Abstract
The development of the nervous system requires complex series of cellular programming and intercellular communication events that lead from the early neural induction to the formation of a highly structured central and peripheral nervous system. Neurogenesis continuously takes place also in select regions of the adult mammalian brain. During the past years, a multiplicity of cellular control mechanisms has been identified, ranging from differential transcriptional mediators to inducers or inhibitors of cell specification or neurite outgrowth. While the identification of transcription factors typical for the stage-specific progression has been a topic of key interest for many years, less is known concerning the potential multiplicity of relevant intercellular signaling pathways and the fine tuning of epigenetic gene regulation. Nucleotide receptors can induce a multiplicity of cellular signaling pathways and are involved in multiple molecular interactions, thus opening the possibility of cross talk between several signaling pathways, including growth factors, cytokines, and extracellular matrix components. An increasing number of studies provides evidence for a role of nucleotide signaling in nervous system development. This includes progenitor cell proliferation, cell migration, neuronal and glial cellular interaction and differentiation, and synaptic network formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the development of the mammalian nervous system, neural stem cells and their derivative progenitor cells generate neurons by asymmetric and symmetric divisions [74]. They are also the source of the two types of macroglial cells of the central nervous system (CNS), astrocytes, and oligodendrocytes [97]. The development of the nervous system requires complex series of cellular programming and intercellular communication events that lead from the neural induction of a specified region of the ectoderm to the formation of a highly structured central and peripheral nervous system, with specified cell types and pathways of cellular interconnection [66]. These processes include the control of proliferation and cell fate determination of neurons, astrocytes, and oligodendrocytes, cell migration and maturation, neurite extension and nerve guidance, synapse formation, plasticity and network implementation and, in addition, abandoning of excessive cellular contacts and apoptosis. During the past years, a multiplicity of cellular control mechanisms has been identified, ranging from differential transcriptional mediators to inducers or inhibitors of cell specification or neurite outgrowth. Furthermore, there is now abundant evidence that neurogenesis continuously takes place in select regions of the adult mammalian forebrain [3]. This has initiated a wealth of studies to understand the mechanisms controlling adult neuron formation and raises hopes to reinitiate neurogenesis under conditions of neural trauma or disease [29, 55, 109].
While the identification of transcription factors typical for the stage-specific progression during nervous system development has been a topic of key interest for many years, less is known concerning the potential multiplicity of intercellular signaling substances and the epigenetic gene regulation that define or modulate individual developmental switches [13, 35, 69, 86]. Regarding nervous system development, nucleotides [32] are among the most recently identified and least investigated group of extracellular signaling substances. The present overview highlights recent evidence for a role of nucleotide signaling in nervous system development with a focus on the mammalian nervous system. Aspects of the role of nucleotides and nucleosides in nervous system development have previously been reviewed [30, 31, 64].
Signaling via extracellular nucleotides
Signaling via extracellular nucleotides is complex (Fig. 1). Nucleotides act via ionotropic or G-protein-coupled receptors (P2 receptors) [146]. The homomeric or heteromeric P2X receptors (seven subtypes, P2X1–7) are stimulated by ATP, are permeable to Na+, K+, and Ca2+, and mediate fast signal transmission [139]. Most of these can combine to form heterooligomers, resulting in altered pharmacological properties. The eight subtypes of P2Y receptors (P2Y1,2,4,6,11,12,13,14) are activated by ATP, ADP, UTP, UDP, or nucleotide sugars, depending on the subtype, and couple to differential intracellular signaling pathways [104]. In rodents, the most common experimental animals, the ligand preference (in brackets) is as follows: P2Y1 (ADP, ATP), P2Y2 (UTP, ATP), P2Y4 (UTP, ATP), P2Y6 (UDP), P2Y11 (ATP), P2Y12 (ADP), P2Y13 (ADP), and P2Y13 (UDP glucose and other nucleotide sugars). The ATP-activated P2Y11 receptor is not apparent in the rodent genome. The potential role in nervous system development of dinucleoside polyphosphates, which can activate various P2 receptors as well as receptors of their own [116], has not yet been investigated. Extracellular nucleotides can be inactivated or interconverted by a multiplicity of enzymes generally referred to as ectonucleotidases. These belong to several enzyme families that vary regarding catalytic properties and cellular expression pattern [201, 203]. Extracellular ATP is eventually degraded to adenosine that may additionally influence neural development via P1 receptors (A1, A2A, A2B, A3) [146, 163]. ATP (and UTP) can be released from essentially every cell type. Release can be constitutive as well as stimulus-evoked and regulated [164].
Potential pathways of extracellular nucleotide metabolism and receptor function. ATP is shown as an example. Released ATP is metabolized by ectonucleotidases to adenosine in a stepwise manner (catabolism). Some of the ectonucleotidases directly degrade ATP to AMP without intermediate formation of ADP. ATP is an agonist at P2X receptors as well as at P2Y1, P2Y2, P2Y4, and P2Y11 receptors whereas UTP only activates P2Y2 and P2Y4 receptors. ADP is an agonist at P2Y1, P2Y12, and P2Y13 receptors and UDP at P2Y6 receptors. Adenosine activates P1 receptors (A1, A2A, A2B, and A3). The surface-located enzymes nucleoside diphosphate kinase and adenylate kinase catalyze the extracellular formation of ATP (anabolism). Ectoprotein kinases can phosphorylate surface-located proteins which in turn are dephosphorylated by ectophosphoprotein phosphatase activity (potentially by alkaline phosphatases)
Nucleotide receptor-induced cellular signaling
Nucleotides and nucleosides mediate a considerable number of trophic effects on neurons and glial cells including cell proliferation, cell differentiation, axonal growth, and the synthesis and release of trophic factors such as fibroblast growth factor-2 (FGF-2), nerve growth factor (NGF), or neurotrophin 3 [132, 147]. Recent evidence suggests that cellular communication via nucleotides can involve a considerable variety of molecular interactions and cellular signaling mechanisms.
P2Y receptors
Depending on subtype, P2Y receptor activation can induce several intracellular signaling cascades, most notably activation of phospholipase C, activation or inhibition of adenylyl cyclase, activation of phospholipase A2, coupling to ion channels, activation of the mitogen-activated (MAP) kinase pathways, and induction of immediate early genes. Transactivation of receptor tyrosine kinases opens up additional avenues for cross talk with other signaling pathways [1, 14, 104, 115, 130]. The outcome of individual P2 receptor activation is likely to vary with cell type, with the coexpression of receptors for additional signaling molecules and with the associated intracellular signaling molecules—that can change during development [12, 57]. In addition, P2Y receptors were shown to form homooligomers or heterooligomers with other G-protein-coupled receptors (GPCRs) that could impact on both ligand properties and intracellular signaling. The P2Y2 receptors can form homooligomers [102], and the P2Y1 receptor forms heterooligomers with the A1 adenosine receptor [123].
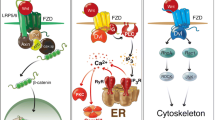
The multiple interactions of the well-investigated UTP- and ATP-activated P2Y2 receptor can serve as an example [190]. Through activation of phospholipase C, it induces a rise in intracellular Ca2+. This may modulate Ca2+ signaling pathways including the generation of intercellular Ca2+ waves that can play a significant role in embryonic development [189]. Furthermore, the human P2Y2 receptor contains two C-terminal consensus Src-homology-3 (SH3) binding domains that promote recruitment and activation of Src. The Src family of tyrosine kinases was found to be important for embryonic stem-cell renewal [8]. Activation of Src is important for P2Y2 receptor-mediated transactivation of soluble tyrosine kinases, the epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and vascular endothelial growth factor receptor (VEGFR)-2 [110, 167], that have all been implicated in the control of neurogenesis [187]. P2Y2 receptor activation increases Src kinase-dependent clustering with the EGFR and VEGFR-2 receptors. Similarly, P2Y2 receptor activation in the presence of NGF leads to the colocalization and association of tyrosine receptor kinase A and P2Y2 receptors [9]. Furthermore, the P2Y2 receptor contains an RGD integrin-binding motif in its first extracellular loop. It colocalizes with αVβ3/β5 integrins [56], suggesting that it can form a large signaling complex containing integrins and proteins associated with integrins such as Src, focal adhesion kinase, Pyk2, EGFR, PDGFR, and the actin cytoskeleton [110]. In accordance, the P2Y2 receptor-mediated astrocyte migration was shown to involve the interaction with αV integrin [10, 186].
P2Y receptors activate MAP kinase pathways in a wide variety of cell types [165, 170]. The functional role of MAP kinase protein cascades in P2Y receptor-mediated trophic actions has been particularly intensively investigated in astrocytes [128, 130]. In these cells, extracellular signal-regulated protein kinases (ERKs) can be activated via P2Y1, P2Y2, or P2Y4 receptors whereby the intracellular signaling cascades vary between receptor subtypes [100, 107, 127, 129]. Nucleotides can exert a synergistic effect on cell proliferation together with growth factors, chemokines, or cytokines in a variety of cell types, including astrocytes and stem cells [88, 106, 117, 126, 181]. This may be initiated by parallel activation of the MAP kinase pathway and/or by transactivation of growth factor receptors [107, 133] (Fig. 2). Activation of P2 receptors (including P2Y2 receptors) can also result in CREB phosphorylation [26, 42, 119]. P2Y2 receptor activation in astrocytes not only induces the expression of bcl-2 and bcl-xl, thus triggering survival signaling cascades, but also stimulates the expression of genes implicated in nervous system development, neuronal differentiation and survival, and the formation and function of synapses [42].
Simplified model of potential pathways for cross talk between ATP-, ADP-, or UTP-activated P2Y receptors and growth factor receptors (e.g., EGF, PDGF, and VEGFR-2). Both signaling pathways can converge at the MAP kinases Erk1/2, inducing rapid transcription and protein synthesis that could augment, e.g., cell proliferation. In addition, the G protein-coupled P2Y receptors may directly phosphorylate and thus activate the receptor tyrosine kinase (receptor transactivation). Furthermore, P2 receptors may activate plasma membrane-bound ectometalloproteases that could cleave and mobilize membrane-bound growth factor precursors
An additional and very interesting potential concerns the stimulation of α-secretase activity by the heterologously expressed P2Y2 receptor, the enhancement of amyloid precursor protein processing, and the concomitant release of the soluble non-amyloidogenic amyloid precursor protein-α (sAPP) [34]. sAPP has been shown to increase proliferation of embryonic and adult neural stem cells [33]. In addition, the P2 receptor-mediated activation of ectometalloproteases carries potential to induce ectodomain shedding of additional transmembrane proteins such as TGF-α (endogenous ligand of the EGF receptor), HB-EGF, or of Notch1 [79, 89]. Growth factor shedding could in turn result in the activation of the corresponding growth factor receptors [115].
It is of interest that EGF can sensitize and potentiate the intracellular Ca2+ response to ATP [49]. This opens up the possibility that the cellular release of small amounts growth factor could prime and increase the impact of nucleotidergic signaling during neural development.
P2X receptors
Activation of P2X receptors by ATP generally induces a robust cellular influx of Na+ and Ca2+. This in turn leads to a rapid cellular depolarization and to the activation of intracellular Ca2+-dependent signaling cascades. In most cases, activation of P2X7 receptors leads to the opening of large conductance channels that have been associated with the induction of apoptosis [139] and may thus be relevant for stage-specific programmed cell death. Activation of this receptor and also of other P2X receptors can also initiate ERK1/2 phosphorylation [71, 92].
P1 receptors
The four different receptors for adenosine (P1 receptors) couple either negatively or positively to adenylyl cyclase [146]. In addition, these receptors can couple to MAP kinases which could give them a role in cell growth, survival, death, and differentiation [163]. In astrocytes, ERK can be activated via P1 purinoceptor in addition to P2Y receptor agonists [131]. Furthermore, P1 receptors crosstalk with other metabotropic receptors, including P2Y receptors [6]. This opens up a considerable potential for an interaction of P2 receptor- and P1 receptor-mediated pathways in multiple functional contexts.
Developmental changes in nucleotide receptor expression
Both P1 and P2 receptors are expressed during nervous system development [31]. P2X family genes are already expressed in the developing zebrafish nervous system [140]. The early and transient expression of P2 receptors in distinct developmental settings can serve as a first indication for an involvement of the nucleotidergic signaling cascade.
P2X receptors
Several P2Y and P2X receptors were found by RT-PCR and immunocytochemistry to be dynamically expressed in the pre- and postnatal central and peripheral nervous system [39–41, 99, 196]. Among all the P2X receptors examined, the homomeric P2X3 receptor was the first to be expressed during neurogenesis in both the rat CNS and peripheral nervous system. P2X3 immunoreactivity was detected in cranial motor neurons as early as E11, when neurons exit the cell cycle and start axon outgrowth. The P2X3 receptor was also identified in (the neural crest cell-derived) sensory ganglia. During further development, the P2X3 receptor appeared in additional distinct brain regions and in peripheral nerves but its expression declined at later stages. From P16 onward, P2X3 immunoreactivity was absent from the facial nucleus, spinal trigeminal tract, the mesencephalic trigeminal nucleus, and the vestibular nucleus. The P2X2 receptors that can form heterodimers with the P2X3 receptor appeared after the P2X3 receptor (E14 onward). In retinal ganglion cells, the P2X3 receptor was detected from E14.5 onward, whereas the P2X2 receptor was not detected in the prenatal retina. P2X7 receptors were detected from E14 onward. While P2X4, P2X5, and P2X6 receptors were expressed from P1 onward, no expression of P2X1 receptors was observed [39, 40]. It is interesting to note that both P2X4 and P2X5 receptors were expressed in the neurogenic subventricular zone, presumably in affiliation with separate, yet-to-be-defined cell types (see below). Similarly, P2X3 receptor immunoreactivity developed transiently in the myenteric plexus of rat stomach. Immunoreactivity appeared first at E12 in the trunk and branches of the vagus nerve and was first demonstrated at P1 in intrinsic neuron cell bodies. It declined after peaking at P14. Within the intraganglionic laminar nerve endings and intramuscular arrays, many growth cone-like structures exhibited strong P2X3 immunoreactivity [195].
The P2X3 receptor is also transiently expressed in the developing mouse brain, and in precursors of spinal motor neurons where it is considered to be a useful cell lineage marker for neural, crest-derived early sensory neurons, sympathetic neurons, and the adrenal medulla [22]. Based on immunohistochemistry, expression of P2X3 receptors is equally transient in mouse sensory ganglia [152].
The P2X7 receptor has previously been shown to induce cytotoxicity at high concentrations of ATP and to be involved in programmed cell death [105, 121]. Indeed, via the P2X7 receptor, endogenous extracellular ATP triggers the death of retinal cholinergic neurons during normal development, thereby controlling the total number, local density, and regular spacing of these neurons [148]. It is possible that this receptor is also involved in apoptosis during neurogenesis in the brain.
Also in sensory organs, the expression of P2X receptors can be developmentally regulated. P2X1, P2X2, P2X3, and P2X7 receptors are expressed in the developing inner ear [27, 84, 85, 87, 95, 134, 135] suggesting a contribution to the establishment of synaptic connections between the primary auditory neurons and the sensory hair cells. The time pattern of expression varies between the P2X1, P2X2, and P2X3 subunits. For example, the expression of the P2X3 receptor becomes apparent in neurites of spinal ganglion neurons already at E16 [87]. It is expressed to synaptic terminals and vanishes again postnatally (Fig. 3). This transient nature of expression and the association with afferent fibers implies that P2X receptor upregulation is linked to the development in particular of the innervation of the organ of Corti. P2X receptors are likely to contribute to the neurotrophic signaling that establishes functional auditory neurotransmission before the likely onset of hearing (P8–P12).
Example for the transient expression of a P2X receptor subunit (P2X3) during the development of the rat cochlear innervation, as revealed by immunohistochemistry (modified from [87]). The receptor is first detected at E16 in the spiral ganglion that provides the major primary afferent innervation to the cochlear sensory hair cells. It disappears at P14. Between E18 and P4, it can be identified in the afferent synaptic terminals that innervate the inner hair cells (IHC). Between P2 and P13, it is associated with the afferent fibers innervating the outer hair cells (OHC). The P2X3 receptor could not be immunodetected in the adult cochlea (in contrast to the P2X2 receptor). The rat is borne deaf and the onset of airborne hearing is around P12. The early postnatal period is thus critical for the synaptic determination of the afferent (and efferent) innervation of the cochlear outer hair cells
P2Y receptors
A novel P2Y receptor was discovered in the Xenopus that is expressed at very early stages of neural plate development. It is no longer detected after neural tube closure [21]. The chick P2Y1 receptor is expressed in a developmentally regulated manner in various embryonic tissues, including the brain [114]. In situ hybridization first detected the receptor at approximately stage 33. The receptor was expressed mostly in regions of postmitotic neurons, including the telencephalon, with smaller patches in the dorsal diencephalon, posterior midbrain, and anterior hindbrain. Expression in the telencephalon, dorsal diencephalon, and posterior hindbrain was strengthened by stage 36.
Using RT-PCR and immunohistochemistry, Burnstock et al. also analyzed the developmental expression of P2Y1, P2Y2, P2Y4, and P2Y6 receptors during rat development [41]. Only P2Y1 and P2Y4 receptor proteins were identified in the developing brain. The P2Y4 receptor appeared to be the dominant P2Y receptor present early in the brain. It appeared first at E14 and was identified in the olfactory system, diencephalon, amygdala, and brain stem. P2Y1, P2Y2, and P2Y4 receptor proteins were all expressed in the spinal cord and the peripheral nervous system from E12. At this stage, the P2Y1 receptor was detected in the floor plate of the spinal neural tube, a transient organization center that profoundly influences the development of the nervous system [52]. The P2Y2 and P2Y4 receptors were expressed in the ventral horns of the embryonic spinal cord and, together with the P2Y1 receptor, may play a role in motor neuron development.
Developmental alterations in P2 receptor signaling
Several studies addressed P2 receptor-mediated signaling in development-specific states. In the chicken retina, ATP-evoked Ca2+ transients were strongest as early as E3 and were drastically reduced at E11–13.5 [178]. In neonatal rats, ATP transiently affected the excitation of motoneurons of the nucleus ambiguus, which are the final output neurons of the swallowing pattern generator [28]. In medullary slices from P0–4 animals, local application of ATP produced large desensitizing inward currents whereas in slices from juvenile animals (P15–21), only negligible currents were recorded. This corresponded to a transient robust expression of the P2X3 receptor and moderate and weak expression of P2X2, P2X5 and P2X4, P2X6 receptors, respectively. The data support the notion that the transient excitatory actions of ATP on neonatal motoneurons impact on the development and/or neuromodulation of the ambigual motor network. Similarly, ATP, operating via distinct P2X and P2Y receptors, directly contributes to modulate rat hippocampal network activity at early stages of postnatal development [154].
In presynaptic terminals of spinal cord substantia gelatinosa neurons, P2X receptors that enhance glycinergic activity become functional late in ontogeny (later than P10–12) [94]. Similarly, in locus coeruleus neurons of the rat, inward currents could be induced by ATP antagonists at P18–23 but hardly at P10–14 [192]. It is apparent that no functional P2 receptors are present at locus coeruleus neurons right after birth but thereafter, P2 purinoceptor function increases with age.
In the rat cerebellar cortex, the neuronal network develops during the first 3 weeks of life. In a recent study, ATP was found to enhance the synaptic activity in rat cerebellar Purkinje neurons by the end of the second postnatal week. From P9/10 to P17/18, ATP was found to increase fourfold the frequency of spontaneous postsynaptic currents (sPSCs) recorded from Purkinje neurons. Functional ATP receptors appeared during P10–12, sPSC frequency was enhanced after inhibition of ecto-ATPases, and application of the P2 receptor antagonist PPADS revealed tonic stimulation of P2 receptors at P14 [37]. Similarly, immunostaining demonstrated a postnatal increase in cerebellar P2X receptor expression [196]. These data imply that purinergic systems mature during the second postnatal week in the rat cerebellum and start to contribute to the modulation of the synaptic activity in Purkinje neurons. They also reveal that the ectonucleotidase pathway is functionally developed at that developmental stage and raise the question whether mechanisms and sites of ATP release are under a comparable developmental control.
A transient expression of P2X receptor function has also been reported for the peripheral nervous system. As revealed by patch clamp recording, sympathetic neurons of the rat superior cervical ganglion are more responsive to ATP at birth and during the early postnatal period due largely to the expression of the P2X3 subunit. These responses are much reduced in mature rats [54]. In the embryonic (E14) chick ciliary ganglion neurons, ATP induced a rapid inward current, inferring ATP-mediated modulation of these parasympathetic neurons [2].
Developmental changes in ectonucleotidase expression
Ligand availability at P2 receptors is tightly controlled by ectonucleotidases. Prolonged exposure can lead to either receptor desensitization or downregulation [146, 182]. Ectonucleotidases may hydrolyze ATP or UTP as agonists of P2 receptors. They may also generate ADP or UDP as agonists of nucleoside diphosphate-sensitive P2Y receptors and eventually produce the P1 receptor agonist adenosine [201]. Developmentally regulated transient receptor expression may be accompanied by alterations in ectonucleotidase expression. Several investigations demonstrate that the expression of individual ectonucleotidases differs in developmental onset and that ectonucleotidases can be transiently expressed in defined regions of the nervous system, including the neurogenic regions.
Alkaline phosphatase
The four mammalian isoforms of alkaline phosphatase (AP) degrade nucleoside 5′-tri-, -di-, and -monophosphates, release inorganic phosphate from a large variety of organic compounds, including proteins, and share an alkaline pH optimum [201]. They are anchored to the cell surface via a glycosylphosphatidyl inositol (GPI) anchor. It is interesting to note that the tissue nonspecific form of alkaline phosphatase (TNAP) is expressed by mouse and human undifferentiated embryonic stem cells [43]. As revealed by enzyme histochemistry, homogeneous TNAP activity in the mouse neuroepithelium becomes apparent at E8.5 [125]. At E9.5, distinctly TNAP-positive cells appear in the brain and spinal cord. At E10.5–12.5, TNAP positivity is observed between the mesencephalon and the rhombencephalon, along the entire spinal cord and the cranial nerves emerging from the myelencephalon, suggesting an association with pioneer growth cones. At E14.5, TNAP expression is considerably reduced in brain tissue and very low in the adult brain. In the late developing rat cerebellar cortex, peak alkaline phosphatase activity was localized to the proliferative external granular cells until P7 [198]. More recent studies allocate TNAP to primary sensory areas in the adult primate sensory cortex, including axonal and dendritic processes and the synaptic cleft. It is most notable that TNAP activity is regulated by sensory experience [59]. In the postnatal marmoset (Callithrix jacchus), expression of TNAP is developmentally regulated both in gray and white matter [60], suggesting that TNAP is involved in the mechanisms regulating maturation of synaptic transmission and axonal conduction in the developing brain.
The functional role of the enzyme during embryonic development has not yet been investigated. In addition to its nucleotidase activity, TNAP can ectodephosphorylate proteins and it has been shown to bind to collagens [193]. Because TNAP can catalyze the entire hydrolysis chain from the nucleoside-5′-triphosphate to the respective nucleoside, it may scavenge or produce ligands of P2 receptors (comp. Fig. 1) and finally produce adenosine as the ligand of P1 receptors. Mice lacking TNAP reveal no gross abnormalities in brain development. However, at approximately 2 weeks after birth, they develop fatal seizures. These have been attributed to a defective metabolism of pyridoxal 5′-phosphate and subsequent reduced levels of the inhibitory neurotransmitter GABA [188]. Deficiency in TNAP is associated with defective bone mineralization [124]. At present, little is known concerning the mechanism controlling TNAP expression. TNAP can be induced by retinoic acid [158] and by activation of the phosphatidyl inositol 3-kinase/Akt pathway [138].
Ectonucleotide pyrophosphatase/phosphodiesterases
Similar to alkaline phosphatases, the ectonucleotide pyrophosphatase/phosphodiesterases (E-NPPs) NPP1, NPP2, and NPP3 share an alkaline pH optimum. They hydrolyze 5′-monodiester bonds in nucleotides and their derivatives, resulting in the release of 5′-nucleotide monophosphates. Physiological substrates include ATP, NAD+, nucleotide sugars, and also dinucleoside polyphosphates. Typically, ATP is directly degraded to AMP with the release of inorganic pyrophosphate. Whereas NPP1 and NPP3 are type II membrane proteins, NPP2 (autotaxin) is secreted [72, 172]. As revealed by in situ hybridization, autotaxin, a splice variant of NPP2, is expressed as early as E9.5 in the floor plate of the neural tube. Expression increases during the following days but is absent at E16.5 [15]. During later brain development, autotaxin has been correlated with intermediate stages of rat brain oligodendrocyte differentiation and myelin formation [68]. It becomes expressed with myelinating oligodendrocytes and participates in the myelination via a novel signaling pathway leading to changes in integrin-dependent focal adhesion assembly and consequently oligodendrocyte extracellular matrix interactions [61, 62]. The related enzyme NPP3/gp130RB13−6 is transiently expressed during rat brain development by a small subset of precursor cells distributed in a specific spatio-temporal pattern in the germinal layers of the ventricular zone of the immature brain. These cells are capable of differentiating in vitro into cells with radial–glial, astrocytic or ependymal cell properties [20]. In the adult brain, immunolabeling for NPP3 is restricted to the ependyma. It is interesting to note that transfection of NPP3 into several cell lines induced cell aggregation, cell motility, and invasion into collagen I. Furthermore, it induced coexpression of both glial and neuronal markers [48]. It has been hypothesized that NPP3 may represent an important factor in the process of glial cell proliferation which is accompanied by migration of glial precursors.
The endogenous substrate relevant in the various E-NPP locations remains to be identified. In particular, NPP2/autotaxin (but not NPP1 and NPP3) also targets lipids, exerting lysophospholipase D activity. NPP2 has been shown to promote tumor cell motility [177], and this property results from the generation of lysophosphatidic acid [184]. The involvement of NPP2/autotaxin in oligodendrocyte development may thus mainly result from its lysophospholipase D activity and an additional functional domain mediating the modulation of oligodendrocyte remodeling and focal adhesion organization [50].
Ectonucleoside triphosphate diphosphohydrolases
NTPDase1–3 and NTPDase8, four members of the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) protein family, are firmly anchored to the plasma membrane via two transmembrane domains and thus represent typical ectoenzymes. All four enzymes hydrolyze nucleoside triphosphates including the physiologically active ATP and UTP but the hydrolysis rates for nucleoside diphosphates vary considerably. They also differ in product formation. This is of considerable relevance for the regulation of nucleotide signaling [19, 202]. NTPDase1 and NTPDase3 were found to be associated with the vasculature and neurons, respectively [17, 151]. They can also be detected by Northern hybridization in the developing nervous system (Zimmermann, unpublished results). In synaptosomes isolated from rat cerebral cortex, a postnatal increase in ecto-ATPase and ecto-ADPase activities was observed [122]. NTPDase2 protein and catalytic activity is associated with neural progenitor cells of the late embryonic and adult rodent brain [24, 168], implicating a role of purinergic signaling pathways in neurogenesis (see below). NTPDase2 is associated also with immature and nonmyelinating Schwann cells of the peripheral nervous system, with satellite glia cells of dorsal root ganglia and sympathetic ganglia, and with enteric glia [25].
Ecto-5′-nucleotidase
Mammalian ecto-5′-nucleotidase catalyzes the hydrolysis of nucleoside monophosphates and is thus of major importance for the extracellular formation of the P1 receptor agonist adenosine [200]. In the adult CNS, ecto-5′-nucleotidase has a predominantly glial (astrocytic, oligodendroglial) location. However, there is considerable evidence that the enzyme is associated with the neural surface during development and plasticity. During postnatal ontogeny of the rat cerebellum, it is expressed to the surface of migrating neuroblasts [58, 159]. It is notable that proliferation of precursors, cell migration and differentiation, and dendritic and axonal growth and remodeling occur equally in the postnatal cerebellum. Ecto-5′-nucleotidase also becomes transiently associated with synapses during synaptogenesis and synapse remodeling [11, 58, 160]. Examples include the developing postnatal cerebellum, the adult olfactory bulb, lesion-induced sprouting within the adult dentate gyrus, and also reactive synaptogenesis in the human brain [108]. Expression of ecto-5′-nucleotidase was found to be mandatory for neurite extension in vitro [80].
An impressive correlation has been found between the development of the kitten visual cortex and the distribution of ecto-5′-nucleotidase [161, 162]. Within postnatal weeks 4 to 6, the enzyme exhibits a patchy distribution that is related to the terminal fields of the afferents from the two eyes that segregate during this phase to form ocular dominance columns. During this developmental phase, ecto-5′-nucleotidase is associated with synaptic contacts and shifts to a glial location only when the processes of synapse formation and remodeling have come to an end. Furthermore, the cell adhesion carbohydrate epitope HNK-1 is transiently associated with the kitten ecto-5′-nucleotidase during the period of its synaptic expression [185]. These observations support the notion that ecto-5′-nucleotidase plays a significant role in neurogenesis and in cellular contact formation within the synaptic cleft.
While it is tempting to assume that the expression of the enzyme indicates the involvement of P1 receptor-mediated mechanisms, additional functional contexts may apply. Ecto-5′-nucleotidase also binds to the extracellular matrix components laminin and fibronectin and may, in addition, mediate cellular adhesion [200]. Nerve growth factor and retinoic acid increase ecto-5′-nucleotidase expression [101, 150]. Wnt and β-catenin signaling target the expression of ecto-5′-nucleotidase and increase extracellular adenosine formation [171]. Wnt signaling is essential for neural development at various stages and also plays a role in axon guidance and neurite outgrowth [90].
Neural progenitors
In the mammalian nervous system, neurogenesis occurs predominantly during embryogenesis whereas glial cells are prevalently generated after birth. During prenatal and early postnatal stages, neurogenesis takes place in two actively proliferating zones, the ventricular zone (VZ) and the subventricular zone (SVZ), two relatively thin layers lining the primitive ventricular cavities [144]. After birth, these layers progressively disappear, leaving the mature nervous tissue in contact with a continuous, ventricle-lining cell monolayer, the ependyma. Both neurons and glia continue to be generated in restricted adult forebrain structures, including the subependymal layer (SEL) (or adult SVZ) of the lateral walls of the lateral ventricles [7] and the subgranular layer (SGL) of the dentate gyrus of the hippocampus [98].
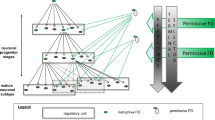
The SEL and its rostral extension, originating after the occlusion of the primitive olfactory ventricle, generate neuroblasts that migrate towards the olfactory bulb (OB), constituting the rostral migratory stream (RMS) (Fig. 4). Within the RMS, the neuroblasts continue to divide and move as a network of tightly associated chains of cells ensheathed by tubes of slowly proliferating astrocyte-like cells [111, 113]. Upon reaching the OB, the neuroblasts move radially and differentiate into granular or periglomerular interneurons [111]. Within the adult SEL, three principal, closely adjacent cell types are distinguished morphologically and functionally. The glial fibrillary acidic protein- (GFAP) and nestin-expressing protoplasmic astrocyte-like type-B cells are thought to represent (at least in part) the actual stem cells. They give rise to transit-amplifying progenitors (type-C cells) that generate type-A cells, representing the neuroblasts migrating to the OB [70]. For comparison, in the hippocampus, radial glia-like cells (residual radial glia) with their cell body in the SGL are considered to function as progenitors and maintain their neurogenic potential into adulthood (Fig. 5). The GFAP- and nestin-expressing residual radial glial elements proliferate and become transformed into neurons via transit-amplifying cells [98, 173]. Cells in the transition state start to express markers of neuronal differentiation and migrate into the granule cell layer where they differentiate into interneurons [53]. There is now increasing evidence for an association of distinct functional purinergic signaling pathways with embryonic and adult neural progenitors.
Stage-specific cellular expression of NTPDase2 during adult neurogenesis in the subependymal layer (SEL) of the rodent lateral ventricles. Top Parasaggital section through a murine forebrain, depicting a lateral ventricle (LV) with the SEL and the corresponding rostral migratory stream (RMS), extending from the SEL into the olfactory bulb (OB). Bottom The nucleoside triphosphate-hydrolyzing enzyme NTPDase2 is selectively associated with the slowly proliferating type-B cells considered to represent (at least in part) adult neural stem cells. The enzyme becomes downregulated on formation of the highly proliferating type-C cells and is also absent from migrating neuroblasts (type-A cells) and mature interneurons that are formed after migration of type-A cells into the OB. Within the RMS, NTPDase2 is associated with astrocyte-like cells ensheathing the migrating neuroblasts but it is absent from cells radially migrating out from the RMS within the OB (according to [117])
Cellular association of NTPDase2 in the neurogenic adult mouse dentate gyrus. NTPDase2 is expressed by slowly proliferating residual radial glia considered to represent the neurogenic progenitors. The cell body of this cell type is situated in the subgranular layer (SGL). It extends strong radial processes throughout the granule cell layer (GCL) and forms bushy arborizations within the inner molecular layer (IML). NTPDase2 is still associated with highly multiplying intermediate cell types [166] that exhibit a horizontal extension in the SGL. Expression of NTPDase2 ceases when young neurons migrate into the GCL and differentiate into mature neurons, with long dendrites spanning the outer molecular layer (OML) and an axon innervating CA3 pyramidal neurons (according to [168])
Association of ectonucleotidase activity with stem cells/progenitor cells
NTPDase2 is the dominant ectonucleotidase expressed by mammalian progenitors in the late embryonic and adult murine brain [24]. The enzyme reveals a high preference for the hydrolysis of nucleoside triphosphates over nucleoside diphosphates. The enzyme thus not only inactivates ligands for nucleoside triphosphate-sensitive receptors but also generates ligands for nucleoside diphosphate-sensitive receptors. In the rat and mouse brain, NTPDase2 is first immunodetected at E18 and E17, respectively. It gradually becomes expressed along the ventricular and subventricular zone of the brain, followed by retraction to the adult expression pattern at P21. In the adult brain, NTPDase2 is selectively expressed by the GFAP, polysialylated neural cell adhesion molecule- (PSA-NCAM) and S-100-positive stem cells (type-B cells) of the SEL and the RMS (Fig. 4). It is also associated with select tanycytes of the third ventricle and by subpial astrocytes. It is interesting to note that neurogenesis has recently also been allocated to the ventricular layer of the adult third ventricle [197]. Furthermore, NTPDase2 selectively marks postnatal cortical radial glia [168]. Radial glia is considered to represent the major source of neurons during development [73]. Cortical immunostaining for NTPDase2 ceases after P5, a state when radial glia transforms into astrocytes [118].
In addition, NTPDase2 is selectively associated with progenitor cells of the mouse dentate gyrus, beginning with the early migration of progenitor cells into the dentate gyrus anlage during embryonic development [168]. The embryonic pattern of expression mirrors that of the dentate migration of neuroblasts. By P23, the immunostaining corresponds to the adult stage. At that stage, the NTPDase2-positive cells correspond to the “residual radial glia”, the presumptive adult progenitors of hippocampal granule neurons. Double immunolabeling of the adult dentate gyrus revealed that NTPDase2 is associated with subpopulations of GFAP-, nestin-, and doublecortin-positive cells and also with the amplifying horizontal D cells [166] (Fig. 5). NTPDase2 is absent from mature granule cells and S100-positive astrocytes. NTPDase2-positive cells proliferate and postmitotic cells preferentially acquire an NTPDase2-positive phenotype.
Association of purinergic receptors with stem cells/progenitor cells
Neural stem cells can successfully be cultured in vitro as floating cell aggregates, referred to as neurospheres. Neurospheres cultured from the adult SEL in the presence of epidermal growth factor (EGF) and fibroblast growth factor-2 (FGF-2) contain multipotent precursors with the characteristics of neural stem cells and the ability to generate neurons, astrocytes, and oligodendrocytes [76, 149]. Neurospheres derived from the adult mouse SEL express the metabotropic P2Y1 and P2Y2 nucleotide receptors, resulting in the generation of rapid Ca2+ transients after the application of the agonists ATP, ADP, or UTP [117]. Agonists of these receptors and also low concentrations of adenosine augmented cell proliferation in the presence of growth factors, inferring nucleotide receptor- and growth factor receptor-mediated synergism with progenitor cell proliferation. Growth factor-stimulated neurosphere cell proliferation was attenuated after application of the P2Y1 receptor antagonist MRS2179 and in neurospheres from P2Y1 receptor knockout mice. These results suggest that nucleotides may be constitutively released from neurosphere cells and exert a synergistic P2 receptor-mediated effect on growth factor-mediated cell proliferation. Furthermore, neurospheres expressed the ectonucleotidases NTPDase2 and TNAP and hydrolyzed extracellular ATP to adenosine. While the data suggest that in vitro progenitors can directly be targeted by nucleotides, the cellular allocation of nucleotide receptors in the SEL is less defined. Using in situ hybridization, the P2Y1 receptor could be allocated not only to striatal neurons but also to select clusters of cells in the SEL [117].
Nucleotides activate hippocampal progenitors in situ. In acute slices, the neural progenitors in the adult dentate gyrus of the hippocampus react with an inward current to application of ATP, implicating the expression of P2X receptors [168]. P2X receptor-mediated inward currents were also detected on individual cultured and undifferentiated adult hippocampal progenitors [82].
There is increasing evidence for a functional role of purinergic signaling pathways also in embryonic neurogenesis. Ntera-2/D1 (NT-2) cells, a pluripotent human embryonal carcinoma-derived clonal cell line, express functional P2Y1 receptors resulting in an ATP- or ADP-mediated increase in intracellular Ca2+ [120]. P2Y1 receptor-mediated Ca2+ transients could be evoked in cells (originally obtained from the embryonic striatum, E14) of connexin43-null mice that had migrated out from neurospheres shifted to growth factor-free medium [156]. In addition, progenitor migration and proliferation may be affected by the P2Y1 receptor. ATP was found to induce an elevation of cytosolic Ca2+ levels and proliferation of precursor cells cultured from immortalized human stem cells derived from the embryonic telencephalon as well as from mouse embryonic and adult neurospheres [153, 181]. The functional impact of nucleotides in embryonic neurogenesis is further corroborated by the observation of P2Y1 receptor-mediated Ca2+ waves through radial glial cells in slices of the embryonic rat ventricular zone. Disrupting Ca2+ waves between these neuronal progenitors reduced ventricular zone cell proliferation during the peak of embryonic neurogenesis [191]. Similarly, ATP stimulates proliferation of neural, retinal progenitor cells in the embryonic chicken retina [143, 179]. Recent investigations using acute slices of postnatal mice further revealed the presence of ATP-induced inward currents in precursors in the SVZ and in particular in the RMS [23].
Neurite outgrowth
Whereas coactivation of P2Y receptors and EGF leads to enhanced cell proliferation, it appears that NGF and P2Y receptor coactivation preferentially results in neurite outgrowth. The effects of nucleotides vary, however, between cell type or cell line investigated. In neural tube explants derived from E12 rat embryos, ATP (but not UTP) had an inhibitory effect on motor axon outgrowth, most likely via the P2X3 receptor [40]. In contrast, ATP, several ATP analogues, and UTP were shown to enhance NGF-mediated survival and neurite outgrowth from pheochromocytoma (PC12) cells [16, 45, 46, 145] and also from cultured neonatal dorsal root ganglion neurons, apparently involving P2Y2 receptor activation [9]. In accordance, P2 receptor antagonists prevented NGF-dependent neuritogenesis [44]. It is interesting to note that a similar stimulatory effect on PC12 neuritogenesis has been described for guanosine and GTP [77, 78] for which no cell surface-located receptors are known to date. The effect of GTP was not mediated by P2 receptors.
In dibutyryl-cAMP-differentiated human neuroblastoma SH-SY5Y cells, a model system to study neuronal differentiation in vitro, UTP acting via the P2Y4 receptor increased the contribution of neurite-bearing cells. Transient transfection with the P2Y4 receptor facilitated neuritogenesis. This was accompanied by an increased transcription of immediate early genes linked to differentiation. However, prolonged exposure to UTP induced cell death [38]. In addition, activation of adenosine A1 and A2A receptors induced neuritogenesis in SH-SY5Y cells in a nonsynergistic manner, apparently via differential intracellular signaling pathways [36]. A similar effect was observed with primary cultures of striatal neurons. 2-Chloroadenosine increased cell number and neurite length in cultured myenteric neurons and synergistically enhanced neurite outgrowth with FGF-2 [157]. In accordance, culturing of PC12 cells on an ecto-5′-nucleotidase substratum stimulated neurite extension of PC12 cells [81]. Inhibition of extracellular adenosine formation by inhibition of ecto-5′-nucleotidase activity via selective antibodies or by knockdown of the enzyme protein via antisense oligonucleotides inhibited neurite formation in PC12 cells and in cultured cerebellar granule neurons [80]. In contrast, A1 receptor activation was found to inhibit NGF-induced neurite outgrowth in PC12 cells and cultured cortical and hippocampal neurons [180].
There may be additional impacts of nucleotides on the interaction of neurons and on neurite outgrowth. Extracellular ATP binds to (and can be hydrolyzed by) the neural cell adhesion molecule NCAM. Neurite outgrowth from hippocampal neurons (prepared from embryonic E17–19 rats) allowing NCAM homophilic interactions was inhibited by high concentrations of ATP [169]. Autotaxin/NPP2 induced neurite retraction of PC12 cells, an effect possibly produced by its lysophospholipase D rather than by its ectonucleotidase activity [155].
Developing glia
Astroglia and retinal Müller cells
Multiple investigations revealed the presence of P2 and P1 receptors on all types of glial cells. During the neurogenic phase of the cerebral cortex, radial glia is considered as the relevant stem cell, generating most of the pyramidal neurons [103, 137]. Towards the end of neurogenesis, the cortical progenitor switches back to symmetric division and gives rise to astrocytes. Astrocytes, generally cultured from postnatal brain, express a wide array of P2X and P2Y receptors, and nucleotides and nucleosides have important roles in the proliferation and differentiation of these cells [63, 67, 93, 100, 132, 142]. As for other cell types, the possibility needs to be considered that the functional properties of cultured cells differ from the in situ situation. But nucleotide-mediated astrocyte proliferation and differentiation were demonstrated in situ [65]. A single cell PCR of astrocytes acutely isolated from the hippocampal CA1 region of P8-12 rats revealed the mRNA of P2Y1, P2Y2, or P2Y4 receptors [199]. In cultured astrocytes, ATP or UTP increased GFAP expression and chemotactic and chemokinetic cell migration [186], and induced intracellular Ca2+ transients and proliferation [130]. Astrocytes share this property with the astrocyte-like stem cells of the embryonic and adult nervous system (see above). For comparison, elevated concentrations of adenosine induce astrocyte apoptosis [51]. Differentiation of Müller cells from radial glial progenitors in the developing rabbit retina is accompanied by a decreasing capability to respond to ATP with an increase in intracellular Ca2+. Whereas postnatally most of the cells respond, the response is minor regarding cell number and amplitude in the adult retina [183].
Oligodendroglia
Oligodendrocytes tend to arise from specific locations within the CNS, in particular the ventral region of the neural tube, and migrate into the white matter of the CNS to myelinate axons [136]. Oligodendrocytes are generated by the proliferation and differentiation of oligodendrocyte progenitors (OPs) [75]. There are multiple studies on the modulation of oligodendrocyte function by nucleotides and nucleosides [4, 5]. The effect of nucleotides and nucleosides on cultured OPs varies between investigations, possibly as a result of variations in culture conditions or source of cells. OPs cultured from the forebrain of P1 rats expressed P2X7 and P2Y1 as the main ionotropic and metabotropic nucleotide receptors, respectively [4]. Their activation induced intracellular Ca2+ transients. P2Y1 receptors were also expressed in NG2-positive OPs in P4 cerebral cortex and in the cerebellar granule cell layer. P2Y receptor activation stimulated OP random motility and to a lesser extent chemotaxis, inhibited the mitogenic response of OPs to platelet-derived growth factor (PDGF) in both purified and organotypic cultures, and promoted OP differentiation. Migration and proliferation was also inhibited by adenosine. Other studies invoke a significant stimulation of OP migration by an A1 adenosine receptor agonist [141].
It is interesting to note that the release of nucleotides and nucleosides from electrically active axons was shown to regulate proliferation and oligodendrocyte development. OP cells cultured from embryonic rat brain were found to express adenosine receptors that were activated in response to action potential firing. Adenosine inhibited OP cell proliferation and promoted the formation of myelin [176]. Adenosine thus appears to be a primary activity-dependent signal promoting the differentiation of premyelinating progenitor cells into myelinating oligodendrocytes.
Schwann cells
Most Schwann cells develop from the neural crest. Their generation involves the formation of two intermediates, the Schwann cell precursor and the immature Schwann cells, originating during later embryonic development. In mature nerves, individual large caliber axons are ensheathed by myelinating Schwann cells, whereas bundles of small caliber axons are loosely ensheathed by the nonmyelinating Schwann cells [96], whose sensitivity to purinergic stimulation differs [112]. Similar to oligodendrocytes, ATP and adenosine were found to act as axonally derived signals mediating activity-dependent communication between neurons and premyelinating cultured mouse Schwann cells, but their effect differed [174, 175]. ATP was found to inhibit proliferation of cultured Schwann cells (in contrast to astrocytes that are stimulated, see above) and to arrest maturation before differentiation into either the myelinating or nonmyelinating phenotypes, thus preventing the formation of myelin. For comparison, adenosine also inhibited Schwann cell proliferation albeit through a different mechanism than ATP. It did not, however, inhibit Schwann cell differentiation and myelination [175].
Microglia
Microglial cells represent the immune effector cells of the CNS. They are derived from mesenchymal precursors that invade the brain during late embryogenesis and early postnatal periods. Several studies have identified P2X and P2Y receptors and a variety of functional responses in cultured microglia [18, 91]. These include eliciting of Ca2+ transients, stimulating proliferation, inducing cell death via activation of P2X7 receptors, or stimulating the release of plasminogen, IL-1β, or tumor necrosis factor. ATP was found to mediate rapid morphological responses in microglia to brain injury in vivo [47] and ATP or ADP induced chemotaxis via P2Y receptors in a microglial cell line [83]. A recent study has investigated the ontogenetic expression of the microglial P2X receptors (P2X1, P2X4, and P2X7) by immunocytochemistry in the rat brain [194]. These receptors were identified on microglial cells from late E16. The expression of individual P2X receptors varied between microglial subtype or functional state and tissue location. At P30, microglial cells with P2X1 receptors had disappeared and microglial cells with P2X7 receptor-immunoreactivity were found to be widely distributed in the forebrain, suggesting that the P2X7 receptor governs the actions of ATP in the resting microglial cells.
Summary and outlook
Signaling pathways employing extracellular nucleotides and adenosine are expressed early on during embryonic development of the nervous system. The transient expression of defined receptor subtypes and of ectonucleotidases implies that nucleotides and nucleosides affect stage-specific developmental processes. Yet, much more needs to be learned concerning the developmental onset of receptor function and ectonucleotidase activity in specific cellular settings. In several cases, the transient expression of P2 receptors suggests that nucleotidergic signaling is of even greater functional significance during development than in the adult nervous system. There is increasing evidence that purinergic signaling has a direct impact on progenitor cell proliferation, cell migration, neuronal and glial cellular interaction and differentiation, and synaptic network formation (Fig. 6).
Very little is presently known concerning sites, cell types, and mechanisms of nucleotide release in the various developmental settings. While it appears possible that ubiquitous and constitutive nucleotide release becomes relevant only when the appropriate receptors are expressed, it is likely that regulated release also plays a significant role. It is now becoming clear that nucleotide receptors can induce a multiplicity of cellular signaling pathways and are involved in multiple molecular interactions. A fascinating possibility concerns the congruence and synergistic interactions of multiple signaling pathways such as those of growth factors, cytokines, and purines/pyrimidines. Preconditioning via one pathway may increase the impact of another. Thus, analyzing the functional role of single signaling pathways on defined developmental processes may fall short of identifying the relevance of multiple and interactive signaling mechanisms in situ.
It can be anticipated that the focus on nucleotide signaling in nervous system development will rapidly broaden. Novel technical approaches are going to facilitate the investigations. These include not only the analysis of knockouts of specific components of the nucleotide signaling pathways but also the knockdown of individual proteins by cell-type specific viral transfection and RNA interference, or the analysis of animals in which the encoding gene can be inactivated or selectively induced in specific tissues by genetic approaches. Transgenic mice expressing fluorescent protein under the promoter of the respective receptor or ectonucleotidase will greatly facilitate the identification of the expression pattern of individual proteins during development. Purines may have several functional roles; they may act as direct functional switches or as mutual modulators or enhancers of signaling pathways mediated by other agonists. The examples provided show that nucleotides possess a broad potential to control multiple developmental cues, alone or together with other signaling substances.
References
Abbracchio MP, Brambilla R, Ceruti S, Cattabeni F (1999) Signalling mechanisms involved in P2Y receptor-mediated reactive astrogliosis. Prog Brain Res 120:333–342
Abe Y, Sorimachi M, Itoyama Y, Furukawa K, Akaike N (1995) ATP responses in the embryo chick ciliary ganglion cells. Neuroscience 64:547–551
Abrous DN, Koehl M, LeMoal M (2005) Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85:523–569
Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonté C, Aloisi F, Visentin S (2005) Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia 50:132–144
Agresti C, Meomartini ME, Amadio S, Ambrosini E, Volonté C, Aloisi F, Visentin S (2005) ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Brain Res Rev 48:157–165
Alloisio S, Cugnoli C, Ferroni S, Nobile M (2004) Differential modulation of ATP-induced calcium signalling by A1 and A2 adenosine receptors in cultured cortical astrocytes. Brit J Pharmacol 141:935–942
Alvarez-Buylla A, García-Verdugo JM (2002) Neurogenesis in the adult ventricular zone. J Neurosci 22:629–634
Annerén C, Cowan CA, Melton DA (2004) The Src family of tyrosine kinases is important for embryonic stem cell self-renewal. J Biol Chem 279:31590–31598
Arthur DB, Akassoglou K, Insel PA (2005) P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA 102:19138–19143
Bagchi S, Liao ZJ, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L (2005) The P2Y2 nucleotide receptor interacts with alphaV integrins to activate Go and induce cell migration. J Biol Chem 280:39050–39057
Bailly Y, Schoen SW, Delhaye-Bouchaud N, Kreutzberg GW, Mariani J (1995) 5′-Nucleotidase activity as a synaptic marker of parasagittal compartmentation in the mouse cerebellum. J Neurocytol 24:879–890
Baker OJ, Camden JM, Ratchford AM, Seye CI, Erb L, Weisman GA (2006) Differential coupling of the P2Y1 receptor to Gα14 and Gαq/11 proteins during the development of the rat salivary gland. Arch Oral Biol (in press)
Bally-Cuif L, Hammerschmidt M (2003) Induction and patterning of neuronal development, and its connection to cell cycle control. Curr Opin Neurobiol 13:16–25
Barnard EA, Simon J, Tsim KWK, Filippov AK, Brown DA (2003) Signalling pathways and ion channel regulations of P2Y receptors. Drug Dev Res 59:36–48
Bächner D, Ahrens M, Betat N, Schröder D, Gross G (1999) Developmental expression analysis of murine autotaxin (ATX). Mech Dev 84:121–125
Behrsing HP, Vulliet PR (2004) Mitogen-activated protein kinase mediates purinergic-enhanced nerve growth factor-induced neurite outgrowth in PC12 cells. J Neurosci Res 78:64–67
Belcher SM, Zsarnovzky A, Crawford PA, Hemani H, Spurling L, Kirley TL (2006) Immunolocalization of ecto-nucleoside triphosphate diphosphohydrolase 3 in rat brain: implications for modulation of multiple homeostatic systems including feeding and sleep wake bahaviors. Neuroscience 137:1331–1346
Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonté C, Matteoli M, Abbracchio MP, Verderio C (2005) Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev 48:144–156
Bigonnesse F, Lévesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJG, Sévigny J (2004) Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry (USA) 43:5511–5519
Blass-Kampmann S, Kindler-Rohrborn A, Deissler H, D’Urso D, Rajewsky MF (1997) In vitro differentiation of neural progenitor cells from prenatal rat brain: common cell surface glycoprotein on three glial subsets. J Neurosci Res 48:95–111
Bogdanov YD, Dale L, King BF, Whittock N, Burnstock G (1997) Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J Biol Chem 272:12583–12590
Boldogköi Z, Schutz B, Sallach J, Zimmer A (2002) P2X3 receptor expression at early stage of mouse embryogenesis. Mech Dev 118:255–260
Bolteus AJ, Liu X, Bordey A (2005) Postnatal neuronal precursors from the subventricular zone express ionotropic ATP receptors. Program No. 595 17 2005, Abstract Viewer/Itinery Planner Washington, DC: Society for Neuroscience, Online
Braun N, Sévigny J, Mishra S, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H (2003) Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci 17:1355–1364
Braun N, Sévigny J, Robson SC, Hammer K, Hanani M, Zimmermann H (2004) Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia 45:124–132
Brautigam VM, Frasier C, Nikodemova M, Watters JJ (2005) Purinergic receptor modulation of BV-2 microglial cell activity: potential involvement of p38 MAP kinase and CREB. J Neuroimmunol 166:113–125
Brändle U, Zenner HP, Ruppersberg JP (1999) Gene expression of P2X-receptors in the developing inner ear of the rat. Neurosci Lett 273:105–108
Brosenitsch TA, Adachi T, Lipski J, Housley GD, Funk GD (2005) Developmental downregulation of P2X3 receptors in motoneurons of the compact formation of the nucleus ambiguus. Eur J Neurosci 22:809–824
Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Götz M (2005) Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A 102:18183–18188
Burnstock G (1996) Purinoceptors: ontogeny and phylogeny. Drug Dev Res 39:204–242
Burnstock G (2001) Purinergic signalling in development. In: Abbracchio MP, Williams M (eds) Purinergic and pyrimidergic singnalling I. Molecular, nervous and urogenitary system function. Springer, Berlin Heidelberg New York, pp 89–127
Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304
Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, Prochiantz A (2004) Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 131:2173–2181
Camden JM, Schrader AM, Camden RE, Gonzalez FA, Erb L, Seye CI, Weisman GA (2005) P2Y2 nucleotide receptors enhance α-secretase-dependent amyloid precursor protein processing. J Biol Chem 280:18696–18702
Campbell K (2003) Dorsal–ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol 13:50–56
Canals M, Angulo E, Casadó V, Canela EI, Mallol J, Vinals F, Staines W, Tinner B, Hillion J, Agnati L, Fuxe K, Ferré S, Lluis C, Franco R (2005) Molecular mechanisms involved in the adenosine A1 and A2A receptor-induced neuronal differentiation in neuroblastoma cells and striatal primary cultures. J Neurochem 92:337–348
Casel D, Brockhaus J, Deitmer JW (2005) Enhancement of spontaneous synaptic activity in rat Purkinje neurones by ATP during development. J Physiol (London) 568:111–122
Cavaliere F, Nestola V, Amadio S, D’Ambrosi N, Angelini DF, Sancesario G, Bernardi G, Volonté C (2005) The metabotropic P2Y4 receptor participates in the commitment to differentiation and cell death of human neuroblastoma SH-SY5Y cells. Neurobiol Dis 18:100–109
Cheung KK, Burnstock G (2002) Localization of P2X3 receptors and coexpression with P2X2 receptors during rat embryonic neurogenesis. J Comp Neurol 443:368–382
Cheung KK, Chan WY, Burnstock G (2005) Expression of P2X purinoceptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience 133:937–945
Cheung KK, Ryten M, Burnstock G (2003) Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn 228:254–266
Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA (2004) P2Y2 receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem 91:119–132
Czyz J, Wiese C, Rolletschek A, Blyszczuk P, Cross M, Wobus AM (2003) Potential embryonic and adult stem cells in vitro. Biol Chem 384:1391–1409
D’Ambrosi N, Cavaliere F, Merlo D, Milazzo L, Mercanti D, Volonté C (2000) Antagonists of P2 receptor prevent NGF-dependent neuritogenesis in PC12 cells. Neuropharmacology 39:1083–1094
D’Ambrosi N, Murra B, Cavaliere F, Amadio S, Bernardi G, Burnstock G, Volonté C (2001) Interaction between ATP and nerve growth factor signalling in the survival and neuritic outgrowth from PC12 cells. Neuroscience 108:527–534
D’Ambrosi N, Murra B, Vacca F, Volonté C (2004) Pathways of survival induced by NGF and extracellular ATP after growth factor deprivation. Prog Brain Res 146:93–100
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758
Deissler H, Blass-Kampmann S, Bruyneel E, Mareel M, Rajewsky MF (1999) Neural cell surface differentiation antigen gp130RB13−6 induces fibroblasts and glioma cells to express astroglial proteins and invasive properties. FASEB J 13:657–666
Delicado EG, Jimenez AI, Carrasquero LMG, Castro E, Miras-Portugal MT (2005) Cross-talk among epidermal growth factor, Ap5A, and nucleotide receptors causing enhanced ATP Ca2+ signaling involves extracellular kinase activation in cerebellar astrocytes. J Neurosci Res 81:789–796
Dennis J, Nogaroli L, Fuss B (2005) Phosphodiesterase-Iα/autotaxin (PD-Iα/ATX): a multifunctional protein involved in central nervous system development. J Neurosci Res 82:737–742
Di Iorio P, Kleywegt S, Ciccarelli R, Traversa U, Andrew CM, Crocker CE, Werstiuk ES, Rathbone MP (2002) Mechanisms of apoptosis induced by purine nucleosides in astrocytes. Glia 38:179–190
Dodd J, Jessell TM, Placzek M (1998) The when and where of floor plate induction. Science 282:1654–1657
Doetsch F (2003) A niche for adult neural stem cells. Curr Opin Genet Dev 13:543–550
Dunn PM, Gever J, Ruan HZ, Burnstock G (2005) Developmental changes in heteromeric P2X2/3 receptor expression in rat sympathetic ganglion neurons. Devel Dyn 234:505–511
Emsley JG, Mitchell BD, Kempermann G, Macklis JD (2005) Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol 75:321–341
Erb L, Liu J, Ockerhausen J, Kong QM, Garrad RC, Griffin K, Neal C, Krugh B, Santiago-Perez LI, Gonzalez FA, Gresham HD, Turner JT, Weisman GA (2001) An RGD sequence in the P2Y2 receptor interacts with aVβ3 integrins and is required for Go-mediated signal transduction. J Cell Biol 153:491–501
Fam SR, Paquet M, Castleberry AM, Oller H, Lee CJ, Traynelis SF, Smith Y, Yun CC, Hall RA (2005) P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA 102:8042–8047
Fenoglio C, Scherini E, Vaccarone R, Bernocchi G (1995) A re-evaluation of the ultrastructural localization of 5′-nucleotidase activity in the developing rat cerebellum, with a cerium-based method. J Neurosci Methods 59:253–263
Fonta C, Negyessy L, Renaud L, Barone P (2004) Areal and subcellular localization of the ubiquitous alkaline phosphatase in the primate cerebral cortex: evidence for a role in neurotransmission. Cereb Cortex 14:595–609
Fonta C, Negyessy L, Renaud L, Barone P (2005) Postnatal development of alkaline phosphatase activity correlates with the maturation of neurotransmission in the cerebral cortex. J Comp Neurol 486:179–196
Fox MA, Alexander JK, Afshari FS, Colello RJ, Fuss B (2004) Phosphodiesterase-Iα/autotaxin and IFAK phosphorylation during controls cytoskeletal organization myelination. Mol Cell Neurosci 27:140–150
Fox MA, Colello RJ, Macklin WB, Fuss B (2003) Phosphodiesterase-Iα/autotaxin: a counteradhesive protein expressed by oligodendrocytes during onset of myelination. Mol Cell Neurosci 23:507–519
Franke H, Bringmann A, Pannicke T, Krügel U, Grosche J, Reichenbach A, Illes P (2001) P2 receptors on macroglial cells: functional implications for gliosis. Drug Dev Res 53:140–147
Franke H, Illes P (2006) Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther 109:297–324
Franke H, Krügel U, Schmidt R, Grosche J, Reichenbach A, Illes P (2001) P2 receptor-types involved in astrogliosis in vivo. Br J Pharmacol 134:1180–1189
Frisén J, Johannson CB, Lothian C, Lendahl U (1998) Central nervous system stem cells in the embryo and adult. Cell Mol Life Sci 54:935–945
Fumagalli M, Brambilla R, D’Ambrosi N, Volonté C, Matteoli M, Verderio C, Abracchio MP (2003) Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43:218–230
Fuss B, Baba H, Phan T, Tuohy VK, Macklin WB (1997) Phosphodiesterase I, a novel adhesion molecule and/or cytokine involved in oligodendrocyte function. J Neurosci 17:9095–9103
Gangemi RMR, Perera M, Corte G (2004) Regulatory genes controlling cell fate choice in embryonic and adult neural stem cells. J Neurochem 89:286–306
Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV (2004) GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci 7:1233–1241
Gendron FP, Neary JT, Theiss PM, Sun GY, Gonzalez FA, Weisman GA (2003) Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am J Physiol Cell Physiol 284:C571–C581
Goding JW, Grobben B, Slegers H (2003) Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta, Mol Basis Dis 1638:1–19
Götz M, Barde YA (2005) Radial glial cells: defined and major intermediates between embryonic stem cells and CNS neurons. Neuron 46:369–372
Götz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6:777–788
Grinspan J (2002) Cells and signaling in oligodendrocyte development. J Neuropathol Exp Neurol 61:297–306
Gritti A, Parati EA, Cova L, Frolichsthal P, Galii R, Wanke E, Faravelli L, Morassutti DJ, Roisen F, Nickel DD, Vescovi AL (1996) Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J Neurosci 16:1091–1100
Guarnieri S, Fano G, Rathbone MP, Mariggio MA (2004) Cooperation in signal transduction of extracellular guanosine 5′ triphosphate and nerve growth factor in neuronal differentiation of PC12 cells. Neuroscience 128:697–712
Gysbers JW, Guarnieri S, Mariggiò MA, Pietrangelo T, Fanò G, Rathbone MP (2000) Extracellular guanosine 5′ triphosphate enhances nerve growth factor-induced neurite outgrowth via increases in intracellular calcium. Neuroscience 96:817–824
Hagg T (2005) Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci 28:589–595
Heilbronn A, Maienschein V, Carstensen C, Gann W, Zimmermann H (1995) Crucial role of 5′-nucleotidase in differentiation and survival of developing neural cells. Neuroreport 7:257–261
Heilbronn A, Zimmermann H (1995) 5′-Nucleotidase activates and an inhibitory antibody prevents neuritic differentiation of PC12 cells. Eur J Neurosci 7:1172–1179
Hogg RC, Chipperfield H, Whyte KA, Stafford MR, Hansen MA, Cool SM, Nurcombe V, Adams DJ (2004) Functional maturation of isolated neural progenitor cells from the adult rat hippocampus. Eur J Neurosci 19:2410–2420
Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S (2001) Extracellular ATP or ADP induce chemotaxis of cultured microglia through GI/o-coupled P2Y receptors. J Neurosci 21:1975–1982
Housley GD, Luo L, Ryan AF (1998) Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. J Comp Neurol 393:403–414
Housley GD, Ryan AF (1997) Cholinergic and purinergic neurohumoral signaling in the inner ear: a molecular physiological analysis. Audiol Neurootol 2:92–110
Hsieh J, Gage FH (2004) Epigenetic control of neural stem cell fate. Curr Opin Genet Dev 14:461–469
Huang LC, Greenwood D, Thorne PR, Housley GD (2005) Developmental regulation of neuron-specific P2X3 receptor expression in the rat cochlea. J Comp Neurol 484:133–143
Huang N, Wang DJ, Heppel LA (1989) Extracellular ATP is a mitogen for 3T3, 3T6, and A431 cells and acts synergistically with other growth factors. Proc Natl Acad Sci USA 86:7904–7908
Huovila APJ, Turner AJ, Pelto-Huikko M, Kärkkäinen I, Ortiz RM (2005) Shedding light on ADAM metalloproteinases. Trends Biochem Sci 30:413–422
Ille F, Sommer L (2005) Wnt signaling: multiple functions in neural development. Cell Mol Life Sci 62:1100–1108
Inoue K (2002) Microglial activation by purines and pyrimidines. Glia 40:156–163
Jacques-Silva MC, Rodnight R, Lenz G, Liao ZJ, Kong QM, Tran M, Kang Y, Gonzalez FA, Weisman GA, Neary JT (2004) P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol 141:1106–1117
James G, Butt AM (2002) P2Y and P2X purinoceptor mediated in the central Ca2+ signalling in glial cell pathology nervous system. Eur J Pharmacol 447:247–260
Jang IS, Rhee JS, Kubota H, Akaike N (2001) Developmental changes in P2X purinoceptors on glycinergic presynaptic nerve terminals projecting to rat substantia gelatinosa neurones. J Physiol (London) 536:505–519
Järlebark LE, Housley GD, Thorne PR (2000) Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X2 receptor subunits in adult and developing rat cochlea. J Comp Neurol 421:289–301
Jessen KR, Mirsky R (1999) Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci 22:402–410
Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, Mckay RDG (1996) Single factors direct differentiation of stem cells from the fetal and adult central nervous system. Genes Dev 10:3129–3140
Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452
Kidd EJ, Miller KJ, Sansum AJ, Humphrey PPA (1998) Evidence for P2X3 receptors in the developing rat brain. Neuroscience 87:533–539
King BF, Neary JT, Zhu Q, Wang S, Norenberg MD, Burnstock G (1996) P2 purinoceptors in rat cortical astrocytes: expression, calcium imaging and signalling studies. Neuroscience 74:1187–1196
Kohring K, Zimmermann H (1998) Upregulation of ecto-5′-nucleotidase in human neuroblastoma SH-SY5Y cells on differentiation by retinoic acid or phorbolester. Neurosci Lett 258:127–130
Kotevic I, Kirschner KM, Porzig H, Baltensperger K (2005) Constitutive interaction of the P2Y2 receptor with the hematopoietic cell-specific G protein Gα16 and evidence for receptor oligomers. Cell Signal 17:869–880
Kriegstein A, Götz M (2003) Radial glia diversity: a matter of cell fate. Glia 43:37–43
Lazarowski ER (2003) Molecular and biological properties of P2Y receptors. In: Schwiebert EM (ed) Extracellular nucleotides and nucleosides: release, receptors, and physiological and pathophysiological effects. Academic, Amsterdam pp 59–96
LeFeuvre R, Brough D, Rothwell N (2002) Extracellular ATP and P2X7 receptors in neurodegeneration. Eur J Pharmacol 447:261–269
Lemoli RM, Ferrari D, Fogli M, Rossi L, Pizzirani C, Forchap S, Chiozzi P, Vaselli D, Bertolini F, Foutz T, Aluigi M, Baccarani M, Di Virgilio F (2004) Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood 104:1662–1670
Lenz G, Gottfried C, Luo ZJ, Avruch J, Rodnight R, Nie WJ, Kang Y, Neary JT (2000) P2Y purinoceptor subtypes recruit different Mek activators in astrocytes. Br J Pharmacol 129:927–936
Lie AA, Blumcke I, Beck H, Wiestler OD, Elger CE, Schoen SW (1999) 5′-nucleotidase activity indicates sites of synaptic plasticity and reactive synaptogenesis in the human brain. J Neuropathol Exp Neurol 58:451–458
Lie DC, Song HJ, Colamarino SA, Ming GL, Gage FH (2004) Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol 44:399–421
Liu J, Liao ZJ, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L (2004) Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem 279:8212–8218
Lois C, Garcia-Verdugo J-M, Alvarez-Buylla A (1996) Chain migration of neural precursors. Science 271:978–981
Mayer C, Quasthoff S, Grafe P (1998) Differences in the sensitivity to purinergic stimulation of myelinating and non-myelinating Schwann cells in peripheral human and rat nerve. Glia 23:374–382
Menezes JRL, Smith CM, Nelson KC, Luskin MB (1995) The division of neural progenitor cells during migration in the neonatal mammalian forebrain. Mol Cell Neurosci 6:496–508
Meyer MP, Clark JDW, Patel K, Townsend-Nicholson A, Burnstock G (1999) Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev Dyn 214:152–158
Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A (2003) P2Y receptor-mediated stimulation of Müller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Investig Ophthalmol Vis Sci 44:1211–1220
Miras-Portugal MT, Gualix J, Mateo J, Díaz-Hernández M, Gómez-Villafuertes R, Castro E, Pintor J (1999) Diadenosine polyphosphates, extracellular function and catabolism. Prog Brain Res 120:397–409
Mishra SK, Braun N, Shukla V, Füllgrabe M, Schomerus C, Korf H-W, Gachet C, Ikehara Y, Sévigny J, Robson SC, Zimmermann H (2006) Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development 133:675–684
Misson JP, Takahashi D, Caviness VSJ (1991) Ontogeny of radial and other astroglia cells in murine cerebral cortex. Glia 4:138–148
Molliver DC, Cook SP, Carlsten JA, Wright DE, McCleskey EW (2002) ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci 16:1850–1860
Moore DJ, Chambers JK, Murdock PR, Emson PC (2002) Human Ntera-2/D1 neuronal progenitor cells endogenously express a functional P2Y1 receptor. Neuropharmacology 43:966–978
Morelli A, Ferrari D, Bolognesi G, Rizzuto R, DiVirgilio F (2001) Proapoptotic plasma membrane pore: P2X7 receptor. Drug Dev Res 52:571–578
Müller J, Rocha JBT, Battastini AMO, Sarkis JJF, Dias RD (1993) Postnatal development of ATPase–ADPase activities in synaptosomal fraction from cerebral cortex of rats. Neurochem Int 23:471–477
Nakata H, Yoshioka K, Kamiya T, Tsuga H, Oyanagi K (2005) Functions of heteromeric association between adenosine and P2Y receptors. J Mol Neurosci 26:233–238
Narisawa S, Fröhlander N, Millan JL (1997) Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn 208:432–446
Narisawa S, Hasegawa H, Watanabe K, Millán JL (1994) Stage-specific expression of alkaline phosphatase during neural development of the mouse. Dev Dyn 201:227–235
Neary JT, Whittemore SR, Zhu Q, Norenberg MD (1994) Synergistic activation of DNA synthesis in astrocytes by fibroblast growth factor and extracellular ATP. J Neurochem 63:490–494
Neary JT, Kang Y, Bu YR, Yu E, Akong K, Peters CM (1999) Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C calcium pathway. J Neurosci 19:4211–4220
Neary JT, Kang Y, Shi YF (2004) Signaling from nucleotide receptors to protein kinase cascades in astrocytes. Neurochem Res 29:2037–2042
Neary JT, Kang Y, Willoughby KA, Ellis EF (2003) Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci 23:2348–2356
Neary JT, Lenz G, Kang Y, Rodnight R, Avruch J (2001) Role of mitogen-activated protein kinase cascades in P2Y receptor-mediated trophic activation of astroglial cells. Drug Dev Res 53:158–165
Neary JT, McCarthy M, Kang Y, Zuniga S (1998) Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci Lett 242:159–162
Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G (1996) Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci 19:13–18
Neary JT, Zhu Q (1994) Signaling by ATP receptors in astrocytes. Neuroreport 5:1617–1620
Nikolic P, Housley GD, Luo L, Ryan AF, Thorne PR (2001) Transient expression of P2X1 receptor subunits of ATP-gated ion channels in the developing rat cochlea. Dev Brain Res 126:173–182
Nikolic P, Housley GD, Thorne PR (2003) Expression of the P2X7 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the developing and adult rat cochlea. Audiol Neuro–Otol 8:28–37
Noble M, Arhin A, Gass D, Mayer-Proschel M (2003) The cortical ancestry of oligodendrocytes: common principles and novel features. Dev Neurosci 25:217–233
Noctor S, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR (2002) Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci 22:3161–3173
Noda T, Tokuda H, Yoshida M, Yasuda E, Hanai Y, Takai S, Kozawa O (2005) Possible involvement of phosphatidylinositol 3-kinase/Akt pathway in insulin-like growth factor-I-induced alkaline phosphatase activity in osteoblasts. Horm Metab Res 37:270–274
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Norton WHJ, Rohr KB, Burnstock G (2000) Embryonic expression of a P2X3 receptor encoding gene in zebrafish. Mech Dev 99:149–152
Othman T, Yan H, Rivkees SA (2003) Oligodendrocytes express functional A1 adenosine receptors that stimulate cellular migration. Glia 44:166–172
Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA (2001) P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci 21:7135–7142
Pearson RA, Dale N, Llaudet E, Mobbs P (2005) ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 46:731–744
Peretto P, Merighi A, Fasolo A, Bonfanti L (1999) The subependymal layer in rodents: a site of structural plasticity and cell migration in the adult mammalian brain. Brain Res Bull 49:221–243
Pooler AM, Guez DH, Benedictus R, Wurtman RJ (2005) Uridine enhances neurite outgrowth in nerve growth factor-differentiated pheochromocytoma cells. Neuroscience 134:207–214
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Rathbone MP, Middlemmis P, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F (1999) Trophic effects of purines in neurons and glial cells. Prog Neurobiol 59:663–690
Resta V, Novelli E, DiVirgilio F, Galli-Resta L (2005) Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development 132:2873–2882
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710
Richter-Landsberg C, Maronde E, Besser A (1993) Ecto-5′-nucleotidase activity in PC 12 cells is synergistically modulated by nerve growth factor and 8-bromo-cAMP. Neurosci Res Commun 12:51–56
Robson SC, Wu Y, Sun XF, Knosalla C, Dwyer K, Enjyoji K (2005) Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost 31:217–233
Ruan HZ, Moules E, Burnstock G (2004) Changes in P2X3 purinoceptors in sensory ganglia of the mouse during embryonic and postnatal development. Histochem Cell Biol 122:539–551
Ryu JK, Choi BH, Hatori K, Heisel RL, Pelech SL, McLarnon JG, Kim SU (2003) Adenosine triphosphate induces proliferation of human neural stem cells: role of calcium and p70 ribosomal protein S6 kinase. J Neurosci Res 72:352–362
Safiulina VF, Kasyanov AM, Sokolova E, Cherubini E, Giniatullin R (2005) ATP contributes to the generation of network-driven giant depolarizing potentials in the neonatal rat hippocampus. J Physiol (London) 565:981–992
Sato K, Malchinkhuu E, Muraki T, Ishikawa K, Hayashi K, Tosaka M, Mochiduki A, Inoue K, Tomura H, Mogi C, Nochi H, Tamoto K, Okajima F (2005) Identification of autotaxin as a neurite retraction-inducing factor of PC12 cells in cerebrospinal fluid and its possible sources. J Neurochem 92:904–914
Scemes E, Duval N, Meda P (2003) Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J Neurosci 23:11444–11452
Schafer KH, Saffrey MJ, Burnstock G (1995) Trophic actions of 2-chloroadenosine and bFGF on cultured myenteric neurones. Neuroreport 6:937–941
Scheibe RJ, Kuehl H, Krautwald S, Meissner JD, Mueller WH (2000) Ecto-alkaline phosphatase activity identified at physiological pH range on intact P19 and HL-60 cells is induced by retinoic acid. J Cell Biochem 76:420–436
Schoen SW, Graeber MB, Tóth L, Kreutzberg GW (1988) 5′-Nucleotidase in postnatal ontogeny of rat cerebellum: a marker for migrating nerve cells? Dev Brain Res 39:125–136
Schoen SW, Kreutzberg GW (1995) Evidence that 5′-nucleotidase is associated with malleable synapses—an enzyme cytochemical investigation of the olfactory bulb of adult rats. Neuroscience 65:37–50
Schoen SW, Kreutzberg GW, Singer W (1993) Cytochemical redistribution of 5′-nucleotidase in the developing cat visual cortex. Eur J Neurosci 5:210–222
Schoen SW, Leutenecker B, Kreutzberg GW, Singer W (1990) Ocular dominance plasticity and developmental changes of 5′-nucleotidase distributions in the kitten visual cortex. J Comp Neurol 269:379–392
Schulte G, Fredholm BB (2003) Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 15:813–827
Schwiebert EM, Zsembery A, Geibel JP (2003) Cellular mechanisms and physiology of nucleotide and nucleoside release from cells: current knowledge, novel assays to detect purinergic agonists, and future directions. In: Schwiebert EM (ed) Extracellular nucleotides and nucleosides: release, receptors, and physiological and pathophysiological effects. Academic, Amsterdam pp 31–58
Sellers LA, Simon J, Lundahl TS, Cousens DJ, Humphrey PPA, Barnard EA (2001) Adenine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J Biol Chem 19:16379–16390
Seri B, Manuel J, García-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A (2004) Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol 478:359–378
Seye CI, Yu NP, Gonzalez FA, Erb L, Weisman GA (2004) The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J Biol Chem 279:35679–35686
Shukla V, Zimmermann H, Wang L, Kettenmann H, Raab S, Hammer K, Sévigny J, Robson SC, Braun N (2005) Functional expression of the ecto-ATPase NTPDase2 and of nucleotide receptors by neuronal progenitor cells in the adult murine hippocampus. J Neurosci Res 80:600–610
Skladchikova G, Ronn LCB, Berezin V, Bock E (1999) Extracellular adenosine triphosphate affects neural cell adhesion molecule (NCAM)-mediated cell adhesion and neurite outgrowth. J Neurosci Res 57:207–218
Soltoff SP (1998) Related adhesion focal tyrosine kinase and epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G-protein-coupled P2Y2 receptor. J Biol Chem 273:23110–23117
Spychala J, Kitajewski J (2004) Wnt and beta-catenin signaling target the expression of ecto-5′-nucleotidase and increase extracellular adenosine generation. Exp Cell Res 296:99–108
Stefan C, Jansen S, Bollen M (2005) NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci 30:542–550
Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G (2004) Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia 46:41–52
Stevens B, Fields RD (2000) Response of Schwann cells to action potentials in development. Science 287:2267–2271
Stevens B, Ishibashi H, Chen JF, Fields RD (2004) Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol 1:23–34
Stevens B, Porta S, Haak LL, Gallo V, Fields RD (2002) Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36:855–868
Stracke ML, Krutzsch HC, Unsworth EJ, Arestad AA, Cioce V, Schiffmann E, Liotta LA (1992) Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem 267:2524–2529
Sugioka M, Fukuda Y, Yamashita M (1996) Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. J Physiol 493:855–863
Sugioka M, Zhou WL, Hofmann HD, Yamashita M (1999) Involvement of P2 purinoceptors in the regulation of DNA synthesis in the neural retina of chick embryo. Int J Dev Neurosci 17:135–144
Thevananther S, Rivera A, Rivkees SA (2001) A1 adenosine receptor activation inhibits neurite process formation by rho kinase-mediated pathways. Neuroreport 12:3057–3063
Tran PB, Ren DJ, Veldhouse TJ, Miller RJ (2004) Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res 76:20–34
Tulapurkar ME, Schafer R, Hanck T, Flores RV, Weisman GA, Gonzalez FA, Reiser G (2005) Endocytosis mechanism of P2Y2 nucleotide receptor tagged with green fluorescent protein: clathrin and actin cytoskeleton dependence. Cell Mol Life Sci 62:1388–1399
Uckermann O, Grosche J, Reichenbach A, Bringmann A (2002) ATP-evoked calcium responses of radial glial (Müller) cells in the postnatal rabbit retina. J Neurosci Res 70:209–218
Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H (2002) Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 158:227–233
Vogel M, Zimmermann H, Singer W (1993) Transient association of the HNK-1 epitope with 5′-nucleotidase during development of the cat visual cortex. Eur J Neurosci 5:1423–1425
Wang M, Kong QM, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA (2005) P2Y2 nucleotide receptor interaction with αV integrin mediates astrocyte migration. J Neurochem 95:630–640
Watts C, McConkey H, Anderson L, Caldwell M (2005) Anatomical perspectives on adult neural stem cells. J Anat 207:197–208
Waymire JC, Mahuren JD, Jaje JM, Guilarte T, Coburn SP, Macgregor GR (1995) Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet 11:45–51
Webb SE, Miller AL (2003) Calcium signalling during embryonic development. Nat Rev Mol Cell Biol 4:539–551
Weisman GA, Wang M, Kong Q, Chorna NE, Neary JT, Sun GY, Gonzalez FA, Seye CI, Erb L (2005) Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Mol Neurobiol 31:1–15
Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR (2004) Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43:647–661
Wirkner K, Franke H, Inoue K, Illes P (1998) Differential age-dependent expression of α2 adrenoceptor- and P2 purinoceptor-functions in rat locus coeruleus neurons. Naunyn–Schmiedeberg’s Arch Pharmacol 357:186–189
Wu LNY, Genge BR, Lloyd GC, Wuthier RE (1991) Collagen-binding proteins in collagenase-released matrix vesicles from cartilage. J Biol Chem 266:1195–1203
Xiang Z, Burnstock G (2005) Expression of P2X receptors on rat microglial cells during early development. Glia 52:119–126
Xiang ZH, Burnstock G (2004) Development of nerves expressing P2X3 receptors in the myenteric plexus of rat stomach. Histochem Cell Biol 122:111–119
Xiang ZH, Burnstock G (2005) Changes in expression of P2X purinoceptors in rat cerebellum during postnatal development. Dev Brain Res 156:147–157
Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C (2005) Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 192:251–264
Yoshioka T, Tanaka O (1989) Histochemical localization of Ca2+, Mg2+-ATPase of the rat cerebellar cortex during postnatal development. Int J Dev Neurosci 7:181–193
Zhu YZ, Kimelberg HK (2004) Cellular expression of P2Y and α-AR receptor mRNAs and proteins in freshly isolated astrocytes and tissue sections from the CA1 region of P8-12 rat hippocampus. Dev Brain Res 148:77–87
Zimmermann H (1996) Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol 49:589–618
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn–Schmiedeberg’s Arch Pharmacol 362:299–309
Zimmermann H (2001) Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev Res 52:44–56
Zimmermann H (2001) Ecto-nucleotidases. In: Abbracchio MP, Williams M (eds) Handbook of experimental pharmacology. Purinergic and pyrimidergic signalling. Springer, Berlin Heidelberg New York, pp 209–250
Acknowledgements
The support given by Deutsche Forschungsgemeinschaft (140/17-1; GRK 361) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zimmermann, H. Nucleotide signaling in nervous system development. Pflugers Arch - Eur J Physiol 452, 573–588 (2006). https://doi.org/10.1007/s00424-006-0067-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0067-4