Abstract
Background
Robotic surgery has the potential to broaden the indications for minimally invasive liver surgery owing to its technical advantages. This paper compares our experience with robotic liver surgery (RLS) with conventional laparoscopic liver surgery (LLS).
Methods
All consecutive liver resections between October 2011 and October 2022 were selected from our prospective database to be included in this cohort study. Patients who underwent RLS were compared with a LLS group for operative and postoperative outcomes.
Results
In total, 629 patients were selected from our database, including 177 patients who underwent a RLS and 452 patients who had LLS. Colorectal liver metastasis was the main indication for surgery in both groups. With the introduction of RLS, the percentage of open resections decreased significantly (32.6% from 2011 to 2020 vs. 11.5% from 2020 onward, P < 0.001). In the robotic group, redo liver surgery was more frequent (24.3% vs. 16.8%, P = 0.031) and the Southampton difficulty score was higher (4 [IQR 4 to 7] vs. 4 [IQR 3 to 6], P = 0.02). Median blood loss was lower (30 vs. 100 ml, P < 0.001), and postoperative length of stay (LOS) was shorter in the robotic group (median 3 vs. 4 days, P < 0.001). There was no significant difference in postoperative complications. Cost related to the used instruments and LOS was significantly lower in the RLS group (median €1483 vs. €1796, P < 0.001 and €1218 vs. €1624, P < 0.001, respectively), while cost related to operative time was higher (median €2755 vs. €2470, P < 0.001).
Conclusions
RLS may allow for a higher percentage of liver resections to be completed in a minimally invasive way with lower blood loss and a shorter LOS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Robot-assisted surgery has found its way into a multitude of surgical specialties [1,2,3], and over the past decade, there has been a steady increase in robotic liver surgery (RLS) [4]. However, there remains a paucity of evidence comparing robotic-assisted and laparoscopic resections [5, 6]. Therefore, some criticism is warranted given the observed increases in procedural time and cost with these procedures [7,8,9]. Well-known advantages of the robotic platform such as 3D visualization and its seven degrees of freedom may provide a benefit over laparoscopic surgery in technically more demanding resections [10, 11]. This is why RLS has the potential to broaden the applications of minimally invasive surgery. In order to identify those patients who would benefit most from a robotic approach, more data are needed. In this paper, we report our experience with RLS in comparison with conventional laparoscopic liver surgery (LLS).
Methods
Study population and design
LLS was first introduced in our center in October 2011, and RLS has been performed since February 2020. All patients undergoing liver surgery are included in a prospectively maintained electronic database regarding patient demographics, indication for surgery, intraoperative details and postoperative course. For the current analysis, we included all minimally invasive liver resections from 2011 to October 2022, aiming to compare robotic-assisted resections with conventional laparoscopic procedures. Additionally, the percentage of open resections before and after the introduction of robotics was compared. Patients who underwent ablation procedures, cyst fenestrations or a biopsy without an additional resection, were not included in the current analysis (Fig. 1). This study received approval from our institutional ethics committee (B3962022000047).
The indication for surgery was discussed by a multidisciplinary team for all cases, and all surgeries were performed by a single surgeon (MD). All patients underwent preoperative evaluation with computed tomography imaging and magnetic resonance imaging with cholangiopancreatography was performed in specific cases to define biliary anatomy.
Surgical technique—conventional laparoscopy
Our technique for both laparoscopic and robotic-assisted resections has been described in detail elsewhere [12]. Ultrasound is performed intraoperatively in all cases. This allows us to assess the exact location and extent of the lesion, as well as its relation to nearby vascular and biliary structures. Ultrasound is repeated during the procedure to assure adequate resection margins. Transection of the parenchyma is typically performed using a sealing device. An ultrasonic cavitation device (CUSA®, Integra LifeSciences, Plainsboro, NJ, USA) is used for lesions located near major vascular or biliary structures, or those where deep parenchymal dissection is necessary [13]. For extensive resections, an intermittent Pringle maneuver is applied. When arterial or venous branches require division, they are clipped and divided using a sealing device. Larger branches that cannot be clipped are divided using a linear vascular stapler (Endo-GIA, Covidien, Mansfield, MA, USA).
Surgical technique–robotic hepatectomy
All robotic procedures are undertaken using the Da Vinci Xi Surgical System® (Intuitive Surgical, Mountain View, CA, USA). Patients are placed in supine position with legs parted and a laparoscopic assistant standing between the legs. Port placement is described in Fig. 2. One or two additional 12-mm assistant trocars are used for blood aspiration and bringing in items such as suturing material and hemostatic aids. The robot is then docked, coming from the patients right side.
Technical aspects of robotic liver resections. a Port placement: robotic port 1: right midclavicular line = 8 mm or 12 mm robotic port (used for robotic stapling), robotic port 2: umbilicus (camera), robotic port 3: left midclavicular line, robotic port 4: left anterior axillary line, 1 or 2 assistant ports: suprapubic region. b Patient installation. Patients are placed in french position with 12° anti-Trendelenburg. A laparoscopic assistant is sitting between the legs. The robot is docked, coming from the patients right side. c Pringle maneuver is performed using a Foley catheter (Huang loop). d Subsegmentectomy for colorectal liver metastasis located in ventral part of Segment VIII (S8vent). Anatomical resection of S8vent was performed. A tributary of the middle hepatic vein between the ventral and dorsal part of segment 8 is being clipped. Note that by stretching the specimen upward, the portal pedicle that was dissected using Kelly–crush technique with SynchroSeal device can be seen (arrow) and is prepared for clipping. e Important for anatomical resections of segment VII or right hemihepatectomy is full mobilization of the right hemiliver. A gauze can be used for pulling the liver downward and exposing the hepatocaval confluence or upward for exposing the posterior leaf of the right coronary ligament and retrohepatic vena cava. Full mobilization allows anticlockwise rotation of segment VII and bringing it in the position of segment VI. f Right hemihepatectomy: hilar dissection exposing the right portal vein. Preparing hanging maneuver and parenchymal transection
Intraoperative ultrasound is performed in all cases, as it is in laparoscopic procedures. Liver parenchymal dissection is done using the Kelly clamp–crush technique with robotic Maryland, robotic vessel sealer or SynchroSeal device. Total pedicular clamping is used during parenchymal transection in complex cases to reduce blood loss. This is performed by applying a Foley catheter around the hepatoduodenal ligament and tightening it using a robotic bulldog clamp (Fig. 2c) [14]. Smaller blood vessels or pedicles are ligated or clipped and divided. Large vessels or pedicles are divided using a robotic stapling device. When an anatomical resection of segment 7 or a right hemihepatectomy is performed, a full mobilization of the right hemiliver is required. A gauze is used to pull the liver downward to expose the hepatocaval confluence or upward for exposing the posterior leaf of the right coronary ligament and retrohepatic vena cava (Fig. 2e).
Operative outcome and follow-up
Minor liver resections were defined as those requiring the removal of less than 3 Couinaud segments. Anatomically major resections comprised the resection of 3 or more segments, and technically major resections were those of segments 1, 4a, 7 or 8. Operative time included the time needed for preparing and docking of the robotic system. Blood loss was measured by recording aspirated fluid and subtracting the fluids used for irrigation. Postoperative complications were classified using the Clavien–Dindo classification [15] and are reported as low- (grade I and II) or high-grade (grade III and IV) complications. After discharge, patients were followed in our outpatient clinic at 1 month after surgery and on indication thereafter.
A cost analysis was performed using data obtained from our hospitals financial and pharmacological departments, and instrument-related costs were derived from our database. We obtained separate data of cost related to hospital stay, operative time and the used instruments during the procedure. The cost of purchase and maintenance of the robotic system was not included in these data as these are shared between multiple surgical disciplines within our institution. Similarly, the purchase of laparoscopic tower components and cameras was not included.
Statistical analysis
Data are described using mean and standard deviation (SD) in case of normally distributed data, and with median and interquartile range (IQR) when data are not normally distributed. Comparison of continuous variables between groups was performed with unpaired Student’s T-test when data were normally distributed and Mann–Whitney U test when data were not normally distributed. Association between categorical variables was evaluated with the (exact) Chi-square test. Differences with a P value < 0.05 were considered statistically significant. All analyses were performed with Statistica 13 (StatSoft Inc., Tulsa, OK, USA).
Results
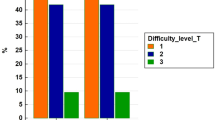
During the study period, a total of 629 patients underwent a minimally invasive hepatectomy. This included 452 patients who underwent a conventional laparoscopic procedure and 177 patients who underwent a robotic procedure. In this period, 220 other patients underwent an open partial hepatectomy. Since the introduction of robotic surgery in our center, 11.5% of patients (31 out of 270) undergoing liver surgery had an open resection versus 32.6% (189 out of 579) in the era of conventional laparoscopy (P < 0.001) (Fig. 3).
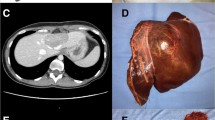
Robotic liver resections which were not performed minimally invasive before the introduction of robotic liver surgery in our center. a Robotic left hemihepatectomy with lymphadenectomy (Groups 1 and 2 lymph nodes: stations 7, 8, 9 and 12). Indication: large intrahepatic cholangiocarcinoma. b Robotic anatomical segment 3 resection—repeat hepatectomy after 3 open liver resections in a patient with large ventral hernia. Indication: colorectal liver metastasis. c Robotic-extended right hemihepatectomy with preservation of segment 4A, lymphadenectomy, wedge resection of stomach and hepaticojejunostomy (reimplantation bile duct of segment 4A and left lobe). Indication: gallbladder carcinoma invasion right hepatic artery, bile duct and invasion right anterior sectoral portal vein. d Left hemihepatectomy with resection of segment 1, extrahepatic bile duct, lymphadenectomy and hepaticojejunostomy (double barrel anastomosis). Indication: perihilar cholangiocarcinoma Bismuth–Corlette type IV
Baseline and operative characteristics
Baseline characteristics were well distributed between both groups (Table 1). Colorectal liver metastasis was the most common indication for the procedure in both groups. Benign lesions accounted for 11.8% of indications in the robotic group and 11.3% in the laparoscopic group (P = 0.84). The amount of minor and major resections was comparable. The classification of procedures according to the Brisbane classification is reported in Table 2. The number of patients who underwent redo liver surgery was higher in the robotic group (24.3% vs. 16.8%, P = 0.031), and the Southampton difficulty score[16] was also higher for robotic resections (4 [IQR 4 to 7] vs. 4 [IQR 3 to 6], P = 0.02). The robotic group included 9 patients undergoing a combined liver and bile duct resection with biliary reconstruction.
A Pringle occlusion was more often used during RLS (Table 3) and was applied for a longer period of time (median 30 min versus 23 min, P < 0.001). Median blood loss was lower in the robotic group (30 mL [IQR 10 to 90 mL] vs. 100 mL [IQR 50 to 250 mL], P < 0.001) with similar rates of blood transfusion. Due to the different surgical techniques, staplers were used significantly less frequently in the robotic group. Operative time was slightly longer for robotic procedures with a median of 145 min (IQR 118 to 190 min) as compared to 130 min (IQR 95 to 175 min) for laparoscopic procedures (P < 0.001).
Postoperative outcome and cost analysis
Median postoperative LOS was 3 days after RLS (IQR 1 to 3 days) and 4 days after LLS (IQR 3 to 6 days) (P < 0.001). Postoperative complications and readmissions were comparable between groups (Table 3). A 90-day postoperative mortality was not significantly different with 3 deaths in the robotic-assisted group and 5 in the laparoscopic group (1.7% vs. 1.1%, respectively, P = 0.69).
A robotic approach was associated with a significant reduction in cost related to hospital stay (median €1218 vs. €1624, P < 0.001) and used instruments (median €1483 vs. €1796, P < 0.001). The minimal difference in operative time did confer a higher cost to RLS (median €2755 vs. €2470, P < 0.001). Total cost was lower for RLS with €5361 as compared to €6270 for LLS (P < 0.001).
Discussion
Early reports comparing RLS to LLS showed similar postoperative morbidity and R1 resection rate but greater blood loss and operative time in the robotic groups [17]. Since these early reports, surgical techniques have evolved and experience with RLS has increased substantially. In the current study, we report our single-center experience of RLS in comparison with conventional LLS.
Overall, we found similar perioperative outcomes for both groups. There were no significant differences in low- or high-grade complications. There was no difference in the occurrence of postoperative bile leak although the CUSA device was not used in the robotic cohort. These findings are supported by the recent meta-analysis by Hu et al. [8] where there were no significant differences in postoperative complications. Their analysis of conversion rates showed a significant difference with 4.3% for robotic procedures and 11.6% for laparoscopic procedures. Our study did not find a significant difference. However, our overall conversion rates were low with 1.1% and 2.4%, respectively. A recent meta-analysis of 2728 resections [6] found no significant difference in the occurrence of complications after RLS or LLS. When considering only major liver resections, there was a slight but significant advantage for the robotic group. LOS was also shorter after major resections performed robotically.
Operative blood loss remains an important consideration in minimally invasive liver surgery. Recent meta-analyses have demonstrated increases in blood loss during RLS compared to LLS [6, 18, 19]. In order to reduce operative blood loss, adaptations of the robotic approach have been proposed to include the laparoscopic CUSA for parenchymal division [20]. While this technique has proved effective, it comes with a higher procedural cost and it requires an additional skilled practitioner to operate the instrument. Our analysis found lower operative blood loss in the robotic group without making use of an ultrasonic cavitation device. We did however apply the Pringle maneuver more often in robotic resections and for a longer period of time.
Longer operative time is considered to be a drawback of robotic procedures. This is due the time needed to prepare and dock the robot, as well as for changing instruments during the procedure [7, 8]. Our series also found longer operative times for robotic procedures with a median of 145 min as compared to 130 min for laparoscopic procedures. While statistically significant, this difference of 15 min does not seem to be clinically relevant. The more complex procedures in the robotic group might also partially explain the observed increase in operative time.
With the start of a robotic program in our institution, we noted a significant decrease in open liver procedures. We believe this is due to technically complex procedures that would have been performed in an open fashion in the laparoscopic era that can now be tackled by a robotic approach (Fig. 3). This view is supported by the observation that our robotic group consisted of more redo surgeries that had a significantly higher Southampton difficulty index. To illustrate this, our robotic group consisted of 9 patients undergoing a combined liver and bile duct resection with biliary reconstruction. No such resections were performed minimally invasive at our center before the introduction of robotic surgery. Previous studies have come to the same conclusion that robotic surgery allowed for a larger percentage of liver resections to be completed in a minimally invasive manner [21, 22]. This finding underlines the importance of robotic-assisted surgery in expanding patient selection for minimally invasive surgery.
As mentioned, a robotic approach may be of particular advantage for technically demanding resections. A recent analysis by Cipriani et al. [10] found an advantage for RLS for highly difficult resections with lower conversion rates, less blood loss and less blood transfusions while maintaining oncological radicality. Postoperative outcomes in this study were similar between both groups. Similarly, procedures on the posterosuperior segments might benefit from a robotic approach as the rib cage and the lateral position of the lesion preclude the free movement of instruments and manipulation of the parenchyma during open or laparoscopic surgery [23]. Labadie et al. [24] performed an analysis of 225 cases and found that the robotic platform aided in applying a parenchymal-sparing technique for the posterosuperior segments while keeping a minimally invasive approach. Further efforts should be made to identify the patient population that benefits the most from a robotic-assisted approach to liver surgery.
The increased cost remains a disadvantage of RLS. This is due to the costs of the robotic system itself, as well as the cost of maintenance and disposables [7, 9, 25]. Previous studies have found robotic resections to be cost-effective as compared to open liver resections [26,27,28]. Despite increased costs in the operating theater, there was a decrease in costs related to the shorter hospitalization, shorter ICU stay and fewer postoperative complications. This is most likely the result of the minimally invasive approach rather than the robotic approach as these observations have also been made for laparoscopic surgery [29]. Previous studies in bariatric surgery have shown a robotic-assisted technique to be cost-effective compared to a laparoscopic approach as anastomotic complications were significantly reduced. Also, a significant reduction in the use of staplers and LOS reduced the cost associated with robotic gastric bypass procedures [30]. Our cost analysis demonstrates a significant reduction related to the used instruments during robotic procedures. This is explained by a reduction in the use of stapler devices as well as our surgical technique that did not include the use of the CUSA as opposed to the laparoscopic group. It should be noted that the purchase and maintenance of the Da Vinci Surgical System were not included in this calculation as these costs are shared between multiple departments at our institution. In addition, the shorter LOS after robotic procedures in our study also did confer a significant cost benefit as compared to laparoscopic resections. However, this advantage has not been confirmed in larger meta-analyses [18, 31]. Operative time during robotic procedures was significantly increased and also came with an increase in cost. A recent propensity-score-matched analysis between laparoscopic and robotic-assisted liver resections found robotic surgery to be significantly more expensive with a cost increase in 21.3% [32]. The investigators found significantly higher costs related to blood transfusions, longer operative time and longer LOS in the robotic group. Another propensity-score-matched study by Aziz et al. [33] found a robotic approach to be more cost-effective as compared to both open and laparoscopic liver resections. This cost benefit was mainly attributed to a lower rate of readmissions. Our study did not find a significant difference in readmissions.
This study comes with some limitations. First, as this is an observational study, it is susceptible to selection bias and one should be cautious in drawing conclusions. Second, we report on operative and perioperative outcomes. To draw firm conclusions on the role of a robotic platform, long-term data and oncological outcomes would be desirable. On the other hand, as many of the potential drawbacks of RLS such as cost and operative time reside in the perioperative period, we do believe these data are of interest in defining the role of RLS.
In conclusion, this single-center analysis shows that RLS may allow for a higher percentage of liver resections to be completed in a minimally invasive way. This approach came with a reduction in operative blood loss and LOS at the cost of slightly longer operative times.
References
Crocerossa F, Carbonara U, Cantiello F et al (2021) Robot-assisted radical nephrectomy: a systematic review and meta-analysis of comparative studies. Eur Urol 80:428–439
Roth AE, Thornley CJ, Blackstone RP (2020) Outcomes in bariatric and metabolic surgery: an updated 5-year review. Curr Obes Rep 9:380–389
Solaini L, Bocchino A, Avanzolini A et al (2022) Robotic versus laparoscopic left colectomy: a systematic review and meta-analysis. Int J Colorectal Dis 37:1497–1507
Görgec B, Zwart M, Nota CL et al (2022) Implementation and Outcome of robotic liver surgery in The Netherlands: a nationwide analysis. Ann Surg 277:e1269
Muaddi H, El Hafid M, Choi WJ et al (2021) Clinical outcomes of robotic surgery compared to conventional surgical approaches (laparoscopic or open): a systematic overview of reviews. Ann Surg 273:467–473
Ciria R, Berardi G, Alconchel F et al (2022) The impact of robotics in liver surgery: a worldwide systematic review and short-term outcomes meta-analysis on 2,728 cases. J Hepatobiliary Pancreat Sci 29:181–197
Kim JK, Park JS, Han DH et al (2016) Robotic versus laparoscopic left lateral sectionectomy of liver. Surg Endosc 30:4756–4764
Hu Y, Guo K, Xu J et al (2021) Robotic versus laparoscopic hepatectomy for malignancy: a systematic review and meta-analysis. Asian J Surg [Internet] 44:615–628
Packiam V, Barlett DL, Tohme S et al (2012) Minimally-invasive liver resection: robotic versus laparoscopic left lateral sectionectomy. J Gastrointest Surg 16:2233–2238
Cipriani F, Fiorentini G, Magistri P et al (2021) Pure laparoscopic versus robotic liver resections: multicentric propensity score-based analysis with stratification according to difficulty scores. J Hepatobiliary Pancreat Sci 23:1–16
Casciola L, Patriti A, Ceccarelli G et al (2011) Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc 25:3815–3824
D’Hondt M, Devooght A, Willems E et al (2022) Transition from laparoscopic to robotic liver surgery: clinical outcomes, learning curve effect, and cost-effectiveness. J Robot Surg. https://doi.org/10.1007/s11701-022-01405-w
D’Hondt M, Willems E, Parmentier I et al (2020) Laparoscopic liver resection for liver tumours in proximity to major vasculature: a single-center comparative study. Eur J Surg Oncol [Internet] 46:539–547
Huang JW, Su WL, Wang SN (2018) Alternative laparoscopic intracorporeal pringle maneuver by huang’s Loop. World J Surg 42:3312–3315. https://doi.org/10.1007/s00268-018-4584-z
Clavien PA, Sanabria JR, Strasberg SM (1992) Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 111:518–526
Halls MC, Berardi G, Cipriani F et al (2018) Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg 105:1182–1191
Montalti R, Berardi G, Patriti A et al (2015) Outcomes of robotic vs. laparoscopic hepatectomy: a systematic review and meta-analysis. World J Gastroenterol 21:8441–8451
Hu L, Yao L, Li X et al (2018) Effectiveness and safety of robotic-assisted versus laparoscopic hepatectomy for liver neoplasms: a meta-analysis of retrospective studies. Asian J Surg 41:401–416
Zhao Z, Yin Z, Li M et al (2021) State of the art in robotic liver surgery: a meta-analysis. Updates Surg 73:977–987
Hawksworth J, Radkani P, Nguyen B et al (2022) Improving safety of robotic major hepatectomy with extrahepatic inflow control and laparoscopic CUSA parenchymal transection: technical description and initial experience. Surg Endosc [Internet] 36:3270–3276
Tsung A, Geller DA, Sukato DC et al (2014) Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 259:549–555
Wu Y-M, Hu R-H, Lai H-S et al (2014) Robotic-assisted minimally invasive liver resection. Asian J Surg 37:53–57
Efanov M, Salimgereeva D, Alikhanov R et al (2023) Comparison between the difficulty of laparoscopic limited liver resections of tumors located in segment 7 versus segment 8: an international multicenter propensity-score matched study. J Hepato-Biliary-Pancreat Sci 30(2):177–191
Labadie KP, Droullard DJ, Lois AW et al (2022) IWATE criteria are associated with perioperative outcomes in robotic hepatectomy: a retrospective review of 225 resections. Surg Endosc 36:889–895
Yu Y-D, Kim K-H, Jung D-H et al (2014) Robotic versus laparoscopic liver resection: a comparative study from a single center. Langenbeck’s Arch Surg 399:1039–1045
Stewart C, Wong P, Warner S et al (2021) Robotic minor hepatectomy: optimizing outcomes and cost of care. HPB 23:700–706
Daskalaki D, Gonzalez-Heredia R, Brown M et al (2017) Financial impact of the robotic approach in liver surgery: a comparative study of clinical outcomes and costs between the robotic and open technique in a single institution. J Laparoendosc Adv Surg Tech 27:375–382
Cortolillo N, Patel C, Parreco J et al (2019) Nationwide outcomes and costs of laparoscopic and robotic vs. open hepatectomy. J Robot Surg 13:557–565
Jackson NR, Hauch A, Tian H, Buell JF, Slakey DP, Kandil E (2015) The safety and efficacy of approaches to liver resection: a meta-analysis. J Soc Laparoendosc Surg 19(1):e2014.00186. https://doi.org/10.4293/JSLS.2014.00186
Hagen ME, Pugin F, Chassot G et al (2012) Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg 22:52–61
Ziogas IA, Giannis D, Esagian SM et al (2021) Laparoscopic versus robotic major hepatectomy: a systematic review and meta-analysis. Surg Endosc 35:524–535
Miller HP, Hakim A, Kellish A et al (2022) Cost-benefit analysis of robotic vs. laparoscopic hepatectomy: a propensity-matched retrospective cohort study of american college of surgeons national surgical quality improvement program database. Am Surg 88:2886–2892
Aziz H, Hanna K, Lashkari N et al (2022) Hospitalization costs and outcomes of open, laparoscopic, and robotic liver resections. Am Surg 88:2331–2337
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have a conflict of interest.
Ethical approval
All authors comply with the journal’s ethical policies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Winckelmans, T., Wicherts, D.A., Parmentier, I. et al. Robotic Versus Laparoscopic Hepatectomy: A Single Surgeon Experience of 629 Consecutive Minimally Invasive Liver Resections. World J Surg 47, 2241–2249 (2023). https://doi.org/10.1007/s00268-023-07060-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-023-07060-y