Abstract
Background
Splenic flexure (SF) cancer is not a common condition and its treatment is still under discussion. Although laparoscopic surgery is well accepted for the treatment of colon cancer at any stage, complete mesocolon excision (CME) with selective vascular ligation using the laparoscopic approach for SF cancer remains technically demanding and represents a real challenge for surgeons.
Methods
We present a single-institution experience of laparoscopic CME for SF cancer. Intra-operative, pathologic, and post-operative data of patients who underwent laparoscopic SF resection were reviewed to assess the technical feasibility and oncologic safety. Technical features, histopathology, morbidity, and mortality were evaluated.
Results
From February 2015 to October 2017, a minimally invasive approach was proposed to 17 patients (M/F 14/3) affected by splenic flexure cancer. In all patients, the procedure was completed by laparoscopy. The anastomosis was completed intra-corporeally in 89% of cases. The distal margin was 3.1 ± 2.6 cm and the proximal margin was 6.5 ± 3.3 cm from the tumor site. The number of mean harvested nodes was 13.9 ± 7. The mean operative time was 215.5 ± 65 min, and blood loss was 80 ± 27. In one case, a laparoscopic partial gastrectomy was associated due to tumor invasion. The mean post-operative stay was 6.7 ± 3.3 days. Readmission was necessary for two patients. No major morbidity was recorded.
Conclusions
Despite the wide spread and increasing confidence in laparoscopic colectomy, SF resection remains one of the most challenging procedures in colorectal surgery with a complex learning curve. SF resection with CME and CVL is feasible and safe for the treatment of early-stage and locally advanced SF cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon cancer is one of the most commonly diagnosed cancers and a leading cause of death from cancer worldwide [1]. Tumors located between proximal descending colon and distal transverse colon at the left colonic angle, known as splenic flexure (SF) cancer, are rare [2].
SF cancers have been excluded from the most important trial evaluations because of technical difficulties, particularly lymphatic drainage, open surgery, and an apparently worse oncological outcome [3, 4].
The complete mesocolic excision (CME) technique with central vascular ligation (CVL) and the mobilization of splenic flexure are well-known procedures but are still being debated in Western surgical society, as they are associated with increased morbidity and doubtful effects in oncological outcome [5,6,7]. Performing this type of operation with minimal invasive approach increases the level of difficulties. Several studies, conducted in an open surgery series, have assessed that, if compared to conventional extended resection, CME with CVL in colon cancer surgery have better clinical results with an equal oncologic outcome [8, 9].
The laparoscopic approach for SF cancer is technically demanding and not fully standardized. In fact, an extended right or left hemicolectomy is often performed by surgeons for technical difficulties related to multiple lymphatic drainage. However, many recent reports have described the laparoscopic approach to SF cancer with interesting results [10,11,12] such as satisfactory surgical margins with a correct number of harvested lymph nodes.
Currently, the laparoscopic approach to SF cancers with CME shows several criticalities. The core of the question includes the appropriate extension of colectomy, lymph node dissection along the superior and inferior mesenteric vessels, the risk of inadvertent injury of the spleen or pancreas tail, and, finally, the type of anastomosis [13]. Performing laparoscopical SF resection requires extensive knowledge on the vascular and lymphatic’s anatomy. However, no study with a high level of evidence has considered the laparoscopic resection of the transverse colon or, specifically, segmental resection of the splenic flexure for cancer. The present study reviews our experience in treating SF tumors by a totally laparoscopic CME with CVL approach, illustrating surgical technique and short-term outcomes.
Methods
Between February 2015 to October 2017, seventeen (17) consecutive patients out of 245 patients with colorectal cancer underwent totally laparoscopic CME with CVL for splenic flexure cancer.
Demographic data (age, sex, body mass index (BMI), comorbidities, Physical Status Classification System/American Society of Anesthesiology (ASA) score, previous surgical procedures), pathological features (number of harvested lymph nodes, tumor diameter, distal and proximal margin, grading and staging according to AJCC Cancer Staging 8th edition), operative time, blood loss, presence of abdominal drainage, operative time, time of passage of the first stool, minor and major complications (reported following the Clavien-Dindo classification) [14], hospital stay, and readmission rate were retrospectively recorded. All patients were evaluated in outpatient setting 30 days after discharge. Preoperative workup included physical examination, colonoscopy with biopsy, toraco-abdominal CT scan, and blood count. On all patients, China ink tattooing was performed.
No patient underwent mechanical bowel preparation. On all patients, we administered short-term antibiotic therapy with 2 g cephazolin and 500 mg metronidazole. All patients received thrombotic prophylaxis with low molecular weight heparin 12 h before surgery and until discharge once a day or more according to the patient’s comorbidities. Nasogastric tube was removed on awakening; the urinary catheter was placed after induction of general anesthesia and removed on the first post-operative day. A perianastomotic penrose drain was routinely used. All patients followed an enhanced recovery after surgery (ERAS) protocol [15]. Criteria for the discharge included absence of symptoms, flatus passage, and acceptable feeding with a light diet.
Surgical technique
All procedures were performed by three surgeons fully trained in laparoscopic colorectal surgery (FFDM, TG, PDS).
The laparoscopic approach was proposed to all patients. All patients underwent SF resection with complete mesocolon excision (CME) and central vascular ligation (CVL).
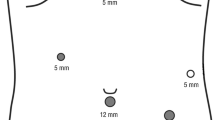
The patient was placed in a slight anti-Trendelenburg position with legs apart and a 10° right rotation. The basic angle was a 30° anti-Trendelenburg with the hips flexed at 15° with leg supports. The surgeon and the first assistant were on the right side. The second assistant was between the patient’s legs. Pneumoperitoneum was achieved by the Hasson’s technique. Usually, three working trocars and an optical trocar near the umbilicus were placed. The position of trocars is shown in Fig. 1. The AirSeal® system (Ab Medica s.p.a., via J.F. Kennedy, 10/12 20,023 Cerro Maggiore—MI) was used in all laparoscopic procedures.
The medial-to-lateral approach was used for both transverse colon and left colon mobilization. Starting the dissection, the primary landmarks were duodenum, ligament of Treitz, pancreas and inferior mesenteric vein. The inferior mesenteric vein (IMV) was identified and isolated below the ligament of Treitz at the duodenojejunal flexure. The mesocolon was lifted by the first assistant and the inferior mesenteric artery (IMA) was dissected at its origin (Bacon’s axilla). The retroperitoneal plane was created from the promontory starting from the first sigmoidal vessel. Left ureter and left gonadal vessels were visualized and left down in the retroperitoneal plane. Then, the left colic vein (LCV) and the left colic artery (LCA) were isolated and ligated at their root after radical lymphadenectomy on the IMA origin (Fig. 2).
The left colon with its mesentery was detached from the retroperitoneal structures resulting in the release of the renal fascia from the anterior perinephric fascia (Toldt’s lamina). Maintaining the operation in this space was an available way to preserve the intact mesocolon and pre-renal fascia which could protect the ureter, gonadal vessels, and pancreas, as well as reduce blood loss during the dissection. This procedure preserves the integrity of the embryological planes around the mesocolon and leads a maximum harvest of lymph nodes. Following this, the lateral attachment of the sigmoid and descending colon to the abdominal wall was divided (white line of Toldt) matching the previous medial dissection of the Toldt’s fascia. The SF was fully mobilized by the division of gastrocolic and splenocolic ligaments. The pancreaticocolic ligament was divided just below the inferior margin of the tail of the pancreas. The left branches of the middle colic vessels were isolated and divided at their roots.
The lymphadenectomy was performed starting from the root of the middle colic artery (lymph node station 15) and following its left side. Afterwards, the dissection was extended along the inferior border of the pancreas with radical lymphadenectomy of the lymph node station 18 (according to The Japanese Research Society for Gastric Cancer) [16] (Fig. 3).
Therefore, IMV was ligated at its root on the inferior pancreatic border. A full mobilization of the descending colon was reached up to the sigmoid colon. The transverse colon was mobilized up to the umbilical ligament as a landmark for the transection. Drummond’s arcade was isolated and sectioned for both transverse and descending colon. Afterwards, the colon was transected using a tri-staple mechanical device with en bloc partial omentectomy, along the gastroepiploic vessels. The transverse and the descending colon were lifted and secured to the left abdominal wall with an intra-corporeal stitch to obtain a correct approximation and orientation of the colonic stumps. Three stitches were positioned among the transverse and descending colon, and this allowed us to achieve symmetrical approximation of the colonic edges during laparoscopy, with an optimal closure of the deepest extremity of the enterotomy. A side-to-side colo-colonic antiperistaltic intra-corporeal anastomosis was performed by a linear stapler with three suture lines (Fig. 4); the remaining enterotomy was closed in a double layer with a running intra-corporeal suture. In two cases, we have accomplished an extracorporeal anastomosis through a left subcostal mini-laparotomy.
The specimen was extracted by suprapubic transverse incision after the positioning of the wall protection device.
Statistical analysis
All variables were expressed as mean ± standard deviation and as percent value, respectively.
Results
The female/male ratio was 4/13, with a mean age of 66.6 ± 12 years (range 37–88). The BMI mean value was 28.9 ± 3.7 (range 22.6–39). Seven out of seventeen (41.2%) patients have had previous abdominal surgery. The patient’s characteristics are summarized in Table 1.
In all patients, the procedure was completed by laparoscopy. An intracorporal anastomosis was completed in 89% of the patients (15/17). In two cases (11.7%), we performed an extracorporeal anastomosis: in one case, side-to-side double-layer manual anastomosis and in another case, an end-to-end double-layer anastomosis due to tissue frailty, suspected vascular failure or extensive adhesiolysis. The mean operative time was 215.5 ± 65 min (range 120–400), and the blood loss was 80 ± 27 ml. In one case, an intra-operative colonoscopy was necessary due to unclear tumoral area demarcation. In one case, an IMA ligation was performed due to vascular anomalies (abnormal LCA with isolated origin to the aorta).
The distal margin was 3.1 ± 2.6 cm and the proximal margin was 6.5 ± 3.3 cm from the tumor site. The mean number of harvested nodes was 13.9 ± 7 (range 2–31). In one case, a synchronous laparoscopic partial gastrectomy was associated due to tumor suspected invasion.
The mean post-operative stay was 6.7 ± 3.3 days (range 4–14). Intra-operative and pathological data are summarized in Table 2. Overall morbidity was 23.5%. Readmission was necessary for two patients (11.7%): in one case due to persistent abdominal pain requiring medical treatment and in a second case due to peripancreatic abdominal fluid collection that was treated by percutaneous drainage (the Clavien-Dindo III). The reintervention rate was zero. All post-operative complications are scheduled in Table 3 according to the Clavien-Dindo classification.
A median follow-up of 13.3 ± 9.9 months shows no local recurrence. Adjuvant chemotherapy was accomplished by 6/17 patients (stage III and IV according to AJCC/TNM cancer staging). Afterwards, 2/17 patients with resectable liver disease underwent hepatic resection with curative intent.
Discussion
The first laparoscopic colectomy was performed in 1991 [17]. Nowadays, several studies have shown that the laparoscopic approach for colon cancer results in less blood loss, a shorter length of hospital stay, and lower post-operative short-term morbidity compared with open resections with a comparable oncological outcome [3, 18].
SF cancers (SFc) represent about 2–5% of all colorectal cancers (CRC) [19]. Unfortunately, SFc are diagnosed at a significantly more advanced stage and more likely as emergency with a similar survival rate compared to the other colon cancer sites at the same disease stage [2, 20, 21].
These cancers’ aggressive behavior was also based on studies which underlined that SFc has a higher incidence of mucinous adenocarcinomas, generally bulky, with an acute angle of the bowel at the splenic flexure predisposing to the early development of intestinal obstruction [22].
Griffith et al. reported that blood supply to the splenic flexure is carried by the inferior mesenteric artery via the left colic artery in most cases, but in 11% of patients, this is carried by the superior mesenteric artery via the left branch of middle colic artery. Making the topic more complex is the fact that for oncological purpose splenic flexure shows a triple lymphatic drainage to both the superior and inferior mesenteric vessels and to the splenic hilum along the pancreatic tail [23].
The lymphadenectomy may be inappropriate if the vascular and lymphatic anatomies are not considered [12].
Based on these considerations, Levien et al. [24] suggest the need for an extended colonic resection in the case of SFc such as extended right hemicolectomy, subtotal colectomy, or extended left hemicolectomy, with or without splenectomy or splenopancreasectomy to achieve oncological principles in colon resection for cancer.
More recently, De Angelis et al. [12] have demonstrated that laparoscopic extended right colectomy and extended left hemicolectomy procedures performed for SF cancers appear to have similar short- and long-term oncologic outcomes. Obviously, a significantly higher number of lymph nodes were retrieved in extended right colectomy than the left colectomy procedures.
Kim et al. [25] published the most extensive record of patients with SFc comparing open subtotal colectomies to isolated resection of distal transverse and proximal descending colon; the left hemicolectomy and subtotal colectomy groups did not differ in terms of mean overall survival or disease-free survival. However, they found that the rates of post-operative complications and transfusion were higher in patients undergoing combined splenectomy or subtotal colectomy as compared to the limited resection ones. Furthermore, most of the studies that have examined the effect of splenectomy on the long-term survival of colorectal cancer patients have reported that splenectomy did not influence long-term survival, but instead is associated with a significant increase in morbidity [13, 26].
Nowadays, extended colon resection should be reserved for patients with concomitant colon comorbidities as synchronous cancers in other segments, complicated diverticular disease, or intestinal obstruction with suffering proximal colon [2, 27].
Nakagoe et al. [2] have demonstrated that segmental resection for SFc is safe and oncologically correct even with the laparoscopic approach. The technical difficulties are well documented by Jamali et al. [28] where they have conducted a mail-in survey of 35 experienced laparoscopic colorectal surgeons reporting the highest score of difficulty for splenic or transverse colectomy. Their results suggest that splenic flexure and transverse colon resection are best left for the later stages of colorectal surgeon’s experience. This explains why the transverse colon resections were excluded from the most cited randomized controlled trials of laparoscopic versus open surgery for colon cancer [4, 29].
In our experience, the laparoscopic approach for SFc appears to be safe and feasible and this agrees with recent studies [27, 30, 31]; all cases achieved an adequate number of lymph nodes harvested (13.9 ± 7) and an oncologically correct tumor free margin of the specimen.
We believe that the laparoscopic approach is the best way to approach the SFc even for invasive tumors in adjacent structures (T4) with the correct technical knowledge.
Regarding the surgical technique, the medial-to-lateral dissection of Toldt fascia from Gerota’s one with CME and CVL has been the technique of choice. A thorough knowledge of embryology and anatomy is needed before performing a complete mesocolon excision in colon cancer. The lack of anatomical and embryological studies on the splenic flexure does not allow the construction of standardized surgical technique [32].
The laparoscopic approach involves a lateral-to-medial approach to the left-sided transverse mesocolon following a medial-to-lateral approach to the left mesocolon. The left branch of middle colic artery and left superior colic artery have been tied and dissected in all cases with contemporary detachment of pancreaticolic ligament and gastrocolic division determining splenic flexure fully mobilized. The resection of splenic flexure was completed with exeresis of the left and transverse mesocolon containing draining lymph nodes with an intact fascial package. Despite two cases of extracorporeal anastomosis, we prefer a totally laparoscopic intra-corporeal antiperistaltic side-to-side one. The advantages of intra-corporeal anastomoses are well known, especially for obese patients, as it avoids the exteriorization of short mesenteries through a much thicker abdominal wall and reduction of the twisting of the stumps [33, 34].
Our experience highlights a longer hospital stay as compared to patients undergoing resection for sigmoid or descending colon cancer due to the delayed passage of first flatus and stool. The type of anastomosis (antiperistaltic versus isoperistaltic) does not explain this difference, as reported by several studies [35, 36].
It seems to be related to the higher rate of clinical or subclinical intestinal obstruction or systemic disease (M1 disease) in patients presenting T3 tumor, which, in turn, may affect the intestinal motility recovery.
The high rate of morbidity is essentially due to the choice of analyzing complications by the Clavien-Dindo classification system, which divided complications into five categories, and also to the small number of patients. In one case, it was necessary to drain percutaneously a peripancreatic collection (the Clavien-Dindo grade III). The results of our series appear to be satisfactory in terms of short-term outcomes and oncologic adequacy even with limited resections, even if the most recent meta-analyses are not conclusive [37].
The principal limitation of our study is its size; moreover, the lack of larger studies on SFc laparoscopic approach does not allow to draw standardized surgical procedures. However, once technical tips have been acquired, laparoscopic resection of SFc could be feasible as a minimally invasive surgery once technical tips have been acquired.
Abbreviations
- SF:
-
Splenic flexure cancer
- CME:
-
Complete mesocolon excision
- CVL:
-
Central vascular ligation
References
WHO. Cancer fact sheet. WHO media center - Cancer fact sheet. http://www.who.int/mediacentre/factsheets/fs297/en/. Published 2017
Nakagoe T, Sawai T, Tsuji T, Jibiki M, Ohbatake M, Nanashima A, Yamaguchi H, Yasutake T, Kurosaki N, Ayabe H, Ishikawa H (2001) Surgical treatment and subsequent outcome of patients with carcinoma of the splenic flexure. Surg Today 31(3):204–209. https://doi.org/10.1007/s005950170169
Hazebroek EJ (2002) COLOR: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Surg Endosc 16(6):949–953. https://doi.org/10.1007/s00464-001-8165-z
Nelson H (2004) Laparoscopically assisted colectomy is as safe and effective as open colectomy in people with colon cancer. Cancer Treat Rev 30(8):707–709. https://doi.org/10.1016/j.ctrv.2004.09.001
Adamina M, Manwaring ML, Park KJ, Delaney CP (2012) Laparoscopic complete mesocolic excision for right colon cancer. Surg Endosc Other Interv Tech. 26(10):2976–2980. https://doi.org/10.1007/s00464-012-2294-4
Siani LM, Pulica C (2015) Laparoscopic complete mesocolic excision with central vascular ligation in right colon cancer: long-term oncologic outcome between mesocolic and non-mesocolic planes of surgery. Scand J Surg 104(4):219–226. https://doi.org/10.1177/1457496914557017
Storli KE, Søndenaa K, Furnes B, Eide GE (2014) Outcome after introduction of complete mesocolic excision for colon cancer is similar for open and laparoscopic surgical treatments. Dig Surg 30(4–6):317–327. https://doi.org/10.1159/000354580
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation - technical notes and outcome. Color Dis 11(4):354–364. https://doi.org/10.1111/j.1463-1318.2008.01735.x
Weber K, Merkel S, Perrakis A, Hohenberger W (2013) Is there a disadvantage to radical lymph node dissection in colon cancer? Int J Color Dis 28(2):217–226. https://doi.org/10.1007/s00384-012-1564-x
Pisani Ceretti A, Maroni N, Sacchi M, Bona S, Angiolini MR, Bianchi P, Opocher E, Montorsi M (2015) Laparoscopic colonic resection for splenic flexure cancer: our experience. BMC Gastroenterol 15:76. https://doi.org/10.1186/s12876-015-0301-7
Kim MK, Lee IK, Kang WK et al (2017) Long-term oncologic outcomes of laparoscopic surgery for splenic flexure colon cancer are comparable to conventional open surgery. Ann Surg Treat Res 93(1):35–42. https://doi.org/10.4174/astr.2017.93.1.35
de’ Angelis N, Hain E, Disabato M et al (2016) Laparoscopic extended right colectomy versus laparoscopic left colectomy for carcinoma of the splenic flexure: a matched case–control study. Int J Color Dis 31(3):623–630. https://doi.org/10.1007/s00384-015-2469-2
Lolle I, Pommergaard H-C, Schefte DF, Bulut O, Krarup P-M, Rosenstock SJ (2016) Inadvertent splenectomy during resection for colorectal cancer does not increase long-term mortality in a propensity score model: a nationwide cohort study. Dis Colon Rectum 59(12):1150–1159. https://doi.org/10.1097/DCR.0000000000000712
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications. Ann Surg 250(2):187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L, Boutros M, McClane J, Steele SR, Feldman LS (2017) Clinical practice guideline for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons (ASCRS) and Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Surg Endosc Other Interv Tech. 31(9):3412–3436. https://doi.org/10.1007/s00464-017-5722-7
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver 4). Gastric Cancer 20(1):1–19
Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1(3):144–150 http://www.ncbi.nlm.nih.gov/pubmed/1688289
Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100(1):75–82. https://doi.org/10.1002/bjs.8945
Roscio F, Bertoglio C, De Luca A, Frattini P, Clerici F, Scandroglio I (2012) Totally laparoscopic resection of the splenic flexure for tumor. Updat Surg 64(3):185–190. https://doi.org/10.1007/s13304-012-0162-3
Shaikh IA, Suttie SA, Urquhart M, Amin AI, Daniel T, Yalamarthi S (2012) Does the outcome of colonic flexure cancers differ from the other colonic sites? Int J Color Dis 27(1):89–93. https://doi.org/10.1007/s00384-011-1292-7
Steffen C, Bokey EL, Chapuis PH (1987) Carcinoma of the splenic flexure. Dis Colon Rectum 30(11):872–874. https://doi.org/10.1007/BF02555427
Awotar GK, Luo F, Zhao Z, Guan G, Ning S, Ren J, Liu Y, Wang G, Liu P (2016) Splenic abscess owing to cancer at the splenic flexure. Medicine (Baltimore) 95(38):e4941. https://doi.org/10.1097/MD.0000000000004941
GRIFFITHS JD (1956) Surgical anatomy of the blood supply of the distal colon. Ann R Coll Surg Engl 19(4):241–256
Levien DH, Gibbons S, Begos D, Byrne BD (1991) Survival after resection of carcinoma of the splenic flexure. Dis Colon Rectum 34(4):401–403
Kim CW, Shin US, Yu CS, Kim JC (2010) Clinicopathologic characteristics, surgical treatment and outcomes for splenic flexure colon cancer. Cancer Res Treat 42(2):69–76. https://doi.org/10.4143/crt.2010.42.2.69
Konstadoulakis MM, Kymionis GD, Leandros E et al (1999) Long-term effect of splenectomy on patients operated on for cancer of the left colon: a retrospective study. Eur J Surg 165(6):583–587. https://doi.org/10.1080/110241599750006505
Fiscon V, Portale G, Migliorini G, Frigo F (2015) Splenic flexure colon cancers: minimally invasive treatment. Updat Surg 67(1):55–59. https://doi.org/10.1007/s13304-015-0282-7
Jamali FR, Soweid AM, Dimassi H, Bailey C, Leroy J, Marescaux J (2008) Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg 143(8):762–767; discussion 768. https://doi.org/10.1001/archsurg.143.8.768
Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM, COlon cancer Laparoscopic or Open Resection Study Group (COLOR) (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6(7):477–484. https://doi.org/10.1016/s1470-2045(05)70221-7
Ceccarelli G, Biancafarina A, Patriti A, Spaziani A, Bartoli A, Bellochi R, Pisanelli MC, Casciola L (2010) Laparoscopic resection with intracorporeal anastomosis for colon carcinoma located in the splenic flexure. Surg Endosc Other Interv Tech 24(7):1784–1788. https://doi.org/10.1007/s00464-009-0853-0
Nakashima M, Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, Noaki R, Yamakawa K, Nagasue Y, Kuroyanagi H, Yamaguchi T (2011) Colon cancer in the splenic flexure: comparison of short-term outcomes of laparoscopic and open colectomy. Surg Laparosc Endosc Percutan Tech 21(6):415–418. https://doi.org/10.1097/SLE.0b013e31823aca96
Matsuda T, Sumi Y, Yamashita K, et al (2017) Anatomical and embryological perspectives in laparoscopic complete mesocoloic excision of splenic flexure cancers. Surgical Endoscopy and Other Interventional Techniques. 1–7
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P (2010) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 28(2):272–278. https://doi.org/10.1200/JCO.2009.24.1448
Carlini M, Spoletini D, Castaldi F, Giovannini C, Passaro U (2016) Laparoscopic resection of splenic flexure tumors. Updat Surg 68(1):77–83. https://doi.org/10.1007/s13304-016-0357-0
Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Takahashi G, Yamada M, Uchida E (2015) Isoperistaltic versus antiperistaltic stapled side-to-side anastomosis for colon cancer surgery: a randomized controlled trial. J Surg Res 196(1):107–112. https://doi.org/10.1016/j.jss.2015.02.059
Ibañez N, Abrisqueta J, Luján J, Hernández Q, Parrilla P (2017) Isoperistaltic versus antiperistaltic side-to-side anastomosis after right laparoscopic hemicolectomy for cancer (ISOVANTI) trial: study protocol for a randomised clinical trial. Int J Color Dis 32(9):1349–1356. https://doi.org/10.1007/s00384-017-2840-6
Martínez-Pérez A, Brunetti F, Vitali GC, Abdalla S, Ris F, De’Angelis N (2017) Surgical treatment of colon cancer of the splenic flexure: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 27(5):318–327. https://doi.org/10.1097/SLE.0000000000000419
Acknowledgements
Thanks to Prof. Roberto Cotellese, Director of General Surgery Training Program at University G.D’Annunzio—Chieti Pescara.
Author information
Authors and Affiliations
Contributions
PP and TG contributed equally to this work and should be considered co-first authors.
Study conception and design: FFDM, TG, PDS, and PP. Acquisition of data: MR and PP. Analysis and interpretation of data: MR, FFDM, and PP. Drafting of manuscript: PP and FFDM. Critical revision: PDS and FFDM
Corresponding author
Ethics declarations
Ethical approval
The study protocol was in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki Declaration and its later amendments. Since this was a retrospective study, formal consent for this study is not required and no approval of the institutional research committee was needed.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Panaccio, P., Grottola, T., Ricciardiello, M. et al. How we do it: totally laparoscopic complete mesocolon excision for splenic flexure cancer. Langenbecks Arch Surg 403, 769–775 (2018). https://doi.org/10.1007/s00423-018-1699-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-018-1699-5