Abstract
Similar to a variety of nucleated cells, human erythrocytes activate a non-selective cation channel upon osmotic cell shrinkage. Further stimuli of channel activation include oxidative stress, energy depletion and extracellular removal of Cl−. The channel is permeable to Ca2+ and opening of the channel increases cytosolic [Ca2+]. Intriguing evidence points to a role of this channel in the elimination of erythrocytes by apoptosis. Ca2+ entering through the cation channel stimulates a scramblase, leading to breakdown of cell membrane phosphatidylserine asymmetry, and stimulates Ca2+-sensitive K+ channels, thus leading to KCl loss and (further) cell shrinkage. The breakdown of phosphatidylserine asymmetry is evidenced by annexin binding, a typical feature of apoptotic cells. The effects of osmotic shock, oxidative stress and energy depletion on annexin binding are mimicked by the Ca2+ ionophore ionomycin (1 µM) and blunted in the nominal absence of extracellular Ca2+. Nevertheless, the residual annexin binding points to additional mechanisms involved in the triggering of the scramblase. The exposure of phosphatidylserine at the extracellular face of the cell membrane stimulates phagocytes to engulf the apoptotic erythrocytes. Thus, sustained activation of the cation channels eventually leads to clearance of affected erythrocytes from peripheral blood. Susceptibility to annexin binding is enhanced in several genetic disorders affecting erythrocyte function, such as thalassaemia, sickle-cell disease and glucose-6-phosphate dehydrogenase deficiency. The enhanced vulnerability presumably contributes to the shortened life span of the affected erythrocytes. Beyond their role in the limitation of erythrocyte survival, cation channels may contribute to the triggering of apoptosis in nucleated cells exposed to osmotic shock and/or oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis is a physiological mechanism eliminating abundant and potentially harmful cells [27, 30]. Hallmarks of apoptosis include nuclear condensation, DNA fragmentation, mitochondrial depolarization, cell shrinkage and breakdown of phosphatidylserine asymmetry of the plasma membrane [27, 30]. The exposure of phosphatidylserine at the cell surface triggers, and the decrease of cell volume facilitates, the engulfment of the dying cells by phagocytes [6, 22]. Thus, apoptosis allows the elimination of the cells without the release of intracellular proteins, which would otherwise cause inflammation [30]. The stimulation of apoptosis modifies the activity of several transport processes at the cell membrane including K+ channels [29, 53, 56, 68, 69], anion channels [56, 70], Ca2+ channels [55], taurine release channels [45, 48, 60] and Na+/H+ exchange [47].
A wide variety of stimuli induce apoptosis, including nitric oxide [33], UV radiation [42, 65], exposure to pathogens [23], osmotic shock [7, 8, 44, 48, 56, 59, 63, 65] and the activation of defined receptors such as CD95 [30, 45, 46], TNFα [50] and somatostatin [71].
Despite their lack of mitochondria and nuclei, intracellular organelles involved in the apoptosis of nucleated cells, erythrocytes exposed to the Ca2+ ionophore ionomycin undergo shrinkage, membrane blebbing and break down of cell membrane phosphatidylserine asymmetry, all typical features of apoptosis in nucleated cells [3, 9, 15]. It is thus fair to say that erythrocytes undergo apoptosis upon increase of intracellular [Ca2+]. The present brief review presents evidence that entry of Ca2+ through a non-selective cation channel is a major mechanism triggering erythrocyte apoptosis.
Properties of erythrocyte cation channels
Osmotic shock [32] and oxidative stress [21] open non-selective cation channels in the erythrocyte cell membrane. The same channels can be activated by removal of intracellular and extracellular Cl− (Fig. 1A, B) [21, 32]. This property is reminiscent of the Na+ and K+ permeability activated by incubating human erythrocytes in low ionic strength (LIS) medium [4, 36, 43]. Incubation in LIS medium also induces permeabilities to organic osmolytes, such as taurine and glutamine, which share many properties of the LIS-induced cation permeability [14]. This feature may be of interest as taurine release is a typical feature of apoptosis in nucleated cells [45, 48, 60]. Similar to what has been shown for the LIS permeability [14, 36], activation of the volume- and oxidant-sensitive cation channel by removal of extracellular Cl− is inhibited by the anion channel/transport inhibitor 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid (DIDS) [21]. The cation channels allow the permeation of Ca2+ [21]. Accordingly, exposure to osmotic shock or oxidative stress triggers erythrocyte Ca2+ uptake [51].
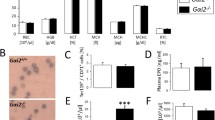
Activation of cell volume-sensitive, non-selective cation channels in the erythrocyte membrane induces annexin binding. A Cation-selective ion channels are activated by removal of extracellular Cl−. Patch-clamp current traces recorded with Na-d-gluconate pipette solution and isotonic NaCl bath solution (left), isotonic Na-d-gluconate bath solution (middle), and after replacement of bath Na+ by the impermeant cation N-methyl-d-glucamine (NMDG +, right). Currents were recorded in the fast, whole-cell, voltage-clamp mode; membrane potential was held at −10 mV and currents were elicited by 400-ms square pulses to test potentials between −100 and +100 mV; currents of the individual voltage sweeps are superimposed; zero current is indicated by the grey line. B Mean (±SE; n=3–16) slope conductance of human erythrocytes recorded under isotonic conditions as in A prior to (NaCl; open bar) and upon activation of the cation channels by extracellular Cl− removal (Na-gluconate; open bar). Hypertonic cell shrinkage (by adding 250 mM sucrose to the bath solution) further activates the cation channels (Na-gluconate; solid bar). C Cell-shrinkage-induced break down of the erythrocyte membrane phospholipid asymmetry is dependent on extracellular Ca2+ and inhibited by amiloride. Mean percentage of annexin binding erythrocytes (±SE; n=3–16) as measured by flow cytometry. Erythrocytes were cultured for 24 h at 37°C either in isotonic (open bar) or in hypertonic Ringer solution (closed bars; osmolarity increased to 850 mOsm by adding sucrose). In some experiments, incubation in hypertonic Ringer solution was performed in the presence of the cation channel inhibitor amiloride (1 mM) or in the absence of extracellular Ca2+. D Summary of the experimental manoeuvres inducing activation or inactivation/inhibition of the Ca2+-permeable non-selective cation channel in human erythrocytes (EIPA ethylisopropylamiloride). Increased channel activity leads to elevated cytosolic free [Ca2+] and subsequently to scramblase activation
Impact of cation channels on cell volume
In general, Na+ entry through cation channels leads to cell membrane depolarization, which should favour cell swelling [44]. Depolarization decreases the electrical driving force for extrusion of negatively charged Cl− and thus leads to accumulation of Cl− in parallel to Na+. In most cells cytosolic Cl− concentration ([Cl−]i) is below 20 mM and thus less than 20% of the extracellular [Cl−] ([Cl−]o). Thus, the equilibrium potential for Cl− (\( E_{{{\text{Cl}}}} {\text{ (in}}\;{\text{mV)}} = 60\log \frac{{[{\text{Cl}}^{ - } ]_{{\text{i}}} }} {{[{\text{Cl}}^{ - } ]_{{\text{o}}} }} \)) is more negative than −40 mV, i.e. any depolarization below −40 mV would drive Cl− into the cells. [Cl−]i in erythrocytes, however, is of the order of 80 mM [76] and the membrane resting potential is at E Cl, which is close to −10 mV [13, 18]. The selectivity of the cation channel is some twofold higher for K+ than for Na+ [21]. In view of the intracellular Na+ and K+ concentrations of approximately 10–20 mM [16, 26, 37, 40] and 140 mM [37], respectively, and the extracellular concentrations of 145 mM and 5 mM, respectively, the equilibrium potential for the channel approaches some −18 mV, i.e. a value more negative than the actual cell membrane potential. Accordingly, the activation of the channel should hyperpolarize the cell membrane and shrink rather than swell the erythrocyte. Moreover, Ca2+ entering the cells though the cation channel will activate Ca2+-sensitive K+ channels (KCNN4) in the erythrocyte cell membrane [31, 53], leading to hyperpolarization of the cell membrane and subsequent loss of KCl from the erythrocyte [17, 20, 25, 28, 54, 61, 67], again rather favouring hyperpolarization and cell shrinkage. Thus, the erythrocyte cation channels probably do not mediate regulatory cell volume increase, even though they are up-regulated by cell shrinkage.
Impact of cation channels on erythrocyte apoptosis
Compelling evidence points to a role of the volume-sensitive cation channels in the induction of erythrocyte apoptosis. Besides triggering cell shrinkage (see above), an increase of [Ca2+]i stimulates a scramblase, thus leading to the breakdown of phosphatidylserine asymmetry [3, 9, 15, 51]. Exposure of phosphatidylserine is detected by determination of annexin binding, together with cell shrinkage, a typical feature of apoptosis in nucleated cells [30].
Erythrocyte annexin binding is triggered by osmotic shock (Fig. 1C) and oxidative stress [51], both manoeuvres that activate the cation channel [21, 32]. Furthermore, energy depletion leads to enhanced annexin binding [51]. Presumably energy depletion impairs the replenishment of GSH and thus weakens the antioxidative defence of the erythrocytes [5, 58]. The annexin binding following osmotic shock and oxidative stress is blunted following chelation of extracellular Ca2+ [51]. Moreover, the annexin binding is blunted by amiloride (Fig. 1C) [51] and ethylisopropylamiloride (EIPA) [52] at concentrations needed to inhibit the cation channel [51, 52]. Thus, it appears safe to conclude that activation of the cell volume- and oxidant-sensitive cation channel and subsequent Ca2+ entry contribute to the stimulation of erythrocyte scramblase following osmotic shock or oxidative stress (Fig. 1D). Interestingly, the Na+/H+ exchange inhibitor ethylisopropylamiloride (EIPA) is effective at a concentration of 1 µM, whereas amiloride, which inhibits both Na+/H+ exchange and cation channels, requires 1 mM to become effective [52].
Further experiments have revealed the enhanced sensitivity of erythrocytes from patients with thalassaemia, sickle-cell anaemia and glucose-6-phosphate dehydrogenase deficiency [49]. Similarly, increased scramblase activity and phosphatidylserine exposure has been demonstrated for erythrocytes in mouse models of sickle cell disease and thalassaemia [38].
Impact of cation channels on erythrocyte ageing
Aged erythrocytes expose more phosphatidylserine, which contributes to the elimination of the senescent cells [6]. The capacity for oxidative defence decreases with erythrocyte age [34, 62] a phenomenon paralleled by increase of passive cation permeability [35] and cytosolic free [Ca2+] [1, 2, 11, 41, 64, 66]. It is thus tempting to speculate that the cation channels sense cell age. Within the ageing erythrocytes, the loss of antioxidative defence can be expected to increase cation channel activity leading to Ca2+ entry, increased Ca2+ pump activity, ATP depletion, further impairment of antioxidative defence, further activation of cation channels and further Ca2+ entry and eventually activation of the scramblase.
Volume-sensitive cation channels in nucleated cells
Cell volume-sensitive cation channels are not only expressed in erythrocytes but are found in a wide variety of nucleated cells, such as airway epithelial cells [12], mast cells [10], macrophages [24], vascular smooth muscle, colon carcinoma and neuroblastoma cells [39], cortical collecting duct [73] and hepatocytes [74, 75]. Moreover, cation channels activated by Cl− removal have been identified in salivary and lung epithelial cells [19, 57, 72]. Cl− influences the channels via a pertussis toxin-sensitive G protein [19]. It is intriguing to speculate that non-selective cation channels are similarly involved in apoptosis of nucleated cells.
References
Aiken NR, Satterlee JD, Galey WR (1992) Measurement of intracellular Ca2+ in young and old human erythrocytes using 19F-NMR spectroscopy. Biochim Biophys Acta 1136:155–160
Allan D, Raval PJ (1987) The role of Ca2+-dependent biochemical changes in the ageing process in normal red cells and in the development of irreversibly sickled cells. Folia Haematol Int Mag Klin Morphol Blutforsch 114:499–503
Berg CP, Engels IH, Rothbart A, Lauber K, Renz A, Schlosser SF, Schulze-Osthoff K, Wesselborg S (2001) Human mature red blood cells express caspase-3 and caspase-8, but are devoid of mitochondrial regulators of apoptosis. Cell Death Differ 8:1197–1206
Bernhardt I, Hall AC, Ellory JC (1991) Effects of low ionic strength media on passive human red cell monovalent cation transport. J Physiol (Lond) 434:489–506
Bilmen S, Aksu TA, Gumuslu S, Korgun DK, Canatan D (2001) Antioxidant capacity of G-6-PD-deficient erythrocytes. Clin Chim Acta 303:83–86
Boas FE, Forman L, Beutler E (1998) Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci USA 95:3077–3081
Bortner CD, Cidlowski JA (1998) A necessary role for cell shrinkage in apoptosis. Biochem Pharmacol 56:1549–1559
Bortner CD, Cidlowski JA (1999) Caspase independent/dependent regulation of K+, cell shrinkage, and mitochondrial membrane potential during lymphocyte apoptosis. J Biol Chem 274:21953–1962
Bratosin D, Estaquier J, Petit F, Arnoult D, Quatannens B, Tissier JP, Slomianny C, Sartiaux C, Alonso C, Huart JJ, Montreuil J, Ameisen JC (2001) Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell Death Differ 8:1143–1156
Cabado AG, Vieytes MR, Botana LM (1994) Effect of ion composition on the changes in membrane potential induced with several stimuli in rat mast cells. J Cell Physiol 158:309–316
Cameron IL, Hardman WE, Smith NK, Fullerton GD, Miseta A (1993) Changes in the concentration of ions during senescence of the human erythrocyte. Cell Biol Int 17:93–98
Chan HC, Goldstein J, Nelson DJ (1992) Alternate pathways for chloride conductance activation in normal and cystic fibrosis airway epithelial cells. Am J Physiol 262:C1273–1283
Cheng K, Haspel HC, Vallano ML, Osotimehin B, Sonenberg M (1980) Measurement of membrane potentials (psi) of erythrocytes and white adipocytes by the accumulation of triphenylmethylphosphonium cation. J Membr Biol 56:191–201
Culliford SJ, Bernhardt I, Ellory JC (1995) Activation of a novel organic solute transporter in mammalian red blood cells. J Physiol (Lond) 489:755–765
Daugas E, Cande C, Kroemer G (2001) Erythrocytes: death of a mummy. Cell Death Differ 8:1131–1133
Deal JE, Shah V, Goodenough G, Dillon MJ (1990) Red cell membrane sodium transport: possible genetic role and use in identifying patients at risk of essential hypertension. Arch Dis Child 65:1154–1157
Del Carlo B, Pellegrini M, Pellegrino M (2002) Calmodulin antagonists do not inhibit IK(Ca) channels of human erythrocytes. Biochim Biophys Acta 1558:133–141
Deutsch CJ, Holian A, Holian SK, Daniele RP, Wilson DF (1979) Transmembrane electrical and pH gradients across human erythrocytes and human peripheral lymphocytes. J Cell Physiol 99:79–93
Dinudom A, Komwatana P, Young JA, Cook DI (1995) Control of the amiloride-sensitive Na+ current in mouse salivary ducts by intracellular anions is mediated by a G protein. J Physiol (Lond) 487:549–555
Dunn PM (1998) The action of blocking agents applied to the inner face of Ca2+-activated K+ channels from human erythrocytes. J Membr Biol 165:133–143
Duranton C, Huber SM, Lang F (2002) Oxidation induces a Cl(-)-dependent cation conductance in human red blood cells. J Physiol (Lond) 539:847–855
Eda S, Sherman IW (2002) Cytoadherence of malaria-infected red blood cells involves exposure of phosphatidylserine. Cell Physiol Biochem 12:373–384
Fillon S, Lang F, Jendrossek V (2002) Pseudomonas aeruginosa triggered apoptosis of human epithelial cells depends on the temperature during infection. Cell Physiol Biochem 12:207–214
Gamper N, Huber SM, Badawi K, Lang F (2000) Cell volume-sensitive sodium channels upregulated by glucocorticoids in U937 macrophages. Pflugers Arch 441:281–286
Gardos G (1958) The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta 30:653–654
Girardin E, Paunier L (1985) Relationship between magnesium, potassium and sodium concentrations in lymphocytes and erythrocytes from normal subjects. Magnesium 4:188–192
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Grygorczyk R, Schwarz W (1983) Properties of the Ca2+-activated K+ conductance of human red cells as revealed by the patch-clamp technique. Cell Calcium 4:499–510
Gulbins E, Szabo I, Baltzer K, Lang F (1997) Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc Natl Acad Sci USA 94:7661–7666
Gulbins E, Jekle A, Ferlinz K, Grassme H, Lang F (2000) Physiology of apoptosis. Am J Physiol 279:F605–F615
Hoffman JF, Joiner W, Nehrke K, Potapova O, Foye K, Wickrema A (2003) The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (Gardos channel) in human red blood cells. Proc Natl Acad Sci USA 100:7366–7371
Huber SM, Gamper N, Lang F (2001) Chloride conductance and volume-regulatory nonselective cation conductance in human red blood cell ghosts. Pflugers Arch 441:551–558
Ibe W, Bartels W, Lindemann S, Grosser T, Buerke M, Boissel JP, Meyer J, Darius H (2001) Involvement of PKC and NF-kappaB in nitric oxide induced apoptosis in human coronary artery smooth muscle cells. Cell Physiol Biochem 11:231–240
Imanishi H, Nakai T, Abe T, Takino T (1985) Glutathione metabolism in red cell aging. Mech Ageing Dev 32:57–62
Joiner CH, Lauf PK (1978) Ouabain binding and potassium transport in young and old populations of human red cells. Membr Biochem 1:187–202
Jones GS, Knauf PA (1985) Mechanism of the increase in cation permeability of human erythrocytes in low-chloride media. Involvement of the anion transport protein capnophorin. J Gen Physiol 86:721–738
Kaji DM, Thakkar U, Kahn T (1981) Glucocorticoid-induced alterations in the sodium potassium pump of the human erythrocyte. J Clin Invest 68:422–430
Kean LS, Brown LE, Nichols JW, Mohandas N, Archer DR, Hsu LL (2002) Comparison of mechanisms of anemia in mice with sickle cell disease and beta-thalassemia: peripheral destruction, ineffective erythropoiesis, and phospholipid scramblase-mediated phosphatidylserine exposure. Exp Hematol 30:394–402
Koch J, Korbmacher C (1999) Osmotic shrinkage activates nonselective cation (NSC) channels in various cell types. J Membr Biol 168:131–139
Korff JM, Siebens AW, Gill JR Jr (1984) Correction of hypokalemia corrects the abnormalities in erythrocyte sodium transport in Bartter's syndrome. J Clin Invest 74:1724–1729
Kramer JJ, Swislocki NI (1985) The effects of pentoxifylline on rat erythrocytes of different age. Mech Ageing Dev 32:283–298
Kulms D, Poppelmann B, Yarosh D, Luger TA, Krutmann J, Schwarz T (1999) Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc Natl Acad Sci USA 96:7974–7979
LaCelle PL, Rothsteto A (1966) The passive permeability of the red blood cell in cations. J Gen Physiol 50:171–188
Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D (1998) Functional significance of cell volume regulatory mechanisms. Physiol Rev 78:247–306
Lang F, Madlung J, Uhlemann AC, Risler T, Gulbins E (1998) Cellular taurine release triggered by stimulation of the Fas(CD95) receptor in Jurkat lymphocytes. Pflugers Arch 436:377–383
Lang F, Szabo I, Lepple-Wienhues A, Siemen D, Gulbins E (1999) Physiology of receptor-mediated lymphocyte apoptosis. News Physiol Sci 14:194–200
Lang F, Madlung J, Bock J, Lukewille U, Kaltenbach S, Lang KS, Belka C, Wagner CA, Lang HJ, Gulbins E, Lepple-Wienhues A (2000) Inhibition of Jurkat-T-lymphocyte Na+/H+-exchanger by CD95(Fas/Apo-1)-receptor stimulation. Pflugers Arch 440:902–907
Lang F, Madlung J, Siemen D, Ellory C, Lepple-Wienhues A, Gulbins E (2000) The involvement of caspases in the CD95(Fas/Apo-1)- but not swelling-induced cellular taurine release from Jurkat T-lymphocytes. Pflugers Arch 440:93–99
Lang KS, Roll B, Myssina S, Schittenhelm M, Scheel-Walter HG, Kanz L, Fritz J, Lang F, Huber SM, Wieder T (2002) Enhanced erythrocyte apoptosis in sickle cell anemia, thalassemia and glucose-6-phosphate dehydrogenase deficiency. Cell Physiol Biochem 12:365–372
Lang KS, Fillon S, Schneider D, Rammensee HG, Lang F (2002) Stimulation of TNF alpha expression by hyperosmotic stress. Pflugers Arch 443:798–803
Lang KS, Duranton C, Poehlmann H, Myssina S, Bauer C, Lang F, Wieder T, Huber SM (2003) Cation channels trigger apoptotic death of erythrocytes. Cell Death Diff 10:249–256
Lang KS, Myssina S, Tanneur V, Wieder T, Huber SM, Lang F, Duranton C (2003) Inhibition of erythrocyte cation channels and apoptosis by ethylisopropylamiloride. Naunyn-Schmiedeberg's Arch Pharmacol 367:391–396
Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM (2003) Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am J Physiol (In press)
Leinders T, van Kleef RG, Vijverberg HP (1992) Single Ca2+-activated K+ channels in human erythrocytes: Ca2+ dependence of opening frequency but not of open lifetimes. Biochim Biophys Acta 1112:67–74
Lepple-Wienhues A, Belka C, Laun T, Jekle A, Walter B, Wieland U, Welz M, Heil L, Kun J, Busch G, Weller M, Bamberg M, Gulbins E, Lang F (1999) Stimulation of CD95 (Fas) blocks T lymphocyte calcium channels through sphingomyelinase and sphingolipids. Proc Natl Acad Sci USA 96:13795–13800
Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y (2000) Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci USA 97:9487–9492
Marunaka Y, Nakahari T, Tohda H (1994) Cytosolic [Cl−] regulates Na+ absorption in fetal alveolar epithelium?: roles of cAMP and Cl− channels. Jpn J Physiol 44 (Suppl 2):S281–S288
Mavelli I, Ciriolo MR, Rossi L, Meloni T, Forteleoni G, De Flora A, Benatti U, Morelli A, Rotilio G (1984) Favism: a hemolytic disease associated with increased superoxide dismutase and decreased glutathione peroxidase activities in red blood cells. Eur J Biochem 139:13–18
Michea L, Ferguson DR, Peters EM, Andrews PM, Kirby MR, Burg MB (2000) Cell cycle delay and apoptosis are induced by high salt and urea in renal medullary cells. Am J Physiol 278:F209–F218
Moran J, Hernandez-Pech X, Merchant-Larios H, Pasantes-Morales H (2000) Release of taurine in apoptotic cerebellar granule neurons in culture. Pflugers Arch 439:271–277
Pellegrino M, Pellegrini M (1998) Modulation of Ca2+-activated K+ channels of human erythrocytes by endogenous cAMP-dependent protein kinase. Pflugers Arch 436:749–756
Piccinini G, Minetti G, Balduini C, Brovelli A (1995) Oxidation state of glutathione and membrane proteins in human red cells of different age. Mech Ageing Dev 78:15–26
Roger F, Martin PY, Rousselot M, Favre H, Feraille E (1999) Cell shrinkage triggers the activation of mitogen-activated protein kinases by hypertonicity in the rat kidney medullary thick ascending limb of the Henle's loop. Requirement of p38 kinase for the regulatory volume increase response. J Biol Chem 274:34103–34110
Romero PJ, Romero EA, Winkler MD (1997) Ionic calcium content of light dense human red cells separated by Percoll density gradients. Biochim Biophys Acta 1323:23–28
Rosette C, Karin M (1996) Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 274:1194–1197
Seidler NW, Swislocki NI (1991) Ca2+ transport activities of inside-out vesicles prepared from density-separated erythrocytes from rat and human. Mol Cell Biochem 105:159–169
Shindo M, Imai Y, Sohma Y (2000) A novel type of ATP block on a Ca2+-activated K+ channel from bullfrog erythrocytes. Biophys J 79:287–297
Szabo I, Gulbins E, Apfel H, Zhang X, Barth P, Busch AE, Schlottmann K, Pongs O, Lang F (1996) Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J Biol Chem 271:20465–20469
Szabo I, Gulbins E, Lang F (1997) Regulation of Kv1.3 during Fas-induced apoptosis. Cell Physiol Biochem 7:148–158
Szabo I, Lepple-Wienhues A, Kaba KN, Zoratti M, Gulbins E, Lang F (1998) Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proc Natl Acad Sci USA 95:6169–6174
Teijeiro R, Rios R, Costoya JA, Castro R, Bello JL, Devesa J, Arce VM (2002) Activation of human somatostatin receptor 2 promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol Biochem 12:31–38
Tohda H, Foskett JK, O'Brodovich H, Marunaka Y (1994) Cl− regulation of a Ca2+-activated nonselective cation channel in beta-agonist-treated fetal distal lung epithelium. Am J Physiol 266:C104–C109
Volk T, Frömter E, Korbmacher C (1995) Hypertonicity activates nonselective cation channels in mouse cortical collecting duct cells. Proc Natl Acad Sci USA 92:8478–8482
Wehner F, Sauer H, Kinne RK (1995) Hypertonic stress increases the Na+ conductance of rat hepatocytes in primary culture. J Gen Physiol 105:507–535
Wehner F, Bohmer C, Heinzinger H, van den Boom F, Tinel H (2000) The hypertonicity-induced Na+ conductance of rat hepatocytes: physiological significance and molecular correlate. Cell Physiol Biochem 10:335–340
Zidek W, Losse H, Lange-Asschenfeldt H, Vetter H (1985) Intracellular chloride in essential hypertension. Clin Sci 68:45–47
Acknowledgements
The authors acknowledge the meticulous preparation of the manuscript by Lejla Subasic. This study was supported by the Deutsche Forschungsgemeinschaft, Nr. La 315/4-3, La 315/6-1, DFG Schwerpunkt Intrazelluläre Lebensformen La 315/11-1 and the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Center for Interdisciplinary Clinical Research) 01 KS 9602 and by the Forschungsschwerpunktprogramm des Landes Baden-Württemberg, Dynamik und Modulation zellulärer Infektionsprozesse.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lang, F., Lang, K.S., Wieder, T. et al. Cation channels, cell volume and the death of an erythrocyte. Pflugers Arch - Eur J Physiol 447, 121–125 (2003). https://doi.org/10.1007/s00424-003-1150-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-003-1150-8