Abstract
Purpose
Currently, there are two laparoscopic stapling techniques to perform the gastrojejunostomy in gastric bypass surgery: the linear stapling and circular stapling techniques. The aim of the study was to compare the two techniques regarding postoperative morbidity and weight loss at an accredited bariatric reference center in Switzerland.
Methods
We compared two consecutive cohorts at a single institution between November 2012 and June 2014 undergoing laparoscopic gastric bypass surgery. The frequency of complications and weight loss at 1 year was assessed in 109 patients with the 21-mm circular stapling technique (CSA) and 134 patients with the linear stapling technique (LSA).
Results
Postoperative complications were more frequent in the CSA group with 23.9 versus 4.5% in the LSA group (p = <0.0001). The main difference was the frequency of strictures, which occurred in 15.6% in the CSA group versus 0% in the LSA group. As a result, endoscopic dilation was required at least once in 15 patients. There was no statistically significant difference in percentage of excessive weight loss (EWL) in both groups; EWL was 74% in the CSA group and 73% in the LSA group (p = 0.68).
Conclusion
Linear stapled laparoscopic gastric bypass had fewer stenotic strictures with similar weight loss at 1 year compared to circular stapling technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is considered the most effective treatment for morbid obesity and results in substantial weight loss as well as in an improvement of the obesity-associated comorbidities, such as arterial hypertension, diabetes mellitus type II, sleep apnea, and hypercholesterolemia [1, 2]. In 2013, a total of 468,609 bariatric procedures were performed worldwide, 95.7% of which were conducted laparoscopically [3]. Today, one of the most frequently performed techniques is still the laparoscopic Roux-en-Y-Gastric Bypass (LRYGB), which was first introduced by Wittgrove et al. in 1993 [4]. The critical step of the operation is the gastrojejunostomy. Currently, there are two standard stapling techniques for the gastrojejunostomy: the circular stapling anastomosis (CSA) as described early by Wittgrove and Clark [4] and the linear stapling anastomosis (LSA) as described by Williams and Champion [5]. The main objective is to achieve a low frequency of complications, such as leaks, strictures, and marginal ulcer at the anastomosis. While the leakage rate has dropped dramatically to less than 3% within the last decades, the occurrence of anastomotic strictures and stenosis has been reported for both techniques and it remains unclear whether one is superior [6,7,8]. Therefore, we assessed the postoperative complication rate and weight loss between the two techniques at our institution.
Materials and methods
Data from all patients who underwent a proximal laparoscopic gastric bypass surgery in our reference center for bariatric surgery were collected from November 2012 to June 2014. After excluding reoperations, conversions (e.g., band to bypass), and patients with previous laparotomy, two consecutive cohorts with 109 patients who underwent a LRYGB using a 21-mm circular stapler and 134 patients who underwent a LRYGB performed by a linear stapling technique were analyzed in the given time period. Two experienced bariatric surgeons, each of whom has performed more than 500 cases, conducted the operations. One surgeon performed LSA and the other one performed CSA according to their preference. The database included patients demographics, comorbidities, weight measurements preoperatively and 1 year postoperatively, operation time, length of hospital stay, 30-day complication rate, secondary interventions, and long-term complications at 1 year. Complications were defined according to the Clavien/Dindo classification [9]. A stenosis was defined if the diameter of the gastrojejunostomy was less than 10 mm [10]. All patients underwent standardized preoperative assessment according to the national guidelines including routine endoscopy with Helicobacter pylorus testing. A thorough follow-up was conducted equally in all patients according to our center guidelines in accordance with the Swiss society for the study of morbid obesity and metabolic diseases (smob.ch). The follow-up is set up as to detect and treat any malnutrition or imbalance of vitamins as well as the adaptation of the medical therapy of the comorbidities. The appointments are held in standardized time intervals (as described below) and they include a thorough physical exam as well as standardized laboratory tests [11].
Operation technique
The LSA technique: A gastric pouch (volume approximately 15 ml) was created using 2–3 Endo GIA tri stapler™ (purple, 60-mm magazines). After identification of the ligament of Treitz and measurement of 50-cm jejunal limb, a side-to-side gastrojejunostomy was performed with an Endo GIA tri stapler™ (purple, 45-mm magazine). Caution was taken to insert the stapler no more than 2 cm. The enterotomy was then closed using a single layer hand-sewn continuous suture with a 3.0 PDS™ (polydiaxonone suture, Ethicon). Measurement of 150-cm Roux limb and creation of the jejuno-jejunal anastomosis side-to-side with an Endo GIA tri stapler™ (tan, 60-mm magazine) was then performed. Closure of the enterotomy was achieved using a single layer hand-sewn continuous suture with a 3.0 PDS™ (polydiaxonone suture, Ethicon). The gastrojejunostomy was separated with an Endo GIA tri stapler™ tan magazine.
The CSA technique: After preparation of the pouch with one horizontal 60-mm Echelon stapler line (Ethicon), a 21-mm circular stapler head (ILS Ethicon) was inserted through a gastrotomy at the greater curvature close to the first stapler line. Then, a vertical stapler line (60 mm) was applied close to the angle of His in order to complete the pouch formation and to separate the gastrotomy from the pouch. The gastric remnant with the gastrotomy was then resected using a small wedge resection with one or two stapler firings. After identification of the ligament of Treitz and measurement of 50-cm jejunal limb, the circular stapler was inserted into the jejunal limb and connected to the stapler head to form the gastrojejunostomy. Then, the 21-mm circular stapler was fired and withdrawn without any plastic sheet. The jejuno-jejunal anastomosis was created side to side using the Echelon stapler after measurement of 150-cm Roux limb. Finally, the gastrojejunostomy was separated from the jejuno-jejunostomy using an Echelon stapler (Ethicon). All mesenteric defects were closed at the end of both procedures. The circular stapler insertion site was cleaned with two sterile gauzes to prevent subcutaneous infection.
Postoperative care and follow-up
After upper gastrointestinal series with contrast swallow on postoperative day one, a diet consisting of pureed foods was introduced and maintained for 1 month. Patients were discharged around day 5 with proton pump inhibitors, temporary thrombosis prophylaxis, and vitamin and mineral supplementation. Follow-up was conducted at 2 and 4 weeks and 3, 6, 9, and 12 months. If a patient showed nausea, vomiting, or food intolerance, an anastomotic stricture or stenosis was ruled out by endoscopy. In case of confirmed stenosis, patients underwent immediate endoscopic balloon dilation up to 15 mm. If a leakage was suspected, a contrast-enhanced abdominal computed tomography and endoscopy was performed. Depending on the findings, a diagnostic laparoscopy was then conducted. All other unspecific complaints were noted in the patient’s medical file.
Data analysis and statistics
Univariate analysis was performed to determine any associations between complications and the gastrojejunostomy technique, using Fisher’s exact test. Weight loss data was assessed with t test. Comorbidities were analyzed with a chi-square test. A p value of <0.05 was being considered as statistically significant.
Results
Demographics
The demographics and length of hospital stay did not differ between the investigated groups (Table 1).
Follow-up
For patients in the CSA group, the follow-up rate was 94.5% and for patients in the LSA group, it was 90.6% at 12 months. The follow-up rate after 30 days was 100% for both groups.
Comorbidities
Apart from a higher incidence of obstructive sleep apnea syndrome in the CSA group (45 versus 27.6%, p < 0.05), the distribution of the comorbidities was comparable in both groups at the time of operation (Table 2). One year after the operation, the remission rate of diabetes mellitus type II (DM II) was similar in both groups. In the CSA group, 17 of 25 (68%) patients had remission. In the LSA group, 15 of 21 (71%) had remission (p = 0.915).
Complications and operative time
The overall complication rate in the CSA group was higher with 23.9% compared to 4.5% (p < 0.0001) in the LSA group (Table 4). The operative time was significantly longer in the CSA group (CSA 130.2 (±33.1) minutes compared to LSA 89.4 (±21.1) minutes; p < 0.05). The most frequent complication in the CSA group was a stenosis at the gastrojejunostomy, which occurred in 15.6% of the patients at a mean of 26.4 days (±6) postoperative. Table 5 shows the number of dilations needed to treat the strictures. There were no patients with a stenotic stricture in the LSA group. Leakage at the gastrojejunostomy occurred in 0.74% (one patient) in the LSA and in 1.8% (two patients) in the CSA group (p = 0.422). The leaks occurred at postoperative days 3, 8, and 30. All three cases needed immediate laparoscopy with reconstruction of the gastrojejunostomy (Tables 3 and 4). In one patient of the LSA group, the esophagus was accidentally injured during the pouch formation. This lesion was recognized immediately during primary surgery and stenting of the esophagus was performed postoperatively. In both groups, there was one patient with an intra-abdominal hematoma. In the CSA group, it occurred spontaneously and was managed conservatively. The patient in the LSA group was bleeding under oral anticoagulants, needed the placement of a computed tomography-guided drainage and was classified as a grade 3a complication. There were two cases of wound infection in the CSA group, none in the LSA group. In one case, it could be treated without any further measures and healed spontaneously and therefore was classified as a grade 1 complication. The other wound needed to be opened and cleaned and antibiotic treatment was initiated; this was classified as a grade 2 complication. There was no further complication/stenosis at 1 year in both groups, and we did not observe any severe hypoglycemia (Table 5).
Weight loss
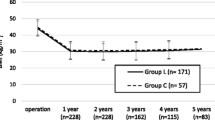
The mean weight and body mass index (BMI) at 1 year postoperative was similar in both groups with 86 kg (±24.6) and 30.2 kg/m2 (±5.1) in the CSA versus 84.2 kg (±24) and 29.9 kg/m2 (±4.7) in the LSA group (p = 0.68). There was no statistically significant difference in percentage of total weight loss (TWL) and percentage of excess weight loss (EWL) in both groups; TWL was 32% in both groups (p = 0.41); EWL was 74% in the CSA group and 73% in the LSA group (p = 0.68) (Table 6).
Discussion
This publication compares the LSA to the CSA technique using a 21-mm stapler head in gastric bypass surgery. It shows that the number of stenotic strictures was higher and the operation time was longer using the CSA technique, while the weight loss at 1 year was comparable in both groups.
When looking at the preferred gastrojejunostomy technique in an online survey of the American Society for Bariatric Surgery, the CSA was preferred by 43% of the surgeons, followed by LSA and hand-sewn anastomosis [12]. This preference implies that the CSA technique may be easier, but we found that operation time was considerably increased with the CSA technique. This is in line with the findings of recently published data by Sima E. et al. 2014 [13] as well as Edholm et al. 2015 [14], who showed that CSA is associated with increased operation time, hospital stay, and incidence in postoperative complications compared to LSA. Prolonged operation time is more costly and may expose the patient to a higher risk of complications such as thromboembolic events [15]. Preoperative BMI, age, and gender were comparable in our groups. Therefore, increased operation time cannot be explained by more challenging conditions. We found no difference regarding length of hospital stay between the two techniques despite an increased complication rate in the CSA group. This can be explained by the fact that most complications were stenotic strictures, which tend to occur after the patient’s discharge. Two large meta-analyses looked at stricture rates in both anastomosis techniques and also found a significantly increased stenosis rate in CSA, although the stapler size was not specified [16, 17]. Most studies compared the 21-mm to the 25-mm stapler head and found that gastrojejunostomy stenosis was increased when using the 21-mm stapler head (17 versus 7%) [18,19,20]. The reported stenosis rates range from 7 to 31% in CSA depending on the literature [19, 21, 22]. In our LSA group, we did not find any strictures, which may be a real benefit of the LSA technique. The stenosis rate in our CSA group was 15.6%, and 15 out of 17 patients required a balloon dilation at least once. The additional endoscopic treatment is cost intensive and an unnecessary burden for the patient. The pathophysiology of the gastrojejunostomy stenosis is still speculative. Some of the reasons may be patient related, such as smoking or excessive scar formation. Additional important factors are most likely the diameter of the gastrojejunostomy and ischemia with or without ulceration causing scarring at the anastomotic junction. Contributing to the tightness of the anastomosis is a small diameter of the anastomosis, created in CSA technique with a stapler head <25 mm [10, 20], whereas the LSA technique results in a wide anastomosis. The different anastomosis techniques did not have an impact on the frequency in leaks. Consistent with the current findings in literature, the anastomotic leakage rate was comparable in both groups [7, 13, 23, 24]. The wound infection rate was slightly higher in the CSA group. In the literature, the infection rate varies between 4.7 and 23% [8, 16, 21]. The passing of the circular stapler and anvil through the abdominal wall is suspected to contaminate the subcutaneous tissue and to cause wound infections. Subcutaneous wound infections may result in a serious threat to the patient. The need for antibiotic treatment and secondary surgical interventions are often required. The reduction of the wound infection rate is therefore a substantial benefit.
It has been propagated to use the CSA technique in order to achieve better restriction and weight loss compared to the LSA technique [25]. However, we found no difference regarding weight loss at 1-year follow-up. While not all of the authors looked at weight loss [7, 18, 26], Gould et al. found similar weight loss when comparing 21-mm to 25-mm stapler [19]. Lee et al. did see a greater weight loss during the first few months after LRYGB in patients with gastrojejunostomy stenosis, independent of the surgical technique, but after 12 months, the difference had disappeared [22]. Bohdjalian et al. and the recent publication of Schneider et al. compared LSA to CSA and found no difference in weight loss after 2 years, which up to now is the longest follow-up time when comparing the LSA to the CSA technique [27, 28]. Therefore, the use of the CSA technique for better weight control seems to be unjustified. Although there was no statistically significant difference, we did see a tendency in better remission of DM II in the LSA group. Long-term data are needed in order to fully clarify this question and to address the role of restriction as well as the amelioration of the comorbidities. Multiple modern concepts other than restriction, that explain weight loss after LRYGB surgery, have been recently identified. Altered bile flow, changes in gastrointestinal hormones, and changes in metabolic rate are most likely more important than restriction and malabsorption [29,30,31,32].
The shortcoming of this study is its retrospective design and potential bias related to these kinds of analyses, such as the comparison of the results of two different surgeons. However, all patients were treated equally in the same institution by the same team in a short period in order to minimize era bias or change of patient care. In addition, our obesity center has a large experience performing over 200 cases per year and each step from the operation to the postoperative care is standardized.
Conclusion
In conclusion, our data show that there is no advantage in using the 21-mm CSA technique compared to the LSA technique. On the contrary, the stricture rate is higher with no difference concerning weight loss at 1 year. Therefore, the LSA technique might be the preferred technique to perform the gastrojejunostomy in LRYGB.
References
Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ (2009) The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technology Assessment 13(41):1–190, 215-357, iii-iv. doi:10.3310/hta13410
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H, Swedish Obese Subjects Study Scientific G (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351(26):2683–2693. doi:10.1056/NEJMoa035622
Buchwald H, Williams SE (2004) Bariatric surgery worldwide 2003. Obes Surg 14(9):1157–1164. doi:10.1381/0960892042387057
Wittgrove AC, Clark GW (2000) Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3-60 month follow-up. Obes Surg 10(3):233–239. doi:10.1381/096089200321643511
Williams MD, Champion JK (2004) Linear technique of laparoscopic Roux-en-Y gastric bypass. Surg Technol Int 13:101–105
Abdel-Galil E, Sabry AA (2002) Laparoscopic Roux-en-Y gastric bypass—evaluation of three different techniques. Obes Surg 12(5):639–642
Bendewald FP, Choi JN, Blythe LS, Selzer DJ, Ditslear JH, Mattar SG (2011) Comparison of hand-sewn, linear-stapled, and circular-stapled gastrojejunostomy in laparoscopic Roux-en-Y gastric bypass. Obes Surg 21(11):1671–1675. doi:10.1007/s11695-011-0470-6
Finks JF, Carlin A, Share D, O’Reilly A, Fan Z, Birkmeyer J, Birkmeyer N, Michigan Bariatric Surgery Collaborative from the Michigan Surgical Collaborative for Outcomes Research E (2011) Effect of surgical techniques on clinical outcomes after laparoscopic gastric bypass—results from the Michigan Bariatric Surgery Collaborative. Surg Obes Relat Dis: Off J Am Soc Bariatric Surg 7(3):284–289. doi:10.1016/j.soard.2010.10.004
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Goitein D, Papasavas PK, Gagne D, Ahmad S, Caushaj PF (2005) Gastrojejunal strictures following laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc 19(5):628–632. doi:10.1007/s00464-004-9135-z
Fried M, Hainer V, Basdevant A, Buchwald H, Deitel M, Finer N, Greve JW, Horber F, Mathus-Vliegen E, Scopinaro N, Steffen R, Tsigos C, Weiner R, Widhalm K, Bariatric Scientific Collaborative Group Expert P (2007) Interdisciplinary European guidelines for surgery for severe (morbid) obesity. Obes Surg 17(2):260–270
Madan AK, Harper JL, Tichansky DS (2008) Techniques of laparoscopic gastric bypass: on-line survey of American Society for Bariatric Surgery practicing surgeons. Surg Obes Relat Dis: Off J Am Soc Bariatric Surg 4(2):166–172; discussion 172-163. doi:10.1016/j.soard.2007.08.006
Sima E, Hedberg J, Ehrenborg A, Sundbom M (2014) Differences in early complications between circular and linear stapled gastrojejunostomy in laparoscopic gastric bypass. Obes Surg 24(4):599–603. doi:10.1007/s11695-013-1139-0
Edholm D, Sundbom M (2015) Comparison between circular- and linear-stapled gastrojejunostomy in laparoscopic Roux-en-Y gastric bypass-a cohort from the Scandinavian Obesity Registry. Surg Obes Relat Dis: Off J Am Soc Bariatric Surg. doi:10.1016/j.soard.2015.03.010
Reames BN, Bacal D, Krell RW, Birkmeyer JD, Birkmeyer NJ, Finks JF (2015) Influence of median surgeon operative duration on adverse outcomes in bariatric surgery. Surg Obes Relat Dis: Off J Am Soc Bariatric Surg 11(1):207–213. doi:10.1016/j.soard.2014.03.018
Giordano S, Tolonen P, Victorzon M (2010) Comparision of linear versus circular stapling techniques in laparoscopic gastric bypass surgery—a pilot study. Scand J Surgery: SJS : Off Org Finn Surg Soc Scand Surg Soc 99(3):127–131
Penna M, Markar SR, Venkat-Raman V, Karthikesalingam A, Hashemi M (2012) Linear-stapled versus circular-stapled laparoscopic gastrojejunal anastomosis in morbid obesity: meta-analysis. Surg Laparosc Endosc Percutaneous Tech 22(2):95–101. doi:10.1097/SLE.0b013e3182470f38
Fisher BL, Atkinson JD, Cottam D (2007) Incidence of gastroenterostomy stenosis in laparoscopic Roux-en-Y gastric bypass using 21- or 25mm circular stapler: a randomized prospective blinded study. Surg Obes Relat Dis: Off J Am Soc Bariatric Surg 3(2):176–179. doi:10.1016/j.soard.2006.11.014
Gould JC, Garren M, Boll V, Starling J (2006) The impact of circular stapler diameter on the incidence of gastrojejunostomy stenosis and weight loss following laparoscopic Roux-en-Y gastric bypass. Surg Endosc 20(7):1017–1020. doi:10.1007/s00464-005-0207-5
Nguyen NT, Stevens CM, Wolfe BM (2003) Incidence and outcome of anastomotic stricture after laparoscopic gastric bypass. J Gastrointest Surg: Off J Soc Surg Alimentary Tract 7(8):997–1003 discussion 1003
Gonzalez R, Lin E, Venkatesh KR, Bowers SP, Smith CD (2003) Gastrojejunostomy during laparoscopic gastric bypass: analysis of 3 techniques. Arch Surg 138(2):181–184
Lee S, Davies AR, Bahal S, Cocker DM, Bonanomi G, Thompson J, Efthimiou E (2014) Comparison of gastrojejunal anastomosis techniques in laparoscopic Roux-en-Y gastric bypass: gastrojejunal stricture rate and effect on subsequent weight loss. Obes Surg 24(9):1425–1429. doi:10.1007/s11695-014-1219-9
Giordano S, Salminen P, Biancari F, Victorzon M (2011) Linear stapler technique may be safer than circular in gastrojejunal anastomosis for laparoscopic Roux-en-Y gastric bypass: a meta-analysis of comparative studies. Obes Surg 21(12):1958–1964. doi:10.1007/s11695-011-0520-0
Stroh CE, Nesterov G, Weiner R, Benedix F, Knoll C, Pross M, Manger T, Obesity Surgery Working G, Competence Network O (2014) Circular versus linear versus hand-sewn gastrojejunostomy in Roux-en-Y-gastric bypass influence on weight loss and amelioration of comorbidities: data analysis from a quality assurance study of the surgical treatment of obesity in Germany. Frontiers Surg 1:23. doi:10.3389/fsurg.2014.00023
Owens ML, Sczepaniak JP (2009) Size really does matter-role of gastrojejunostomy in postoperative weight loss. Surg Obes Relat Dis: Off J Am Soc Bariatric Surg 5(3):357–361. doi:10.1016/j.soard.2008.08.020
Qureshi A, Podolsky D, Cumella L, Abbas M, Choi J, Vemulapalli P, Camacho D (2015) Comparison of stricture rates using three different gastrojejunostomy anastomotic techniques in laparoscopic Roux-en-Y gastric bypass. Surg Endosc 29(7):1737–1740. doi:10.1007/s00464-014-3888-9
Bohdjalian A, Langer FB, Kranner A, Shakeri-Leidenmuhler S, Zacherl J, Prager G (2010) Circular- vs. linear-stapled gastrojejunostomy in laparoscopic Roux-en-Y gastric bypass. Obes Surg 20(4):440–446. doi:10.1007/s11695-009-9998-0
Schneider R, Gass JM, Kern B, Peters T, Slawik M, Gebhart M, Peterli R (2016) Linear compared to circular stapler anastomosis in laparoscopic Roux-en-Y gastric bypass leads to comparable weight loss with fewer complications: a matched pair study. Langenbeck’s Arch Surg/Deutsche Gesellschaft fur Chirurgie 401(3):307–313. doi:10.1007/s00423-016-1397-0
Bueter M, Lowenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T, Lutz T, le Roux CW (2010) Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg 20(5):616–622. doi:10.1007/s11695-010-0075-5
le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR (2006) Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243(1):108–114
Corteville C, Fassnacht M, Bueter M (2014) Surgery as pluripotent instrument for metabolic disease. What are the mechanisms? Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen 85(11):963–968. doi:10.1007/s00104-014-2796-9
Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, Lonroth H, Fandriks L, Olbers T (2012) Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes 36(3):348–355. doi:10.1038/ijo.2011.217
Author information
Authors and Affiliations
Contributions
Study conception and design: Marc Schiesser; acquisition of data: Marc Schiesser and Thomas Frick; analysis and interpretation of data: Marc Schiesser and Larissa Vines; Drafting of manuscript: Larissa Vines; and critical revision of manuscript: Marc Schiesser, Stefan Aczel, Dagmar L’Allemand, and Jan Borovicka.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study were in accordance with the ethical standard of our institution and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Vines, L., Frick, T., Aczél, S. et al. Linear stapled gastrojejunostomy results in fewer strictures compared to circular stapled gastrojejunostomy in laparoscopic gastric bypass surgery. Langenbecks Arch Surg 402, 911–916 (2017). https://doi.org/10.1007/s00423-017-1598-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-017-1598-1