Abstract
Background and aim
The latest Barcelona Clinic Liver Cancer (BCLC) staging system suggests considering surgery in patients with resectable BCLC stage 0/A hepatocellular carcinoma (HCC) and clinically significant portal hypertension (CSPH). This study aimed to evaluate the safety and short- and long-term outcomes of laparoscopic hepatectomy for BCLC stage 0/A HCC patients with CSPH.
Methods
We retrospectively reviewed the medical records of 647 HCC patients in BCLC stage 0/A who were treated at five centers between January 2010 and January 2019. Among these patients, 434 underwent laparoscopic hepatectomy, and 213 underwent open hepatectomy. We used Kaplan–Meier analysis to compare the overall survival (OS) rate and recurrence-free survival (RFS) rate between patients with and without CSPH before and after propensity score matching (PSM). Multivariate Cox regression analysis was performed to identify prognostic factors for BCLC stage 0/A patients, and subgroup analyses were also conducted.
Results
Among the 434 patients who underwent laparoscopic hepatectomy, 186 had CSPH and 248 did not. The Kaplan–Meier analysis showed that the OS and RFS rates were significantly worse in the CSPH group before and after PSM. Multivariate Cox regression analyses identified CSPH as a prognostic factor for poor OS and RFS after laparoscopic hepatectomy. However, CSPH patients treated laparoscopically had a better short- and long-term prognosis than those treated with open surgery.
Conclusions
CSPH has a negative impact on the prognosis of BCLC stage 0/A HCC patients after laparoscopic hepatectomy. Laparoscopic hepatectomy is still recommended for treatment, but careful patient selection is essential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma has the sixth-highest global incidence of malignancies [1, 2]. Hepatectomy has long been considered one of the best treatments for HCC patients with preserved liver function. However, in previous studies, it has been controversial whether hepatic resection can be performed in patients with clinically significant portal hypertension (CSPH) [3, 4]. The 2022 BCLC recommendation concluded that the mortality for BCLC stage 0/A HCC patients with portal hypertension is increased even if non-surgical treatment is performed; therefore, surgery is considered[5].
The BCLC group has demonstrated that CSPH is indeed one of the predictive factors affecting patients’ postoperative outcomes. Choi et al. [6]. found that CSPH was significantly associated with liver function decompensation and a poor prognosis after HCC resection in patients with Child–Pugh A cirrhosis. On the other hand, Casellas-Robert et al. [7]. concluded that laparoscopic liver resection (LLR) was feasible in selected patients. However, liver-specific complications, as well as the length of stay, were significantly higher.
Since the first LLR was performed in 1991, the range of indications for LLR has gradually expanded, and even tumors in the superior and posterior positions (segments IVa, VII, VIII) can be safely resected laparoscopically [8]. LLR offers many advantages, including substantially reduced duration of hospital stay, less intraoperative trauma, and reduced postoperative morbidity. In addition, previous multicenter RCTs and other studies have demonstrated that laparoscopic surgery has a similar prognosis to open surgery for HCC patients [9,10,11,12,13]. Therefore, LLR has become routine in many centers in Asia; however, whether early-stage HCC patients with CSPH can achieve a comparable prognosis to patients without CSPH after LLR is controversial.
Previous studies on the impact of CSPH after hepatectomy for HCC considered the entire spectrum of HCC patients or those with Child–Pugh grade A disease. Currently, there is limited evidence regarding its effect on postoperative outcomes in BCLC stage 0/A HCC patients. This multicenter study aims to compare the safety and outcomes of laparoscopic hepatectomy in HCC patients with and without CSPH and provide more specific recommendations for this patient population.
Materials and methods
Patients
We retrospectively analyzed 647 hepatocellular carcinoma (HCC) patients who underwent hepatectomy at five centers between December 2010 and December 2018. Among them, 434 patients with BCLC stage 0/A HCC underwent laparoscopic hepatectomy, including 186 with clinically significant portal hypertension (CSPH) and 248 without CSPH. CSPH was defined based on gastroscopic evidence of esophageal or gastric varices or significant splenomegaly (abdominal CT showing a maximum spleen diameter ≥ 12 cm) with a preoperative platelet count below 100,000/mm3, without invasive portal venous pressure measurement [14, 15]. The postoperative pathology report included information on cirrhosis, tumor differentiation, microvascular invasion(MVI), satellite foci, and tumor resection margin. The inclusion criteria were diagnosis of HCC by two experienced pathologists, Child–Pugh A or B classification, and first-time diagnosis. Patients were excluded if they had incomplete or missed follow-up or received antitumor therapy preoperatively. This study was approved by the Ethics Committees of Wuhan Tongji Hospital, Zhongshan People’s Hospital, Huangshi Central Hospital, Shenzhen Baoan District People’s Hospital, and Shenzhen Longhua District People’s Hospital, and informed consent was obtained from all patients.
Laparoscopic surgical proceeding
All patients underwent preoperative hematology tests (complete blood count, liver function, kidney function, and coagulation) and imaging tests such as enhanced CT and MRI of the abdomen before surgery. Each HCC patient was evaluated from multiple perspectives by a team of experienced hepatic surgeons, medical oncologists, imaging physicians, and anesthesiologists. The indications for laparoscopic liver resection (LLR) were similar to those for open liver resection (OLR), and the details of each resection were determined based on the tumor's location. The final decision to operate was primarily based on the patient's liver function (Child–Pugh A/B). All patients underwent 3D liver reconstruction before surgery to analyze the location of the tumor and its anatomy regarding major vascular structures, with intraoperative ultrasonography used to modify the resection during surgery. A residual liver volume > 40% was simulated by 3D modeling. The tumor’s size or number and the presence or absence of CSPH were not absolute criteria for surgical treatment of resectable tumors.
A standardized laparoscopic hepatectomy procedure was performed by experienced liver surgery teams at all five centers. Resection was performed purely laparoscopically without manual assist devices, and the surgical approach depended on tumor characteristics.
Anatomical liver resection is the preferred method for treating large tumors, tumors located in specific areas of the liver, or patients with multiple independent tumors. Patients are placed in a supine position (for left or right hepatectomy, as well as segmental resections of segments 1, 5, and 8) or a left lateral decubitus position (for right posterior segmentectomy or segmental resections of segments 6 and 7). The incision site is located above or to the right of the navel. Layer-by-layer dissection is performed, separating and mobilizing the ligaments around the liver. The location of the lesion is confirmed, and the resection site is identified under ultrasound guidance. The Pringle maneuver is used to block hepatic blood flow temporarily. The extent of resection is determined based on the specific situation, and the tissues around the lesion are dissected. The first hepatic hilum is identified, and blood flow is blocked along the vessels. The entire branch and thrombus are removed when venous branch tumor thrombi are found. After the surgery, the incision is sutured, and a drainage tube is placed.
For patients who are not suitable for anatomical liver resection, non-anatomical liver resection is performed. The preoperative preparation, incision selection, and lesion exposure are similar to patients undergoing anatomical liver resection. Intra-abdominal pressure was controlled between 10 and 14 mm Hg, and the procedure was carried out using four or five orifices. Ultrasound was used as needed intraoperatively for tumor location confirmation and determination of hepatic vein course.
According to the 2000 Brisbane system [16], major hepatectomy involves resecting three or more Couinaud segments, while minor hepatectomy involves fewer than three segments. The decision to block the hepatic hilum by the Pringle method was made on a case-by-case basis. R1 resection refers to the microscopic detection of tumor cells at the cut margin.
Definition of postoperative complications
The International Study Group of Liver Surgery (ISGLS) has proposed the definition of Post-Hepatectomy Liver Failure (PHLF) as an elevation of INR on or after postoperative day 5 (POD5) combined with concurrent hyperbilirubinemia [17]. The grading system includes the following:
-
Grade A, which requires no deviation from standard postoperative care.
-
Grade B, which necessitates a non-invasive deviation from standard postoperative clinical care.
-
Grade C, which demands invasive interventions.
The Comprehensive Complication Index (CCI) is calculated based on weighted scores using the Clavien-Dindo grading system for each complication experienced by the patient [18].
IWATE scoring system
Total IWATE score using six difficulty measures:
-
1.
Tumor location (score, 1–5).
-
2.
Extent of hepatic resection (score, 0–4).
-
3.
Tumor size (score 0 or 1).
-
4.
Proximity to a major vessel (score 0 or 1).
-
5.
Liver function (score 0 or 1).
-
6.
HALS/hybrid (score 0 or − 1).
These measures lead to a total score, which is categorized into 12 difficulty levels, grouped into four types: low (0–3), intermediate (4–6), advanced (7–9), and expert (10–12). Using the IWATE system, hepatectomy procedures are scored to determine their complexity, with low and intermediate scores considered uncomplicated hepatectomy and advanced and expert scores denoting complex procedures [19, 20].
Clinical variables
We collected 22 variables potentially affecting HCC prognosis for statistical analysis, including patient demographics, liver and tumor characteristics, pathological factors, surgical-related information, and perioperative variables. These variables were age, gender, maximum tumor length, ALT, preoperative AFP, preoperative ALB, preoperative ALP, preoperative AST, preoperative GGT, CSPH, HBsAg, Edmondson-Steiner grade, MVI, satellite foci, blood loss, perioperative blood transfusion, type of hepatectomy (anatomical vs. non-anatomical), resection margin, extent of hepatectomy (major vs. minor), hepatectomy time, and inflow occlusion time.
Propensity score matching analysis
We used propensity score matching(PSM) to reduce selection and confounding bias. We included ten variables to balance the baseline between patients with and without clinically significant portal hypertension (CSPH), including tumor number, AFP, ALP, GGT, ALB, cirrhosis, perioperative blood transfusion, blood loss, tumor maximum length, and extent of hepatectomy. 1:1 matching was performed using SPSS 25.0 software, with a caliper width of 0.2 chosen for the best trade-off.
Follow-up
Patients were followed up every three months in the first year after discharge and every six months thereafter. Imaging examinations (such as enhanced CT and abdominal MRI) and laboratory tests (including liver and renal function tests, electrolytes, and tumor markers) were performed at each visit. Overall survival (OS) was defined as the time from the date of surgery date to the date of death; recurrence-free survival (RFS) was defined as the time from the surgery date to the date of first recurrence or death before recurrence or the last follow-up for patients without recurrence or death. Follow-up continued until June 30, 2022.
Data analysis
Continuous variables were reported as median (range) and categorical variables as frequency (percentage). Chi-square or Fisher’s exact test was used for comparing categorical variables. Kaplan–Meier curves with the log-rank test were used to analyze survival rates for OS and RFS. Univariate and multivariate Cox regression analyses were performed to evaluate factors affecting OS and RFS after laparoscopic hepatectomy. Variables having P < 0.05 in univariate analysis were included in the multivariate regression analysis. The statistical analyses were conducted using SPSS 25.0 software, and P values < 0.05 (both sides) were considered statistically significant. The sample size was estimated using PASS software (version: 11.0). Kaplan–Meier curves were generated using R software.
Results
Baseline information for BCLC stage 0/A HCC patients before and after PSM
All patient data were collected from five Chinese centers (see Supplementary Fig. 1 for inclusion and exclusion criteria). Before PSM, 186 patients (42.9%) had CSPH. There was an imbalance in variables such as tumor number, preoperative alpha-fetoprotein (AFP), preoperative alkaline phosphatase (ALP), preoperative gamma-glutamyl transferase (GGT), preoperative albumin (ALB), cirrhosis, and perioperative blood transfusion between the two groups. Patients with CSPH had a higher frequency of blood loss and perioperative blood transfusion and a significantly higher percentage of cirrhosis. The IWATE criteria were distributed differently in the two groups, with higher surgical difficulty in the CSPH group than in the Non-CSPH group, and higher proportions of Advanced and Expert difficulty. After 1:1 PSM, the baseline was balanced between the two groups, with 186 patients in each group (Table 1). Of particular note, although the number of intraoperative conversions to open resection was 3.1% higher in the CSPH group [26(14%) vs. 19(10.9%) in the non-CSPH group] before PSM, there was no statistical significance between the two groups (P = 0.375).
Effects of CSPH on OS and RFS after laparoscopic hepatectomy in BCLC stage 0/A HCC patients before and after PSM
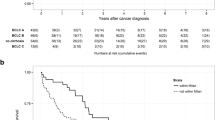
We evaluated the effect of CSPH on OS and RFS after laparoscopic hepatectomy in BCLC stage 0/A HCC patients before and after PSM. Before PSM, there was a significant difference in OS curves between the CSPH and non-CSPH groups (P < 0.001, HR = 2.26 [1.79–2.84]), with 1-, 3-, and 5-year OS rates of 79.0%, 40.1%, and 24.4%, respectively, and a median survival time of 34.3 months in the CSPH group, and rates of 96.8%, 69.7%, and 49.4%, respectively, and a median survival time of 58.0 months in the non-CSPH group. There was also a significant difference in RFS curves between the two groups (P < 0.001, HR = 2.05 [1.63–2.58]), with 1-, 3-, and 5-year RFS rates of 69.4%, 29.5%, and 17.7%, respectively, and a median recurrence time of 23.0 months in the CSPH group, and rates of 79.4%, 59.9%, and 43.8%, respectively, and a median recurrence time of 53.0 months in the non-CSPH group (Fig. 1A and B). After PSM, the overall survival rate and recurrence-free survival rate of patients in the CSPH group were significantly worse compared to the non-CSPH group, with HR values of 2.98 [2.29–3.86] and 2.49 [1.93–3.21], respectively (Fig. 2A, B). These findings suggest that CSPH remains a significant prognostic factor for both OS and RFS after laparoscopic hepatectomy in BCLC stage 0/A HCC patients, even after PSM (Fig. 2).
Subgroup analysis based on the presence of cirrhosis
The subgroup analysis according to the presence of cirrhosis revealed that among 138 patients without cirrhosis, those in the CSPH group had significantly worse OS and RFS rates than those in the non-CSPH group, with HRs of 4.40 (2.67–7.25) and 3.99 (2.46–6.45), respectively, both statistically significant (P < 0.001). Similarly, among 296 patients with cirrhosis, CSPH also significantly influenced the prognosis of patients, with HRs of 2.20 (1.66–2.93) for OS and 1.84 (1.38–2.45) for RFS, both statistically significant (P < 0.001) (Supplementary Fig. 2). The findings suggest that regardless of cirrhosis, CSPH is a significant prognostic factor for both OS and RFS after laparoscopic hepatectomy in BCLC stage 0/A hepatocellular carcinoma patients.
Cox regression analysis for prognostic factors of laparoscopic surgery in BCLC stage 0/A HCC
Univariate analysis identified factors with a P value < 0.05, which were included in the multivariate analysis. In the multivariate regression analysis for OS, CSPH (p < 0.001; HR = 2.051 [1.609–2.615]), tumor differentiation (P < 0.001; HR = 1.927 [1.489–2.495]), AFP (P = 0.006; HR = 1.424 [1.108–1.829]), ALP (P = 0.047; HR = 1.379 [1.004–1.894]), and extent of hepatectomy (P < 0.001; HR = 1.642 [1.312–1.988]) were found to be significant prognostic factors for OS (Table 2).
Similarly, in the multivariate regression analysis for RFS, AFP (P = 0.001; HR = 1.509 [1.179–1.930]), differentiation grade (p = 0.003; HR = 1.474 [1.138–1.909]), satellite foci (P = 0.047; HR = 1.353 [1.004–1.823]), GGT (P = 0.004; HR = 1.535 [1.147–2.054]), resection margin (P = 0.003; HR = 1.412 [1.178–1.831]), CSPH (P < 0.001; HR = 1.860 [1.453–2.37]), and extent of hepatectomy (P < 0.001; HR = 1.475 [1.231–2.011]) were significant prognostic factors for RFS (Table 3). These findings suggest that in addition to CSPH, other variables such as AFP, tumor differentiation, ALP, GGT, resection margin, and extent of hepatectomy also affect the postoperative prognosis of BCLC stage 0/A hepatocellular carcinoma patients.
Subgroup analysis of all patients to understand the effect of CSPH on patients’ OS in different subgroups
Forest plots were constructed to evaluate the prognostic impact of CSPH on patient subgroups. As shown in Supplementary Fig. 3, CSPH did not significantly affect the prognosis of patients with MVI (HR = 0.887 [0.566–1.389]). However, in all other subgroups, including those with early-stage HCC, CSPH had a statistically significant impact on patient prognosis.
Short-term outcomes and complications in all patients treated laparoscopically
Among patients without CSPH, five experienced recurrence, and no deaths occurred within 90 days after surgery. In contrast, among those in the CSPH group, 12 experienced recurrence, and seven died within 90 days after surgery, representing statistically significant differences in postoperative recurrence and mortality rates between the two groups. Patients in the CSPH group also had a higher rate of postoperative liver failure, which was statistically significant (7.0% vs. 2.4%). Moreover, within the CSPH group, there was a higher proportion of grades B and C PHLF. The median CCI score was 57.7, which was significantly higher than the non-CSPH group's score of 28.6 (P < 0.001) (Table 4). Univariate and multivariate logistic regression analyses showed that CSPH was a risk factor for PHLF in patients (OR = 2.178 [1.703–2.710], P < 0.001) (Supplementary Table 1).
Laparoscopic versus open hepatectomy in BCLC stage 0/A HCC patients
Two hundred thirteen patients who underwent open hepatectomy during the same period were included for comparison, and no significant differences in general variables were observed between the laparoscopic and open groups (P > 0.05). However, statistically significant differences were found in perioperative variables such as hepatectomy time, the extent of hepatectomy, and blood loss, with longer hepatectomy times, more extensive hepatectomies, and more significant bleeding in the open hepatectomy group, as shown in Supplementary Table 2. The OS rates were comparable between the two groups (P = 0.087, HR = 1.24 [1.03–1.50]). However, patients who underwent laparoscopic surgery had a higher RFS rate (P < 0.001, HR = 2.06 [1.70–2.48]). Supplementary Fig. 4 shows the survival curves for both groups. Among all HCC patients who underwent surgery, those who underwent laparoscopic surgery had a lower incidence of PHLF. No significant differences were observed in the remaining short-term complications, which are detailed in Supplementary Table 3.
Discussion
With the recent improvement in laparoscopic techniques, liver tumors located in any segment can be safely treated with laparoscopic hepatectomy [8, 21]. Currently, most liver surgery centers in China are gradually adopting laparoscopy as the preferred approach for treating HCC. CSPH has traditionally been considered a contraindication to hepatectomy due to the high postoperative mortality and potential for hepatic failure. However, the latest Barcelona Clinic Liver Cancer (BCLC) staging recommends that hepatectomy may be considered for patients with BCLC stage 0/A HCC [5]. Therefore, it is essential to understand the impact of CSPH on postoperative outcomes in BCLC stage 0/A patients, which will significantly facilitate treatment planning and improve guidelines. Our study is the largest multicenter investigation of BCLC stage 0/A HCC patients with CSPH who underwent laparoscopic liver resection to date.
The Kaplan–Meier curve demonstrates that CSPH significantly impacts the prognosis of HCC. To minimize selection and confounding biases, we employed propensity score matching to achieve an effect similar to that of a randomized controlled trial, and our results suggest that CSPH adversely affects early-stage HCC patients after laparoscopic liver resection. In a study by Casellas-Robert et al.[7, 22], researchers compared short-term outcomes after laparoscopic liver resection in patients with Child–Pugh class A liver function with or without CSPH. They concluded that the laparoscopic approach was feasible in selected HCC patients with CSPH but at the cost of significantly increased liver-specific complications and longer hospital stays. A meta-analysis by Choi et al.[23] found that postoperative mortality, complications, liver-related morbidity, liver failure, and overall survival were significantly worse in the CSPH group, indicating that surgical indications should be more precisely defined. However, a PSM study by Zheng et al. [24] concluded that CSPH had no impact on postoperative outcomes following laparoscopic liver resection, suggesting that indications for surgery could be expanded. Nevertheless, the Kaplan–Meier curves from their study revealed a trend in prognosis between CSPH and non-CSPH groups, likely due to the small sample size (only 24 patients in each group after PSM). Giannini et al. [25] concluded that CSPH did not affect survival in patients with well-compensated cirrhosis. However, this result may be due to a lack of baseline information for the CSPH versus non-CSPH groups and selection bias. Similarly, our multivariate Cox regression analysis demonstrated that CSPH remained a significant prognostic factor for both OS and RFS after laparoscopic liver resection. Thus, more rigorous management is needed for early-stage HCC patients with CSPH.
Of course, liver transplantation is the most effective strategy for patients with early-stage HCC with CSPH. However, the shortage and high cost of donors in China largely limit the feasibility of liver transplantation. Therefore, resection can alleviate CSPH and can be a radical treatment. Previous studies [26,27,28] comparing LLR with open surgery for HCC patients with portal hypertension have shown that LLR provides a comparable long-term prognosis to open surgery and a better short-term prognosis, such as fewer postoperative complications and shorter hospital stays. Less mobilization of the liver and more delicate treatment of the vessels can lead to a lower risk of intractable ascites. Thus, the overall incidence of postoperative liver failure can be reduced. Azoulay and colleagues [22] concluded that laparoscopic surgery was a protective factor in the prognosis of patients with CSPH; Harada et al. [29] compared the prognosis of patients with concurrent portal hypertension treated with LLR, open hepatectomy, and radiofrequency ablation (RFS), showing that the LLR group was the most feasible approach, with a better prognosis than RFA and fewer postoperative complications than open surgery. Therefore, laparoscopic surgery is the best option for patients with early-stage HCC who cannot undergo liver transplantation.
Our results suggest that patients with CSPH have higher and statistically significant rates of death and recurrence within 90 days after surgery than those without CSPH and a relatively higher rate of PHLF after laparoscopy. Previous studies [30, 31] have also concluded that the presence of CSPH makes postoperative complications more likely, and Wang et al. [32] constructed a nomogram to predict the risk of PHLF using preoperative indicators, of which CSPH was a vital variable and played a pivotal role in the model. Azoulay et al. [22] suggested that CSPH may lead to an increased probability of postoperative liver failure. However, the low incidence in the postoperative period (8%) was methodologically hampered by the use of multivariate analysis to identify it as an independent predictor. The incidence of other postoperative complications was not affected by the presence or absence of CSPH, and the proportion of other postoperative complications arising after LLR was lower in both groups (mean 2%). The common view is that the presence of PHLF means that the prognosis will be poor, and therefore, early intervention after LLR is still required for patients with preoperative CSPH.
The hepatic venous pressure gradient (HVPG) is the gold standard for detecting portal hypertension in cirrhosis, but it is invasive and specialized. In Asia, preoperative HVPG measurement is rarely performed due to its invasiveness, which may cause physical and psychological harm to patients. To overcome this limitation, several non-invasive techniques have been developed to evaluate HVPG. For instance, Yu et al. [33] developed an artificial intelligence model for quantitative and multi-stage assessment of HVPG using imaging and histology, while Liu et al. [34] employed deep convolutional neural networks to identify patients with CSPH. Although we currently rely on endoscopy or imaging to detect CSPH, machine learning and imaging technology may lead to more accurate and convenient detection methods in the future.
This study still has some limitations. As a retrospective study, it is susceptible to selection and confounding biases. However, we used statistical methods to limit these biases as much as possible. Our study was not validated using data from non-Asian regions, where most HCC patients are infected with HBV, which differs from those in non-Asian countries.
Conclusion
CSPH significantly impacts the prognosis of HCC patients with BCLC stage 0/A disease who undergo LLR. Despite this, we still recommend LLR for early-stage HCC patients with CSPH but emphasize the importance of early postoperative intervention in these patients to improve their short- and long-term outcomes.
Data availability
The datasets generated and/or analyzed in the current study are not publicly available [These data are used in other studies by our team] but are available from the corresponding author on reasonable request.
Abbreviations
- CSPH:
-
Clinically significant portal hypertension
- BCLC:
-
Barcelona clinic liver cancer
- AFP:
-
Alpha-fetoprotein
- HCC:
-
Hepatocellular carcinoma
- RFA:
-
Radiofrequency ablation
- PHLF:
-
Posthepatectomy liver failure
- TACE:
-
Transcatheter arterial chemoembolization
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- MVI:
-
Microvascular invasion.
- HBsAg:
-
Hepatitis B surface antigen
- ALB:
-
Albumin
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- GGT:
-
γ-Glutamyl transpeptidase
References
Chen Z et al (2020) Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res 10:2993–3036
Couri T, Pillai A (2019) Goals and targets for personalized therapy for HCC. Hepatol Int 13:125–137
Gunarathne LS et al (2020) Cirrhotic portal hypertension: from pathophysiology to novel therapeutics. World J Gastroenterol 26:6111–6140
Iwakiri Y, Trebicka J (2021) Portal hypertension in cirrhosis: pathophysiological mechanisms and therapy. JHEP Rep: Innov Hepatol 3:100316
Reig M et al (2022) BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 76:681–693
Choi GH et al (2011) Predictive factors for long-term survival in patients with clinically significant portal hypertension following resection of hepatocellular carcinoma. Liver Int 31:485–493
Casellas-Robert M et al (2020) Laparoscopic Liver resection for hepatocellular carcinoma in Child-Pugh a patients with and without portal hypertension: a multicentre study. World J Surg 44:3915–3922
D’Silva M et al (2022) Limited liver resections in the posterosuperior segments: international multicentre propensity score-matched and coarsened exact-matched analysis comparing the laparoscopic and robotic approaches. Br J Surg 109(11):1140–1149
Aghayan DL et al (2019) Laparoscopic versus open liver resection in the posterosuperior segments: a sub-group analysis from the OSLO-COMET randomized controlled trial. HPB (Oxford) 21:1485–1490
El-Gendi A et al (2018) Laparoscopic versus open hepatic resection for solitary hepatocellular carcinoma less than 5 cm in cirrhotic patients: a randomized controlled study. J Laparoendosc Adv Surg Tech A 28:302–310
Fretland ÅA et al (2018) Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 267:199–207
Robles-Campos R et al (2019) Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc 33:3926–3936
Xia F et al (2023) Clinically Significant Portal Hypertension (CSPH) on early-stage HCC following hepatectomy: what’s the impact? Eur J Surg Oncol 49:771–779
EASL Clinical Practice Guidelines (2018) Management of hepatocellular carcinoma. J Hepatol 69:182–236
Vogel A et al (2018) Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv238–iv255
Pang YY (2002) The Brisbane 2000 terminology of liver anatomy and resections. HPB 4:99–100
Rahbari NN et al (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149:713–724
Slankamenac K et al (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258:1–7
Barron JO et al (2022) Validation of the IWATE criteria as a laparoscopic liver resection difficulty score in a single North American cohort. Surg Endosc 36:3601–3609
Labadie KP et al (2022) IWATE criteria are associated with perioperative outcomes in robotic hepatectomy: a retrospective review of 225 resections. Surg Endosc 36:889–895
Ishizawa T et al (2012) Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 256:959–964
Azoulay D et al (2021) Liver resection for hepatocellular carcinoma in patients with clinically significant portal hypertension. JHEP Rep: Innov Hepatol 3:100190
Choi SB et al (2014) Influence of clinically significant portal hypertension on surgical outcomes and survival following hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci 21:639–647
Zheng J et al (2021) Safety and feasibility of laparoscopic liver resection for hepatocellular carcinoma with clinically significant portal hypertension: a propensity score-matched study. Surg Endosc 35:3267–3278
Giannini EG et al (2013) Influence of clinically significant portal hypertension on survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Liver Int 33:1594–1600
Hobeika C et al (2020) Influence of surgical approach and quality of resection on the probability of cure for early-stage HCC occurring in cirrhosis. JHEP Rep: Innov Hepatol 2:100153
Levi Sandri GB et al (2018) Laparoscopic liver resection for large HCC: short- and long-term outcomes in relation to tumor size. Surg Endosc 32:4772–4779
Reddy SK et al (2011) Laparoscopic liver resection. World J Surg 35:1478–1486
Harada N et al (2016) Laparoscopic liver resection is a feasible treatment for patients with hepatocellular carcinoma and portal hypertension. Anticancer Res 36:3489–3497
Søreide JA, Deshpande R (2021) Post hepatectomy liver failure (PHLF)—recent advances in prevention and clinical management. Eur J Surg Oncol: J Eur Soc Surg Oncol British Assoc Surg Oncol 47:216–224
Sposito C et al (2021) Preoperative predictors of liver decompensation after mini-invasive liver resection. Surg Endosc 35:718–727
Wang YY et al (2021) Development and Validation of a nomogram to preoperatively estimate post-hepatectomy liver dysfunction risk and long-term survival in patients with hepatocellular carcinoma. Ann Surg 274:e1209–e1217
Yu Q et al (2022) An imaging-based artificial intelligence model for non-invasive grading of hepatic venous pressure gradient in cirrhotic portal hypertension. Cell Rep Med 3:100563
Liu Y et al (2020) Deep convolutional neural network-aided detection of portal hypertension in patients with cirrhosis. Clin Gastroenterol Hepatol 18:2998-3007.e5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Feng Xia, Qiao Zhang, Elijah Ndhlovu, Jun Zheng, Minggang Yuan, Hengyi Gao, and Guobing Xia declare no conflicts of interest.
Ethical approval
The Ethics Committees of Wuhan Tongji Hospital, Zhongshan People’s Hospital, Huangshi Central Hospital, Shenzhen Baoan District People's Hospital, and Shenzhen Longhua District People’s Hospital approved this retrospective observational study.
Informed consent
All patients provided informed consent to the use and publication of their information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.




Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, F., Zhang, Q., Ndhlovu, E. et al. Prognosis and safety of laparoscopic hepatectomy for BCLC stage 0/A hepatocellular carcinoma with clinically significant portal hypertension: a multicenter, propensity score-matched study. Surg Endosc 38, 799–812 (2024). https://doi.org/10.1007/s00464-023-10589-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10589-7