Abstract
Purpose
According to current treatment guidelines, surgical resection of hepatocellular carcinoma (HCC) is mostly restricted to a limited subgroup of patients. Due to improved surgical techniques and perioperative management, liver resections may also be performed more extendedly and also in cirrhotic livers with clinical signs of portal hypertension in selected patients. In this study, the clinical and long-term outcomes of liver resection in HCC patients with or without liver cirrhosis were evaluated.
Methods
One hundred fifty-eight patients undergoing liver resection for primary HCC at our institution were identified. Logistic and Cox regression analyses were used to identify prognostic parameters for postoperative complications and survival.
Results
In our cohort of patients, there was no association between clinical parameters or extent of surgical resection and postoperative morbidity. Only Barcelona Clinic Liver Cancer (BCLC) stage C patients were at significantly higher risk for major complications (OR 5.27, P = 0.009). Risk factors influencing long-term survival were patient age (HR 1.026, P = 0.027) and BCLC stage C (HR 3.47, P = 0.002). Compared to patients without liver cirrhosis, BCLC stage A and B patients undergoing resection were at similar risk for the development of severe complications and long-term mortality.
Conclusion
Liver resection as potentially curative therapy can be performed in selected patients in BCLC stage B, as well as in patients with clinical signs of portal hypertension. The resection of HCC-classified BCLC stage C is feasible but associated with significant morbidity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current guidelines for the management of hepatocellular carcinoma (HCC) include a variety of treatment modalities integrating different medical specialties [1–3]. One of the most accepted treatment algorithms for HCC patients with liver cirrhosis is the Barcelona Clinic Liver Cancer (BCLC) guideline [4]. Here, surgical resection is the suggested therapy for single cancer lesions smaller than 5 cm or up to 3 nodules smaller than 3 cm in diameter. In patients with small tumors but diminished liver reserve, liver transplantation is the treatment of choice, whereas patients with more advanced HCC are recommended to undergo non-surgical therapies without curative intent. Although the BCLC staging system is innovative and includes several aspects of HCC biology and underlying liver disease, its general application remains a matter of ongoing discussion, especially in the case of potentially resectable disease [5].
The restriction of surgical resection to a small subgroup of patients as suggested by the BCLC classification can be disputed for several reasons. First, the safety of extended liver surgery has markedly improved over the last years, making liver resection a technically feasible option even for patients with cirrhosis or large tumor lesions [6, 7]. Second, liver transplantation is a non-projectable therapy option comprising the risk of cancer progress while waiting for a suitable organ [8, 9]. And finally, the sensitivity and specificity of current diagnostic means are both limited [10], leading to erroneous classifications with serious consequences on therapeutic goals (“curative” versus “palliative”).
Thus, surgery might be an appropriate therapy option for a greater group of patients than currently postulated by the BCLC criteria [11], especially in patients classified BCLC stage B [12, 13]. To evaluate this hypothesis, we retrospectively analyzed the postoperative course of patients undergoing liver resection for HCC according to their clinical cirrhosis state and BCLC stage. We further set out to identify factors associated with increased morbidity and mortality, to eventually reveal key factors influencing either of these.
Taken together, we show that postoperative morbidity is acceptable in patients with BCLC A and B and comparable to the complication rates of patients without histological cirrhosis. Clinical signs of portal hypertension are poor predictors of both postoperative complications and survival in our cohort of patients. By that, we postulate that liver resection for intermediate and advanced HCC can be offered to a well-selected patient population in specialized centers. Therapy decisions should always be guided by an expert panel involving hepatologists, radiologists, and surgeons experienced in hepatobiliary procedures and liver transplantation.
Patients and methods
In this retrospective cohort study, 190 adult patients with HCC undergoing hepatic resection between February 1997 and May 2011 were identified in our university hospital’s database. Of these, patients with recurrent HCC (n = 8), fibrolamellar HCC (n = 7), simultaneous additional malignancies (n = 7), mixed HCC/cholangiocellular carcinoma (CCC; n = 4), and patients with ruptured HCC (n = 3) were excluded, as well as patients with whom the BCLC stage could not be defined (n = 3). In total, 158 patients with primary resection of histological confirmed HCC were included in our analysis. The study was approved by the local ethics committee (Ethikkommission an der Universität Regensburg, No. 14-101-0088).
All therapeutic decisions, especially whether or not to perform surgery, were made by the hospital’s expert oncology board, including hepatologists and hepatobiliary surgeons. Treatment decisions dissenting from existing algorithms (BCLC classification) were based on individual features of each patient’s history, type of disease, and therapy intention.
The definition of “portal hypertension” was based on the presence of either esophageal varices or a low platelet count (less than 100,000 per microliter) with splenomegaly, as applied by the European Association for the Study of the Liver (“surrogates of portal hypertension”) [14]. As the portal vein pressure gradient is not measured routinely in our center, it was not included in our analysis.
Stratification according to the BCLC system was based on laboratory tests and imaging before surgery, as well as histopathological examination post surgery. The existence of cirrhosis was also based on the analysis by a certified pathologist.
Postoperative complications were recorded according to the classification by Clavien and Dindo [15]. Data were extracted from the hospital’s computer archive and patient charts. Patients were followed up by the University Hospital outpatient clinic or elsewhere. In the latter case, regular status reports were sent to our institution.
Statistics
All statistical analyses were performed under the supervision of a professional biostatistician (FZ) using SPSS software (IBM, version 21) and R software (version 3). Continuous data are expressed as mean (standard deviation (SD)) or, if clinically more relevant, as median (interquartile range (IQR)). Categorical data is stated as frequency counts (percentages). Complications were assessed using the classification system developed by Clavien and Dindo [15] and normalized to number of events per 100 patients (except for mortality, which is given in percent of total cases). Uni- and multivariable logistic regression analyses were performed to correlate BCLC stage, parameters of liver function, surrogate parameters of clinically relevant portal hypertension and extent of surgery with major postoperative complications, defined as °III to °V (death) complications. The same variables and patient age were included in a multivariable Cox proportional hazards regression model. Odds ratios (OR), hazard ratios (HR), and corresponding 95 % confidence intervals (CI) were calculated. A two-sided P value of ≤0.05 was considered to indicate statistical significance. Absolute patient numbers are given if variables could not be retrieved for every individual patient retrospectively.
Results
Patient characteristics
One hundred fifty-eight patients were included into this analysis and were classified as stage A (n = 43), stage B (n = 46), or stage C (n = 14), according to the BCLC staging system. Fifty-five patients did not show any sign of cirrhosis in the histological examination by expert pathologists. The mean age of the whole study population was 63.3 years (SD 10.9), with a total of 132/158 (83.5 %) being male. The majority of patients with cirrhotic livers had chronic liver diseases, mostly due to alcohol abuse or chronic hepatitis C. In non-cirrhotic patients, a chronic liver disease was known in 13/55 (23.6 %) cases.
At least one surrogate parameter of clinically relevant portal hypertension (defined as either esophageal varices or low platelet count associated with splenomegaly) was reported in 11/43 (25.6 %) BCLC A, 9/46 (19.6 %) BCLC B, 3/14 (21.4 %) BCLC C, and in no patient without cirrhosis. Laboratory features of disturbed liver function, as elevated bilirubin levels or prolonged INR, could be found in all groups. The prevalence of ascites was low in the non-cirrhosis group (1/55 patients, 1.8 %) and rose with BCLC stage, 3/43 (7.0 %) in BCLC A, 6/46 (13.0 %) in BCLC B, and 3/14 (21.4 %) in BCLC C. A detailed overview is shown in Table 1.
Tumor and surgery characteristics
Tumor characteristics were specified based on the postoperative histological examination (Table 2). HCCs were predominantly singular lesions in BCLC stage A (38/43, 88.4 %) and in patients without cirrhosis (35/55, 63.6 %), whereas the majority of patients displayed more than one tumor nodule in BCLC stage B (26/46, 56.5 %) and C (8/14, 57.1 %). The mean maximum diameter of the largest lesion increased with BCLC stage from 2.7 cm in BCLC stage A to 7.9 cm in BCLC B and C, and 9.7 cm in patients without cirrhosis. While patients with microscopic vascular invasion could be identified in all subgroups, macroscopic vascular invasion was (in conformity with the BCLC system) only observed in BCLC stage C and in patients without cirrhosis. Elevated AFP levels were found in 21/43 (48.8 %) BCLC A, 16/44 (36.4 %) BCLC B, 3/14 (21.4 %) BCLC C, and 27/54 (50.0 %) non-cirrhotic patients. The extent of surgery was increasing with BCLC stage, together with the mean duration of surgery, ICU, and hospital stay. All cancer and surgery details are listed in Table 2.
Postoperative morbidity
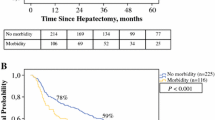
We next analyzed the incidence of postoperative morbidity in relation to BCLC stage, surrogate parameters of portal hypertension (esophageal varices or low platelet count with splenomegaly), and type of surgery. Complications were classified as published by Clavien and Dindo [15]. The number of events for each complication normalized to 100 patients is shown in Table 3. While the number of severe complications (°III to °V) was increasing with BCLC stage, there was no trend in the frequency of complications in patients with surrogates of portal hypertension or extended surgery. Accordingly, neither surrogate parameters for portal hypertension nor the type of surgery was associated with a significantly increased risk for severe complications (Clavien-Dindo °III to °V) in a univariable logistic regression analysis (Table 4). Compared to patients resected without cirrhosis, patients classified BCLC A had no significantly increased risk for severe postoperative morbidity (HR 1.27; 95 % CI 0.52–3.08; P = 0.600). While patients classified BCLC B showed a trend towards an increased risk without reaching statistical significance (HR 2.25; 95 % CI 0.97–5.22; P = 0.059), patients in BCLC stage C had a significantly increased risk for developing complications after surgery (HR 5.27; 95 % CI 1.51–18.4; P = 0.009).
Long-term survival following hepatic resection
Survival analysis was performed using the Kaplan-Meier model (Figure 1) and Cox regression (Table 5). The median follow-up was 2.01 years. The median survival was 5.73 (95 % CI 3.20–8.26) years in the BCLC A group, compared to 3.03 (95 % CI 0.82–5.24) years in BCLC B, 0.73 (95 % CI 0.26–1.19) years in BCLC C, and 3.38 (95 % CI 1.15–5.60) years in patients without histopathological cirrhosis. Survival in BCLC C patients was significantly worse compared to all other groups (P < 0.028; Fig. 1a). The stratification of patients according to the Milan criteria did not result in statistically different survival (P = 0.065; Figure 1b). The analysis of patients with singular versus more than one HCC lesion showed a significant survival benefit for patients with singular lesions (median survival 4.90 years (95 % CI 1.76–8.04) versus 1.93 years (95 % CI 1.28–2.56), P = 0.002; Fig. 1c). Vascular invasion was associated with worse survival, with macroscopic vascular invasion being the worst predictor of overall survival (P < 0.001). Patients without vascular invasion had a median survival of 7.74 years (95 % CI 4.39-11.09), compared to 2.12 years (95 % CI 0.51–3.73) in patients with microvascular or 0.76 years (95 % CI 0.60–0.93) in patients with macrovascular disease (Fig. 1d).
Survival analysis using the Kaplan-Meier plot. a Survival according to BCLC stage. Patients classified BCLC C showed a significantly decreased probability of survival (P values according to the univariable Cox regression analysis compared to BCLC C, 0.002 (BCLC A), 0.28 (BCLC B), 0.11 (no cirrhosis)). b When stratified by Milan criteria, the probability of survival for patients outside Milan was worse (without reaching statistical significance, P = 0.062). c, d The presence of a singular or more than one tumor lesion (c), or vascular invasion (d) was significantly associated with worse survival (P = 0.002 and P < 0.01, respectively)
Applying a multivariable Cox regression analysis, the parameters age (HR 1.026; 95 % CI 1.003–1.050; P = 0.027) and BCLC stage C (HR 3.47; 95 % CI 1.57–7.67; P = 0.002) were identified as statistically significant prognostic parameters for mortality. Interestingly, the prevalence of surrogate parameters of portal hypertension was associated with an increased risk for death in the Cox regression analysis (Tables 5 and 6).
Discussion
Surgery is the mainstay of HCC therapy with curative intent. Following the widely accepted BCLC criteria, surgical resection is frequently restricted to small carcinomas in livers with minor cirrhosis and absent portal hypertension. However, the significance of currently used surrogate parameters of portal hypertension can be disputed, especially as surgical techniques and perioperative care have dramatically improved over the last decades [16]. Apart from the extent of liver cirrhosis, tumor size and distribution are considered to determine the feasibility of surgical resection. In view of the ongoing debate on the interpretation of the aforementioned criteria and whether their limits are well chosen, we investigated their predictive value on postoperative morbidity and long-term survival.
In our cohort of patients, treatment decision was made by an expert board comprising at least one board certified hepatologist, oncologist, interventional radiologist, and a highly qualified hepatobiliary surgeon with transplant experience. Cases were retrospectively assigned to the appropriate BCLC stage involving the final histopathological report. Some cases differed from the preoperative BCLC classification due to the limitations of preoperative diagnostic modalities (data not shown), especially regarding (the well published) difficulties in correctly diagnosing small lesions in cirrhotic livers [17, 18].
Most patients resected for HCC in our specialized center belonged to BCLC stage A or B. When directly compared, these two groups did not show any significant difference in postoperative morbidity or long-term survival. This observation is in accordance with a publication by Vauthey et al., who showed that survival did not differ between patients with T1 or T2 HCC [19]. Consequently, Torzilli and colleagues found that extended indications for hepatic resection are applied throughout the world, with acceptable short- and long-term results [20]. This is in line with a consensus statement by Jarnagin et al., who consider resection as reasonable therapy in selected patients even with large or multifocal HCC [11]. This recommendation is supported by a retrospective study including 45 cirrhotic patients undergoing extended hepatectomy, resulting in up to standard morbidity and long-term survival [21]. Accordingly, a recent retrospective study by the Italian Liver Cancer group showed that liver resection could be beneficial across all BCLC stages compared to loco-regional therapy [22, 23].
While BCLC classification showed a trend towards increasing numbers of major complications from stage A to B (albeit not significant) and to C, our data also indicate that widely applied surrogate parameters of liver cirrhosis are poor predictors of morbidity and mortality following surgery. Other methods, especially the measurement of the hepatic venous pressure gradient [24], might be much more accurate, but have not been applied routinely in our cohort of patients. Associated tests to determine the residual liver function, as the LiMAx test or the indocyanine green test, have not become the standard of care [25]. On the other hand, however, the results of current studies using these tests underscore their role in the future [26, 27]. Our clinical practice is in line with the German national HCC guideline, which recommends that resectability should be defined through a joint assessment of surgeons and hepatologists [28].
Although no definitive conclusion on an oncologic benefit can be drawn from our data without a non-resection control group, both Kaplan-Meier analysis and multivariable Cox regression analysis did not show a significant difference in long-term survival comparing BCLC A to BCLC B patients. BCLC stage C, however, was associated with an increased risk for mortality together with age and extended hemihepatectomy. A limitation of our study is that no comparison with other treatment modalities was performed, as the focus was on postsurgical morbidity. Still, our data show that surgical resection represents a feasible option for this cohort of patients, as long-term survivorship can be achieved. Liu and colleagues observed similar results in patients with advanced HCC, further showing that surgical resection was more successful than transarterial chemoembolization in these patients [29]. Ultimately, randomized controlled trials are needed to clarify the impact of each therapy on survival for each disease stage.
Our study is not suitable for developing a new treatment algorithm for HCC patients. According to our institute’s experience, patients undergoing hepatic resection for HCC outside BCLC A should be in good general condition (ECOG 0–1), and open patient counseling concerning potential pros and cons is mandatory. Resection should be considered when technically and functionally feasible, or after failure of non-surgical treatment. In some cases, resection could also be considered as a palliative approach. Details are given in Table 7 and exemplified in Fig. 2a–c.
Examples for hepatic resection outside BCLC A. a 56-year-old patient requiring limited (bi-segmental) resection for HCC segment VI and VII (see MRI image). b 60-year-old patient with HCC infiltrating the right branch of the portal vein, leading to atrophy of the right liver lobe (see CT image). Only little functional liver tissue was lost by right hemihepatectomy. c 53-year-old patient status post several local ablational therapies and TACE. Diffused HCC including infiltration of the vena cava, requiring right hemihepatectomy with atypical resection of segment IV and I, plus vena cava construction (see CT image). Patient recovered well and is still alive after 1 year following surgery
In conjunction with the aforementioned observations on postoperative morbidity, hepatic resection should clearly be considered for patients classified BCLC B. Surrogate parameters to estimate portal hypertension should be interpreted with care, as specialized centers can perform even extended liver resections safely despite signs of portal hypertension. In our hands, individual patient selection by an expert panel of experienced liver specialists appears to be more reliable than existing classification systems for the prediction of safe resection in HCC patients.
References
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–1022. doi:10.1002/hep.24199
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42(5):1208–1236. doi:10.1002/hep.20933
Verslype C, Rosmorduc O, Rougier P, Group EGW (2012) Hepatocellular carcinoma: ESMO-ESDO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii41–vii48. doi:10.1093/annonc/mds225
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362(9399):1907–1917. doi:10.1016/s0140-6736(03)14964-1
Henderson JM, Sherman M, Tavill A, Abecassis M, Chejfec G, Gramlich T (2003) AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB (Oxford) 5(4):243–250. doi:10.1080/13651820310015833
Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Horbelt R, Kroemer A, Loss M, Rummele P, Scherer MN, Padberg W, Konigsrainer A, Lang H, Obed A, Schlitt HJ (2012) Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 255(3):405–414. doi:10.1097/SLA.0b013e31824856f5
Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J (2001) Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg 234(1):63–70
Earl TM, Chapman WC (2013) Hepatocellular carcinoma: resection versus transplantation. Semin Liver Dis 33(3):282–292. doi:10.1055/s-0033-1351783
Thomas MB, Zhu AX (2005) Hepatocellular carcinoma: the need for progress. J Clin Oncol 23(13):2892–2899. doi:10.1200/JCO.2005.03.196
El-Serag HB, Marrero JA, Rudolph L, Reddy KR (2008) Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 134(6):1752–1763. doi:10.1053/j.gastro.2008.02.090
Jarnagin W, Chapman WC, Curley S, D’Angelica M, Rosen C, Dixon E, Nagorney D, American Hepato-Pancreato-Biliary A, Society of Surgical O, Society for Surgery of the Alimentary T (2010) Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 12(5):302–310. doi:10.1111/j.1477-2574.2010.00182.x
Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, Brouquet A, Adams RB, American Hepato-Pancreato-Biliary A, Society of Surgical O, Society for Surgery of the Alimentary T (2010) Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 12(5):289–299. doi:10.1111/j.1477-2574.2010.00181.x
Yamakado K, Kudo M (2014) Treatment strategies of intermediate-stage hepatocellular carcinomas in Japan (Barcelona Clinic Liver Cancer stage B). Oncology 87(Suppl 1):78–81. doi:10.1159/000368149
European Association For The Study Of The L, European Organisation For R, Treatment Of C (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56(4):908–943. doi:10.1016/j.jhep.2011.12.001
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Wong TC, Lo CM (2013) Resection strategies for hepatocellular carcinoma. Semin Liver Dis 33(3):273–281. doi:10.1055/s-0033-1351782
Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, Massironi S, Della Corte C, Ronchi G, Rumi MG, Biondetti P, Colombo M (2010) The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 59(5):638–644. doi:10.1136/gut.2009.187286
Willatt JM, Hussain HK, Adusumilli S, Marrero JA (2008) MR imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology 247(2):311–330. doi:10.1148/radiol.2472061331
Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A, Cleary KR, Nagorney DM (2002) Simplified staging for hepatocellular carcinoma. J Clin Oncol 20(6):1527–1536
Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M, Morenghi E, Makuuchi M (2013) A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 257(5):929–937. doi:10.1097/SLA.0b013e31828329b8
Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J (2002) Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg 236(5):602–611. doi:10.1097/01.SLA.0000033038.38956.5E
Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, Volk M, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Cabibbo G, Felder M, Gasbarrini A, Sacco R, Foschi FG, Missale G, Morisco F, Svegliati Baroni G, Virdone R, Cillo U, Italian Liver Cancer Group (2015) Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol 62(3):617–624. doi:10.1016/j.jhep.2014.10.037
Farinati F, Vanin V, Giacomin A, Pozzan C, Cillo U, Vitale A, Di Nolfo AM, Del Poggio P, Benvegnu L, Rapaccini G, Zoli M, Borzio F, Giannini EG, Caturelli E, Trevisani F, Italian Liver Cancer Group (2015) BCLC stage B hepatocellular carcinoma and transcatheter arterial chemoembolization: a 20-year survey by the Italian Liver Cancer group. Liver Int 35(1):223–231. doi:10.1111/liv.12649
Berzigotti A, Seijo S, Reverter E, Bosch J (2013) Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol 7(2):141–155. doi:10.1586/egh.12.83
Colombo M, Raoul JL, Lencioni R, Galle PR, Zucman-Rossi J, Banares R, Seehofer D, Neuhaus P, Johnson P (2013) Multidisciplinary strategies to improve treatment outcomes in hepatocellular carcinoma: a European perspective. Eur J Gastroenterol Hepatol 25(6):639–651. doi:10.1097/MEG.0b013e32835e33bb
Stockmann M, Lock JF, Malinowski M, Niehues SM, Seehofer D, Neuhaus P (2010) The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB (Oxford) 12(2):139–146. doi:10.1111/j.1477-2574.2009.00151.x
Seyama Y, Kokudo N (2009) Assessment of liver function for safe hepatic resection. Hepatol Res 39(2):107–116. doi:10.1111/j.1872-034X.2008.00441.x
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) (2013) Diagnostik und Therapie des hepatozellulären Karzinoms, Langversion 1.0, AWMF Registrierungsnummer: 032-053OL.
Liu PH, Hsia CY, Lee YH, Hsu CY, Huang YH, Su CW, Lee RC, Lin HC, Huo TI (2015) Surgical resection versus transarterial chemoembolization for BCLC stage C hepatocellular carcinoma. J Surg Oncol 111(4):404–409. doi:10.1002/jso.23854
Author contributions
PR, JS, and SAF had full access to study data and took responsibility for the integrity and accuracy of data analysis. PR, ML, SAL, EKG, HJS, and SAF were responsible for the concept and design of the study. PR, JS, and SAF were responsible for the acquisition of data. PR, JS, AK, FZ, ML, SAL, EKG, HJS, and SAF performed the analysis and interpretation of data. PR, FZ, EKG, HJS, and SAF were responsible in the drafting of the manuscript. AK, ML, SAL, EKG, and HJS conducted the critical revision of the manuscript for important intellectual content. FZ, PR, and JS completed the statistical analysis. .EKG, HJS, SAF were responsible for the study supervision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors declare no conflict of interest related to this work. P. Renner receives funding from the German Research Society and Novartis. The retrospective analysis of our study cohort was in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of interest
PR is financially supported by Novartis and the German Research Society (DFG) (not in relation with the manuscript).
Rights and permissions
About this article
Cite this article
Renner, P., Schuhbaum, J., Kroemer, A. et al. Morbidity of hepatic resection for intermediate and advanced hepatocellular carcinoma. Langenbecks Arch Surg 401, 43–53 (2016). https://doi.org/10.1007/s00423-015-1359-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-015-1359-y