Abstract
Background
Recently, novel intracorporeal esophagojejunostomy using a linear stapler after laparoscopic total gastrectomy (LTG) was reported and termed as the overlap method. In this study, we evaluated the feasibility and safety of the overlap method for esophagojejunostomy or esophagogastrostomy after LTG or laparoscopic proximal gastrectomy (LPG), respectively.
Methods
Twenty-five patients underwent anastomosis using a linear stapler during esophagojejunostomy and esophagogastrostomy after LTG and LPG, respectively. Clinicopathological data and surgical outcomes were evaluated.
Results
The average surgical duration for LTG was 236.8 min compared with 224.1 min for LPG. Postoperative complications were observed in four patients (16.0%); these included a wound infection, an intestinal obstruction, an afferent loop syndrome, and a reflux symptom. The average postoperative hospital stay of the patients was 12.5 days. There was no case of conversion to open surgery, anastomotic leakage or stenosis, or mortality.
Conclusions
The overlap method for esophagojejunostomy or esophagogastrostomy after LTG or LPG is safe and feasible and does not require an additional minilaparotomy, which may result in less pain and favorable cosmetic outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Kitano et al. [1] reported the first laparoscopy-assisted gastrectomy for gastric cancer in 1991, this technique has become increasingly popular in Japan. Although laparoscopic distal gastrectomy is a popular choice of treatment for early gastric cancer, laparoscopic total gastrectomy (LTG) or proximal gastrectomy (LPG) is still uncommon. Indeed, 40.1% of all gastrectomies were being performed using conventional open techniques for total gastrectomy, whereas only 19.2% of these surgeries were laparoscopy assisted [2]. This may be attributed to the technical difficulty of performing laparoscopy-assisted esophagojejunostomy or esophagogastrostomy.
Circular staplers are frequently used during open esophagojejunostomy, and extensive efforts have been made to apply this device to laparoscopic surgery [3–8]. However, these procedures require additional small incisions to insert the circular stapler into the peritoneal cavity; in addition, there are some technical difficulties in handling the circular stapler and inserting the anvil head into the esophagus because of the limited working space.
Recently, Inaba and colleagues reported a novel method of intracorporeal esophagojejunostomy using a linear stapler after LTG, and they termed this procedure as the overlap method [9]. In this study, we evaluated the feasibility and safety of the overlap method for esophagojejunostomy or esophagogastrostomy after LTG or LPG, respectively.
Methods
Patients
A total of 98 patients with gastric cancer underwent laparoscopic gastrectomy at the Department of Surgery, National Defense Medical College Hospital, between 2008 and 2010. Of the total, 15 patients (15.3%) underwent LTG, and 10 (10.2%) underwent LPG. Anastomosis using a linear stapler during esophagojejunostomy and esophagogastrostomy was performed for all 25 patients after LTG and LPG, respectively.
The clinicopathological findings of the patients were evaluated on the basis of the Japanese Classification of Gastric Carcinoma (second English edition) published by the Japanese Gastric Cancer Association [10]. Gastric cancer staging was based on the preoperative assessment of depth of wall invasion, which was performed using upper gastrointestinal tract endoscopy, barium radiology, and endoscopic ultrasonography. Nodal involvement was determined by preoperative computed tomography [11].
Indication for LTG and LPG
Indications for all laparoscopic gastrectomies were as per those of the gastric cancer treatment guidelines in Japan [12], i.e., clinically mucosal or submucosal carcinoma without lymph node metastasis (cT1, cN0) was an indication for laparoscopic gastrectomy.
LPG was indicated for tumors in the upper part of the stomach and when more than half of the stomach could be preserved. LTG was indicated for tumors in the upper and/or middle thirds of the stomach and when tumors did not meet the criteria for LPG.
Anastomosis using a linear stapler during esophagojejunostomy and esophagogastrostomy
Following induction of general anesthesia, each patient was placed in a supine position. The surgeon was positioned on the left side of the patient, first assistant on the right, and the laparoscopist between the abducted legs of the patient. A camera port was inserted into a median umbilical incision. Next, a pneumoperitoneum of 12 mmHg was induced, and four additional ports (two ports with 12 mm diameter and two with 5 mm diameter) were inserted under laparoscopic imaging into the left upper, right lower, left lower, and right upper quadrants. An ultrasonically activated sealing device (Harmonic Scalpel Ace; Ethicon, Tokyo, Japan) and a vessel sealing device (Ligasure V; Tyco Healthcare, Tokyo, Japan) were used.
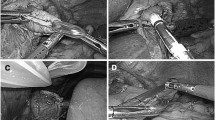
After gastrectomy was performed along with lymphadenectomy as per the tumor stage, a small opening was made in the right side of the esophagus where transection would occur (Fig. 1), following which mucosal and submucosal layers were sutured to the muscular layers with an absorbent monofilament suture (3-0 monocryl, Ethicon). The esophagus was transected with a 60-mm endoscopic linear stapler (Endo-GIA; Covidien, blue cartridge) (Fig. 2). The right diaphragmatic crus was partially divided using a vessel sealing device to widen the surgical field for reconstruction, if necessary. In total gastrectomy, tumors along with the surrounding tissues were pulled out through the umbilical trocar incision, which was extended by enlarging the median fascia and skin incision. Via a similar approach, in proximal gastrectomy, the upper third of the stomach and the surrounding tissue were drawn up.
During LTG, the jejunum was intracorporeally transected 20 cm distal to the ligament of Treitz using an Endo-GIA stapler (blue cartridge). The distal side of the jejunum (approximately 10 cm long) was additionally removed to avoid excessive tension at the site of anastomosis in esophagojejunostomy. A small enterotomy was made 7 cm distal to the stapler line on the antimesenteric side of the jejunal limb. After one fork of the 45-mm Endo-GIA stapler (blue cartridge) was inserted through this opening toward the oral side of the lumen, the jejunal limb was brought up to create an esophagojejunostomy in an antecolic fashion. Another fork of the linear stapler was inserted carefully into the hole of the esophagus under the guidance of a nasogastric tube (Fig. 3). After each fork was completely inserted into each lumen, the two limbs were mated to fashion the side-to-side esophagojejunal anastomosis (Fig. 4). A completion of esophagojejunal anastomosis and intraluminal hemostasis were then confirmed (Fig. 5). The entry hole of the stapler was closed using an intracorporeal interrupted hand-sewn technique with absorbable monofilament sutures (3-0 monocryl; Ethicon) (Fig. 6). Interrupted suturing was usually done 7–10 times, and the intracorporeal esophagojejunostomy anastomosis was completely established (Fig. 7). A jejunojejunostomy was performed using a previously reported procedure [13]. Closure was confirmed by laparoscopic examination after insufflating air into the jejunum and submerging the suture line in water. A closed suction drain (19 Fr J-VAC drain; Ethicon) was placed behind the esophagojejunostomy. A representative postoperative view of the surgical wound 6 months after LTG is shown in Fig. 8.
A small enterotomy is made 7 cm distal to the stapler line on the antimesenteric side of the jejunal limb. After one fork of the 45-mm Endo-GIA stapler (blue cartridge) is inserted through this opening toward the oral side of the lumen, another fork is inserted carefully into the hole of the esophagus under the guidance of a nasogastric tube
During LPG, the upper side of the stomach, which had an adequate surgical margin, was cut using a linear stapler (TL 90 mm; Ethicon), and a small gastrostomy was made 5 cm distal to the stapler line on the anterior wall of the stomach. An esophagogastrostomy was performed by the same procedure as described previously using a 45-mm Endo-GIA stapler. Fundoplication was performed as an antireflux procedure as previously described [14].
Statistical analysis
Statistical calculations were performed using StatView version 5.0 (SAS Institute, Inc., Cary, NC, USA). The data are expressed as mean ± standard deviation. Statistical analyses were performed using the Mann–Whitney U test or chi-square test with Fisher's exact test, whichever was considered appropriate. P values of <0.05 were considered statistically significant.
Results
The demographic data of patients who underwent LTG and LPG for early gastric cancer are depicted in Table 1. The average age of the patients was 69.3 years (range 40–88 years), and 19 of the 25 patients were male. The patients had an average body mass index (BMI) of 21.7 kg/m2 (range 13.8–31.9 kg/m2). All patients with tumors located in the middle part of the stomach underwent LTG; these patients had larger tumors and longer distal margin, and required extended lymphadenectomy, and a higher number of lymph nodes were harvested from these patients than that from patients who underwent LPG. The average surgical duration of LTG was 236.8 min (range 179–334 min), whereas that of LPG was 224.1 min (range 159–299 min) (Table 2). No difference was observed in the incidence of comorbidity between the two surgical procedures. Postoperative complications were observed in four patients (16.0%), of which one developed a wound infection and one developed an intestinal obstruction that required conservative therapy; after which, the patient was discharged on postoperative day (POD) 20. Of the remaining two, one developed afferent loop syndrome that was successfully treated by endoscopic drainage, and the patient was discharged on POD 42, and one LPG patient developed reflux symptoms that were treated by prolonged proton pump inhibitor therapy. In this patient, proximal esophageal resection was required because the tumor involved the distal esophagus. The average postoperative hospital stay of the patients was 12.5 days (range 7–42 days). No cases of conversion to open surgery, anastomotic leakage or stenosis, or mortality were observed.
Tumor recurrence during a mean follow-up period of 18.9 months (range 6–28 months) was not observed in any patient.
Previous reports describing surgical outcomes of esophagojejunostomy or esophagogastrostomy using laparoscopic staplers after LTG or LPG, respectively, are shown in Table 3 [15–19].
Discussion
In this study, we demonstrated the safety and feasibility of anastomosis using the overlap method during esophagojejunostomy and esophagogastrostomy after LTG and LPG, respectively.
In conventional open total gastrectomy, esophagojejunal and esophagogastric anastomoses are mostly performed using circular stapling devices. According to previous reports, esophagojejunostomy after LTG has also been performed using circular staplers to reproduce the results of open surgery [3, 6, 20, 21]. However, there are still potential problems in performing these procedures with a circular stapling device. Placing a purse-string suture in the distal esophagus and inserting an anvil head are occasionally problematic because the esophagus is shortened after it is transected, which may lead to disruption of the esophageal wall. In addition, this procedure requires 3–6 cm of additional minilaparotomy to insert the circular stapler into the peritoneal cavity (Table 3) [3, 21–24]. In the anastomosis presented here, minilaparotomy for esophagojejunostomy or esophagogastrostomy was not necessary, and it was easy to confirm the intraluminal hemostasis after the first firing of the stapler.
We believe that there are two factors that contribute to the successful achievement of this anastomosis. First, a small opening of the esophagus was made before transection of the esophagus. As mentioned previously, the esophagus is shortened after it is transected, and it is also difficult to maintain sufficient tension for making a small enterotomy. Second, we put a single suture through all the layers so that there were no gaps between the submucosal and muscular layers. This facilitated adequate insertion of the stapler into the esophageal lumen to lift the distal esophagus after transection (Fig. 2).
In this study, no case required conventional open surgery, and no technical difficulties were encountered while achieving anastomosis using these procedures. Even with obese patients (BMI > 25), we did not encounter any difficulties during the surgeries, and there was no difference in the duration of surgeries between patients with high and low BMI (BMI > 25: 246.2 ± 59.0 min, BMI ≤ 25: 227.0 ± 35.9 min, respectively). Furthermore, no patients with BMI > 25 had postoperative complications. These findings suggest that this procedure is not affected by intraabdominal and subcutaneous fat.
If a tumor involves the distal esophagus and/or is an advanced esophagogastric cancer, peritoneal seeding may occur [25]. In these cases, therefore, resection of the esophagus must be performed without an enterotomy before the transection of the esophagus [9].
The results of this study are comparable to those of previous studies in terms of surgical duration, hospitalization, and incidence of leakage and stenosis (Table 3). In this context, the overlap method using a linear stapler for esophagojejunostomy or esophagogastrostomy after LTG or LPG, respectively, is safe and feasible; in addition, it provides satisfactory outcomes. This procedure does not require an additional minilaparotomy, which may result in less pain and favorable cosmetic outcomes. We believe that this procedure will facilitate the acceptance of LTG and LPG as surgical options for patients with early proximal gastric cancer.
References
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted billroth I gastrectomy. Surg Laparosc Endosc 4(2):146–148
10th nationwide survey of endoscopic surgery in Japan (2010). J Jpn Soc Endosc Surg 15 (5):565-679
Jeong O, Park YK (2009) Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc 23(11):2624–2630. doi:10.1007/s00464-009-0461-z
Omori T, Oyama T, Mizutani S, Tori M, Nakajima K, Akamatsu H, Nakahara M, Nishida T (2009) A simple and safe technique for esophagojejunostomy using the hemidouble stapling technique in laparoscopy-assisted total gastrectomy. Am J Surg 197(1):e13–17. doi:10.1016/j.amjsurg.2008.04.019
Kim YW, Han HS, Fleischer GD (2003) Hand-assisted laparoscopic total gastrectomy. Surg Laparosc Endosc Percutan Tech 13(1):26–30
Mochiki E, Kamimura H, Haga N, Asao T, Kuwano H (2002) The technique of laparoscopically assisted total gastrectomy with jejunal interposition for early gastric cancer. Surg Endosc 16(3):540–544. doi:10.1007/s00464-001-8219-2
Hiki N, Kaminishi M (2005) Pylorus-preserving gastrectomy in gastric cancer surgery—open and laparoscopic approaches. Langenbecks Arch Surg 390(5):442–447. doi:10.1007/s00423-005-0573-4
Meyer L, Meyer F, Dralle H, Ernst M, Lippert H, Gastinger I (2005) Insufficiency risk of esophagojejunal anastomosis after total abdominal gastrectomy for gastric carcinoma. Langenbecks Arch Surg 390(6):510–516. doi:10.1007/s00423-005-0575-2
Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I (2010) Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg 211(6):e25–29. doi:S1072-7515(10)01072-0
Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer 1(1):10–24. doi:10.1007/s101209800016
Tsujimoto H, Sugasawa H, Ono S, Ichikura T, Yamamoto J, Hase K (2010) Has the accuracy of preoperative diagnosis improved in cases of early-stage gastric cancer? World J Surg 34(8):1840–1846. doi:10.1007/s00268-010-0587-0
Nakajima T (2002) Gastric cancer treatment guidelines in Japan. Gastric Cancer 5(1):1–5
Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A (1999) Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer 2(4):230–234. doi:10.1007/s101209900041
Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Moriya H, Hirai K, Watanabe M (2009) Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg 209(3):344–351. doi:S1072-7515(09)00410-4
Okabe H, Obama K, Tanaka E, Nomura A, Kawamura J, Nagayama S, Itami A, Watanabe G, Kanaya S, Sakai Y (2009) Intracorporeal esophagojejunal anastomosis after laparoscopic total gastrectomy for patients with gastric cancer. Surg Endosc 23(9):2167–2171. doi:10.1007/s00464-008-9987-8
Okabe H, Satoh S, Inoue H, Kondo M, Kawamura J, Nomura A, Nagayama S, Hasegawa S, Itami A, Watanabe G, Sakai Y (2007) Esophagojejunostomy through minilaparotomy after laparoscopic total gastrectomy. Gastric Cancer 10(3):176–180. doi:10.1007/s10120-007-0432-9
Ziqiang W, ZhiMin C, Jun C, Xiao L, Huaxing L, PeiWu Y (2008) A modified method of laparoscopic side-to-side esophagojejunal anastomosis: report of 14 cases. Surg Endosc 22(9):2091–2094. doi:10.1007/s00464-008-9744-z
Bracale U, Marzano E, Nastro P, Barone M, Cuccurullo D, Cutini G, Corcione F, Pignata G (2010) Side-to-side esophagojejunostomy during totally laparoscopic total gastrectomy for malignant disease: a multicenter study. Surg Endosc 24(10):2475–2479. doi:10.1007/s00464-010-0988-z
Kinoshita T, Oshiro T, Ito K, Shibasaki H, Okazumi S, Katoh R (2010) Intracorporeal circular-stapled esophagojejunostomy using hand-sewn purse-string suture after laparoscopic total gastrectomy. Surg Endosc 24(11):2908–2912. doi:10.1007/s00464-010-1041-y
Usui S, Nagai K, Hiranuma S, Takiguchi N, Matsumoto A, Sanada K (2008) Laparoscopy-assisted esophagoenteral anastomosis using endoscopic purse-string suture instrument “Endo-PSI (II)” and circular stapler. Gastric Cancer 11(4):233–237. doi:10.1007/s10120-008-0481-8
Hiki N, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Ohyama S, Seto Y, Muto T (2007) Laparoscopic esophagogastric circular stapled anastomosis: a modified technique to protect the esophagus. Gastric Cancer 10(3):181–186. doi:10.1007/s10120-007-0433-8
Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, Cho SJ, Lee JY, Kim CG, Choi IJ, Kook MC, Park SR, Kim MJ, Lee JS (2009) Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol 100(5):392–395. doi:10.1002/jso.21345
Tonouchi H, Mohri Y, Tanaka K, Kobayashi M, Kusunoki M (2006) Hemidouble stapling for esophagogastrostomy during laparoscopically assisted proximal gastrectomy. Surg Laparosc Endosc Percutan Tech 16(4):242–244. doi:00129689-200608000-00009[pii]
Usui S, Inoue H, Yoshida T, Fukami N, Kudo SE, Iwai T (2003) Hand-assisted laparoscopic total gastrectomy for early gastric cancer. Surg Laparosc Endosc Percutan Tech 13(5):304–307
Hao YX, Zhong H, Yu PW, Qian F, Zhao YL, Shi Y, Tang B (2010) Influence of laparoscopic gastrectomy on the detection rate of free gastric cancer cells in the peritoneal cavity. Ann Surg Oncol 17(1):65–72. doi:10.1245/s10434-009-0703-2
Conflicts of interest
All authors certify that they have no commercial associations that might pose a conflict of interest in connection with the submitted article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsujimoto, H., Uyama, I., Yaguchi, Y. et al. Outcome of overlap anastomosis using a linear stapler after laparoscopic total and proximal gastrectomy. Langenbecks Arch Surg 397, 833–840 (2012). https://doi.org/10.1007/s00423-012-0939-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-012-0939-3