PFE occurs at long muscle/fiber length, where passive force is naturally occurring; it is long lasting (>25 s) and increases with stretch magnitude and initial muscle length, but is independent of the speed of stretch [10, 13]. These characteristics, combined with the fact that PFE occurs after muscle/fiber deactivation, suggest that PFE might be caused by a passive structure. Structures involved in passive force in skeletal muscle include the weakly bound cross bridges [9], the connective tissue [17], the sarcolemma [25], the intermediate filaments [14], and titin [2, 7, 16]. However, because PFE increases with stretch magnitude where the number of available cross bridges is reduced, the contribution of cross bridges to the PFE seems unlikely.
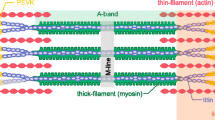
In this study, we wanted to gain further insight into PFE by testing whether PFE occurs in single myofibrils. Myofibrils are formed of sarcomeres arranged in series. Therefore, if isolated myofibrils showed PFE, this would suggest that PFE originates, at least partially, on the sarcomere level. Titin spans the half sarcomeres, connecting the A-bands to the I-bands and Z-lines [16], and it is considered the primary and virtually exclusive source of passive force in myofibrils [3, 5, 21]. Therefore, PFE in myofibrils would likely be related to titin.
Materials and methods
Myofibril preparation
Rabbits were euthanized by an intravenous injection of 1 ml of a pentobarbital solution (240 mg/ml), a protocol approved by the University of Calgary’s Animal Care and Ethics Committee. Strips of psoas muscle were dissected, tied to small wooden sticks, and stored in rigor solution (see “Solutions”) for 12 h at 4°C, then in a rigor-glycerol (50:50) solution at −20°C for 2 weeks. On the day of the experiments, a small piece of muscle tissue (2-mm length) was cut using a fine razor blade, and subsequently blended in rigor solution. A small amount of the blended mixture was placed in a chamber positioned on top of a movable stage mounted on an inverted microscope (Zeiss Axiovert 200 M, Germany). After 5 min of stabilization, the rigor solution was replaced by a relaxing solution (see “Solutions”), and myofibrils in suspension were washed away, leaving those settled on the bottom of the chamber.
A myofibril with a good striation pattern was fixed to a glass needle at one end and to a nanolever (stiffness, 154 pN/nm) [2, 27] at the other end, allowing for length change and force measurement, respectively. The striation pattern of the myofibril was projected onto a linear photodiode array (10,680 elements), which generated a signal with light and dark peaks representing the sarcomere banding pattern. The centroids of the A-bands were determined, and sarcomere lengths were calculated as the distance between adjacent A-band centroids by an algorithm that tracks the signal peak positions continuously during the experiments. The experiments were simultaneously visualized using a charge-coupled device (CCD) camera and recorded by a video recorder. Images of the myofibrils were subsequently used for measurement of the myofibril diameter and force calculation (stiffness of the nanolever × displacement). The displacement of the nanolever was evaluated using the software motion capture plus.
Mechanical tests
Myofibrils (n = 6) were fixed at an average sarcomere length (SL) of 2.4 μm, and then stretched passively in a relaxing solution. The stretch magnitude was 1 μm/sarcomere at a speed of 0.1 μm s−1 sarcomere−1. The myofibrils were then held isometrically for 1 min. After 10 min, myofibrils were activated (see “Solutions”), then stretched (1 μm/sarcomere at a speed of 0.1 μm s−1 sarcomere−1) and held isometrically for 1 min; deactivation was induced 30 s after the end of the stretch (Fig. 1).
Passive force enhancement in myofibrils. Typical myofibril response when stretched passively (●) and actively ( ) from sarcomere lengths 2.4 to 3.4 μm. Fp and Fa are the passive forces obtained following passive and active stretching respectively. The dark arrow indicates the time when the myofibril was deactivated. Passive force enhancement, PFE, is the average difference between Fa and Fp for the last 10 s of the isometric phase following stretch. Fi is the isometric force obtained after activation at a SL of 2.4 μm, Fs is the steady-state force reached after an active stretch from a SL of 2.4 μm to a SL of 3.4 μm
) from sarcomere lengths 2.4 to 3.4 μm. Fp and Fa are the passive forces obtained following passive and active stretching respectively. The dark arrow indicates the time when the myofibril was deactivated. Passive force enhancement, PFE, is the average difference between Fa and Fp for the last 10 s of the isometric phase following stretch. Fi is the isometric force obtained after activation at a SL of 2.4 μm, Fs is the steady-state force reached after an active stretch from a SL of 2.4 μm to a SL of 3.4 μm
Forces were normalized by myofibril cross-sectional area and expressed as stress (nN/μm2). PFE was measured as the difference between the passive forces obtained after active (Fa) and passive stretching (Fp) of the myofibril (Fig. 1). Fa and Fp were determined as the mean value of force recorded during the last 10 s of the isometric phase after active and passive stretching, respectively. Fp and Fa were compared using a paired t test. For comparisons across myofibrils, the nonparametric Mann–Whitney test was used.
Solutions
Rigor solution
The rigor solution includes Tris (50 mM), sodium chloride (100 mM), potassium chloride (2 mM), magnesium chloride (2 mM), and ethylene glycol bis(2-aminoethyl ether)-N,N,N′N′-tetraacetic acid (EGTA; 10 mM) at pH = 7.0.
Relaxing solution
The relaxing solution consists of 3-(N-morpholino) propanesulfonic acid (MOPS; 10 mM), potassium propionate (64.4 mM), magnesium proprionate (5.23 mM), sodium sulfate (9.45 mM), EGTA (10 mM), calcium chloride (0.188 mM), adenosine triphosphate (ATP; 7 mM), and creatine phosphate (10 mM) at pCa = 8.0 and pH = 7.0.
Activating solution
The activating solution consists of MOPS (10 mM), potassium propionate (45.1 mM), magnesium proprionate (5.21 mM), sodium sulfate (9.27 mM), EGTA (10 mM), calcium chloride (9.91 mM), ATP (7.18 mM), and creatine phosphate (10 mM) at pCa = 3.5 and pH = 7.0.
One tablet of protease inhibitors (Complete®, Roche Diagnostics) was added to each 50-ml solution.
Results
Figure 1 shows force–time histories of active and passive stretches in a typical experiment. In all myofibrils, the passive force produced after the active stretch was higher than the force produced after the passive stretch, indicating the presence of PFE. Fp varied from 29 to 40 nN/μm2, while Fa varied from 54 to 84 nN/μm2. PFE ranged between 86 and 145% of Fp (Table 1).
We did not activate the myofibrils at a SL of 3.4 μm, and therefore, we were not able to calculate the total force enhancement as it is classically calculated [4, 10, 11]. However, according to the force–length relationship [6] scaled to the thin and thick filament lengths in rabbit psoas muscle [12], the force at a SL of 3.4 μm should be about 33% of the force produced at 2.4 μm. Interestingly, the force after stretch of activated myofibrils, where the SL was 3.4 μm, was higher than or similar to the force produced at a SL of 2.4 μm (Fig. 1). The steady-state isometric forces produced by activation at a SL of 2.4 μm (Fi) and after the stretch (Fs) are presented in Table 2.
Discussion
The purpose of this study was to gain further insight into PFE observed in skeletal muscle by testing whether PFE occurs in single myofibrils. We determined the force produced by myofibrils after a passive and an active stretch. Passive force was increased after active stretch, suggesting that PFE, previously observed in muscle and fiber preparations [10, 26, 28], also occurs in single myofibrils.
Myofibrils are formed of sarcomeres arranged in series. Therefore, as myofibrils showed PFE, we suggest that at least one of the origins of PFE exists in the sarcomere. The characteristics of the PFE, combined with the fact that it occurs after deactivation, suggest that a passive structure is responsible for PFE. In myofibrils, titin is the major source of passive force [7, 15, 22]. The expression of different titin isoforms has been reported as responsible for myofibrillar passive elastic diversity [3, 5, 7, 21, 31]. For example, the higher passive force of cardiac myofibrils compared to that of skeletal muscle has been correlated to the expression of a short titin isoform in cardiac myofibrils [22]. A similar argument was used for explaining differences in passive stiffness between psoas and soleus rabbit fibers. Furthermore, degradation or extraction of titin from myofibrils leads to a rapid drop in passive force [8, 24, 31] in cardiac and skeletal myofibrils. Accordingly, titin is also the likely source of PFE in single myofibrils.
If titin is the source of PFE in myofibrils, then the passive force increases after an active stretch through an activation-induced increase in titin’s stiffness. In our experiments, activation was induced by a solution containing a high concentration of calcium, and so titin’s increase in stiffness and associated passive force after active stretching might be associated with the presence of calcium. Titin might act like a spring whose elastic modulus increases or whose characteristic length decreases with the increase in calcium concentration. In this case, titin would be directly responsible for the PFE observed when a muscle is actively stretched. This mechanism would be consistent with previous results showing that titin has specific calcium binding sites [30] and that the binding of calcium changes the stiffness properties of titin and also affects titin binding to actin [18], thereby, affecting titin’s natural resting length. This effect would be long lasting; the rate constant of calcium dissociation from titin would be very small, and the effect of calcium on titin stiffness would persist after deactivation and decrease in calcium concentration.
In addition to the presence of PFE, our data showed residual force enhancement in single myofibrils. This finding is consistent with earlier observations in muscles [4, 10, 20, 23] and fibers [19, 26, 28, 29].
References
Abbott BC, Aubert XM (1952) The force exerted by active striated muscle during and after change of length. J Physiol 117:77–86
Bartoo ML, Linke WA, Pollack GH (1997) Basis of passive tension and stiffness in isolated rabbit myofibrils. Am J Physiol 273:C266–C276
Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitas K, Labeit S, Granzier H (2000) Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res 86:59–67
Edman KA, Elzinga G, Noble MI (1982) Residual force enhancement after stretch of contracting frog single muscle fibers. J Gen Physiol 80:769–784
Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, Kolmerer B, Witt C, Beckmann JS, Gregorio CC, Granzier H, Labeit S (2000) Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res 86:1114–1121
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184:170–192
Granzier H, Helmes M, Cazorla O, McNabb M, Labeit D, Wu Y, Yamasaki R, Redkar A, Kellermayer M, Labeit S, Trombitas K (2000) Mechanical properties of titin isoforms. Adv Exp Med Biol 481:283–300
Granzier HL, Irving TC (1995) Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J 68:1027–1044
Granzier HL, Wang K (1993) Passive tension and stiffness of vertebrate skeletal and insect flight muscles: the contribution of weak cross-bridges and elastic filaments. Biophys J 65:2141–2159
Herzog W, Leonard TR (2002) Force enhancement following stretching of skeletal muscle: a new mechanism. J Exp Biol 205:1275–1283
Herzog W, Leonard TR (2000) The history dependence of force production in mammalian skeletal muscle following stretch-shortening and shortening-stretch cycles. J Biomech 33:531–542
Herzog W, Leonard TR, Renaud JM, Wallace J, Chaki G, Bornemisza S (1992) Force-length properties and functional demands of cat gastrocnemius, soleus and plantaris muscles. J Biomech 25:1329–1335
Herzog W, Schachar R, Leonard TR (2003) Characterization of the passive component of force enhancement following active stretching of skeletal muscle. J Exp Biol 206:3635–3643
Higuchi H, Umazume Y (1985) Localization of the parallel elastic components in frog skinned muscle fibers studied by the dissociation of the A- and I-bands. Biophys J 48:137–147
Horowits R (1992) Passive force generation and titin isoforms in mammalian skeletal muscle. Biophys J 61:392–398
Horowits R, Kempner ES, Bisher ME, Podolsky RJ (1986) A physiological role for titin and nebulin in skeletal muscle. Nature 323:160–164
Kovanen V, Suominen H, Heikkinen E (1984) Mechanical properties of fast and slow skeletal muscle with special reference to collagen and endurance training. J Biomech 17:725–735
Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H (2003) Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA 100:13716–13721
Lee EJ, Joumaa V, Herzog W (2007) New insights into the passive force enhancement in skeletal muscles. J Biomech 40:719–727
Lee HD, Herzog W (2002) Force enhancement following muscle stretch of electrically stimulated and voluntarily activated human adductor pollicis. J Physiol 545:321–330
Linke WA, Ivemeyer M, Olivieri N, Kolmerer B, Ruegg JC, Labeit S (1996) Towards a molecular understanding of the elasticity of titin. J Mol Biol 261:62–71
Linke WA, Popov VI, Pollack GH (1994) Passive and active tension in single cardiac myofibrils. Biophys J 67:782–792
Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U (2000) Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J Physiol 522(Pt 3):503–513
Opitz CA, Kulke M, Leake MC, Neagoe C, Hinssen H, Hajjar RJ, Linke WA (2003) Damped elastic recoil of the titin spring in myofibrils of human myocardium. Proc Natl Acad Sci USA 100:12688–12693
Rapoport SI (1973) The anisotropic elastic properties of the sarcolemma of the frog semitendinosus muscle fiber. Biophys J 13:14–36
Rassier DE, Herzog W (2004) Active force inhibition and stretch-induced force enhancement in frog muscle treated with BDM. J Appl Physiol 97:1395–1400
Rassier DE, Herzog W, Pollack GH (2003) Dynamics of individual sarcomeres during and after stretch in activated single myofibrils. Proc Biol Sci 270:1735–1740
Rassier DE, Herzog W, Wakeling J, Syme DA (2003) Stretch-induced, steady-state force enhancement in single skeletal muscle fibers exceeds the isometric force at optimum fiber length. J Biomech 36:1309–1316
Rassier DE, Lee EJ, Herzog W (2005) Modulation of passive force in single skeletal muscle fibres. Biol Lett 1:342–345
Tatsumi R, Maeda K, Hattori A, Takahashi K (2001) Calcium binding to an elastic portion of connectin/titin filaments. J Muscle Res Cell Motil 22:149–162
Wang K, McCarter R, Wright J, Beverly J, Ramirez-Mitchell R (1991) Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension. Proc Natl Acad Sci USA 88:7101–7105
Acknowledgment
The authors would like to thank H. M. Brattberg and A. Jinha for their technical assistance. The financial support of Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes of Health Research (CIHR), and the Canada Research Chair Program is greatly acknowledged.
The nanolevers used in this study were constructed at the Cornell NanoScale Facility, which is supported by the National Science Foundation (grant ECS 03-35765).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joumaa, V., Rassier, D.E., Leonard, T.R. et al. Passive force enhancement in single myofibrils. Pflugers Arch - Eur J Physiol 455, 367–371 (2007). https://doi.org/10.1007/s00424-007-0287-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-007-0287-2