Abstract
Introduction
BRAF mutations and RET or NTRK1 rearrangements were identified as causing events that drive the malignant transformation of the thyroid follicular cell. The impact of these alterations on the course of papillary thyroid carcinoma (PTC) is still unsettled.
Patients and methods
Tumor tissues of 290 (98 male, 192 female) patients were intra-operatively snap frozen or harvested from archival paraffin-embedded blocks and used for extraction of DNA and RNA. Comprehensive analysis of RET/PTC and NTRK1 rearrangements was carried out by multiplex screening RT-PCR, hybrid-specific RT-PCR and sequencing of detected hybrids. A mutation-specific PCR was used for BRAF analysis.
Results
The BRAF V600E mutation was detected in 122/290 (42%), RET rearrangements in 20/137 (14.6%), and NTRK1 rearrangements in 15/93 (16.1%) PTCs. One hundred forty one out of 290 (48.6%) PTCs demonstrated none of the genetic alterations studied. Eight PTCs expressed two different mutations (1 RET/PTC + BRAF, 6 NTRK1 + BRAF, 1 RET/PTC + NTRK1). Tumor-specific survival analysis (mean follow-up, 5.5 years) demonstrated no significant difference, but a tendency toward worse prognosis of BRAF-positive patients compared to BRAF-negative patients or rearrangement-positive patients, respectively.
Conclusion
Long-term follow-up data on large tumor panels are needed to disclose significant survival differences of prognostic predictors on PTC. This study provides further evidence that patients harboring BRAF-V600E-positive PTCs may experience an unfavorable course of the disease compared to patients with tumors carrying other genetic alterations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of papillary thyroid carcinoma (PTC) is closely associated with rearrangements of the receptor tyrosine kinases RET and NTRK1 as well as activating point mutations of BRAF, a member of the downstream signaling cascade activated by the membrane bound receptors. RET and NTRK1 both encode for transmembrane receptors for neural growth factors: RET was identified as one of the receptors for the glial cell line-derived neurotrophic factor (GDNF) and neurturin (NTN) while NTRK1—synonymously designated TrkA—is one of the receptors for the nerve growth factor (NGF) [20].
BRAF mutations constitute the predominant genetic alteration and are detected in 30–50% of PTCs [5, 8, 11, 14, 18, 27]. In certain regions, the frequency can increase up to 80% [6, 10]. RET and NTRK1 hybrid oncogenes are expressed in 10–25% of PTCs in Germany. However, significant regional differences are also described and the frequency of RET rearrangements can increase to more than 80% in selected cohorts with a history of irradiation [13].
The identification of these genetic alterations already gained significant attention for the pre-operative diagnosis of PTC as well as for the post-operative classification of thyroid carcinomas with untypical histological patterns [16, 24]. Moreover, correlations of certain mutations with distinct histological patterns have already been described, although there is a great overlap between groups [1, 18, 22, 23]. In addition, the RET/PTC expression, especially RET/PTC1 seems to be restricted to well-differentiated PTC and has not been detected in undifferentiated thyroid carcinomas while BRAF mutations were observed in both histological types, implicating that predominantly PTC harboring BRAF mutations progress to undifferentiated phenotype [23, 26].
It is, therefore, evident that different genetic alterations induce significant differences of the phenotype of the malignant cells resulting in diverse biological behavior or growth patterns that should have an impact on the course of PTCs and patient's survival [12].
However, due to the slow-growing nature of PTC and the overall good prognosis a great number of patients with long follow-up data have to be analyzed in order to evaluate this hypothesis. To date, only retrospective studies including archival tissue of patients with PTC meet these prerequisites.
The determination of the prognostic impact of the genetic alterations on PTC and patient's survival is of great interest for the surgical treatment as well as for the adjuvant radioiodine therapy or possible new chemotherapeutic strategies in case of advanced disease [27].
Patients and methods
Thyroid tissues
From 1988 to 2010, tumor tissues of 290 patients (192 female, 98 male) who underwent surgery for primary or recurrent papillary thyroid cancer were intra-operatively harvested and immediately snap frozen or retrieved from archival paraffin-embedded blocks. The diagnosis of PTC was verified by histological examination of the surgical specimens. Histopathological confirmation of tumor presence was also performed on the frozen tissues in areas directly adjacent to the tissue used for RNA extraction. According to the recommendations of the institute's ethics committee, informed consent was obtained from all patients whose frozen tissues were used. The patient group consisted of predominantly Caucasians originating from different parts of Germany. Results of the analysis of the proto-oncogenes RET and NTRK1 of 119 patients were already published previously [17]. The materials of these patients were used for the additional analysis of BRAF. Additional archival and fresh frozen tissue was used in 165 patients. In these patients, BRAF mutation analysis was carried out first. Amplification of RET/PTC1-specific hybrids was performed especially in cases with negative BRAF result. NTRK1 rearrangements were not analyzed in these patients.
Clinical data and statistics
Clinical data of surgical procedures performed, intra-operative findings, and histopathology data as well as pre-operative and post-operative status of the PTC patients studied were documented and analyzed. Follow-up information on the patients were derived from personal telephone inquiries as well as regular disease-specific examinations including neck ultrasound, determination of basal and stimulated TG levels, whole body scintigraphy and, in case of lacking iodine uptake, fluorodeoxyglucose-PET scans. Due to the long follow-up time, the fifth edition of the TNM classification was used for all patients.
For statistical analysis, patients with double mutations were assigned to the assumed dominant mutation. The BRAF mutation was always considered a dominant mutation. Patients with RET and NTRK1 rearrangements were grouped to the RET-positive patient cohort. Cause-specific survival of patients was calculated by the Kaplan–Meier analysis while significant differences were tested with either the Chi-square test, the log-rank test, or the Student's t test, utilizing the software package SPSS 18.0 (SPSS Inc., Chicago, IL).
Molecular genetic analyses
Multiplex RT-PCR analysis of RET and NTRK1 using fresh frozen tumor tissue was described previously [16, 17]. Considering the fact that the examined patient population predominantly expressed RET/PTC1 rearrangements and only sporadically other rearrangements are found, the analysis was restricted to a hybrid-specific amplification of the RET/PTC1 since 2003. The protocol for the detection of the RET/PTC1 rearrangement was changed after cDNA synthesis essentially according to Sapio et al. [24]. The quality of the cDNA preparations was confirmed using a cDNA-specific GAPDH assay (Applied Biosystems, Darmstadt, Germany).
Screening for the BRAF V600E Mutation (GTG → GAG) was performed with two parallel wild type-specific and mutation-specific PCR reactions [25], using the following primers:
-
BRAF-Forward-WTGTGATTTTGGTCTAGCTACAGT
-
BRAF-Forward-MutGTGATTTTGGTCTAGCTACAGA
-
BRAF-ReverseGGCCAAAAATTTAATCAGTGGA
In order to ensure optimal sensitivity and specificity, a modified touchdown PCR program was used with the first ten cycles (touchdown of 1°C per cycle) 95°C, 30 s/72°C → 63°C, 30 s/72°C, 45 s followed by 30 cycles of 95°C, 30 s/60°C, 30 s/72°C, 45 s.
A maximum of 250 ng of whole nucleic acid in a volume of 2.5 μl was used as a template for a single PCR reaction of 25 μl of total volume. Samples were run, at least, in duplicates. In each PCR run, a sample containing 2.5 ng of whole nucleic acid preparation from a BRAF V600E mutated case diluted in 250 ng of high-quality nucleic acid preparation from a wild type-BRAF case served as positive control. As a negative control, 250 ng of high-quality nucleic acid preparation from a wild type-BRAF case was used in addition to a no-DNA control (master mix only). PCR primers and Platinum Taq Polymerase were purchased from Invitrogen (Karlsruhe, Germany). In some positive cases, an additional 224-nt fragment of BRAF Exon 15 was amplified in order to confirm the V600E mutation by direct sequencing [25] using Beckman CEQ-8000 equipment and reagents.
Results
Tumor tissues from 290 patients (98 men, 192 women) with histologically verified PTCs were available. The BRAF V600E-mutation was detected in 122/290 (42%) patients while 20/137 (14.6%) and 15/93 (16.1%) expressed rearrangements of the proto-oncogenes RET or NTRK1, respectively. In four patients with either RET (three) or NTRK1 (one) rearrangement, the analysis of BRAF was inconclusive. One hundred forty one out of 290 (48.6%) PTCs demonstrated none of the genetic alterations studied (Table 1). Eight PTCs expressed two different mutations (1 RET/PTC + BRAF, 6 NTRK1 + BRAF, 1 RET/PTC + NTRK1).
Patients with PTC that expressed mutated BRAF were significantly older than patients with other mutations or without detectable mutations (Table 2). The mean age of patients with and without BRAF mutation was significantly different (51.4 and 46.5 years, p = 0.024). Only patients with a BRAF-positive tumor were less than 20 years of age at the time of diagnosis. Patients with RET rearrangements were diagnosed with a mean age of 41.85 years and were, therefore, younger than all other patients.
For the calculation of the tumor-specific survival and the impact of the extent of the disease, patients with two detected mutations were assigned to the probably dominant mutation pursuing the following order of significance: BRAF > RET > NTRK1. As a result, the group of patients with RET rearrangements is reduced by one and the number of patients with NTRK1 rearrangements by six patients, in favor of the patients with mutations of BRAF (Table 1).
The TNM classification for each group of patients is summarized in Table 3. Most conspicuous is the fact that all patients harboring RET/PTC hybrids did present with lymph node metastases at the time of diagnosis. Consequently, a significant difference was calculated with the Chi-square test for the presence or absence of lymph node metastases dependent on the type of underlying mutation (p = 0.004).
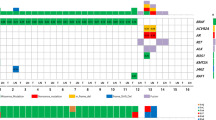
Follow-up data were available from 280 patients with a mean follow-up time of 5.5 years ranging from 0.02 to 22 years (Table 2). Of the 290 patients, 140 were free of disease at the time of the last follow-up while in 73/290 patients an increasing thyroglobulin level or imaging studies revealed residual tumor growth. In 77/290 patients this information was not available (Table 3). During the follow-up, 32 patients died because of progressive disease. Tumor-specific survival analysis demonstrated no significant difference in patient survival for either group of patients if all mutations were included (log-rank test, p = 0.514) or just patients with and without BRAF mutation were compared (log-rank test, p = 0.159) (Figs. 1, 2). However, while the survival curves were congruent for the first 10 years, they continuously separated thereafter.
Tumor-specific survival of patients dependent on the underlying mutation. Patients with double mutation were assigned to the assumed dominant mutation. The difference between the groups is not significant. However, after a follow-up of 10 years, a tendency towards a worse prognosis for BRAF-positive patients seems to become apparent
Tumor-specific survival of patients with and without a BRAF V600E mutation. The survival analysis confirms the impression of the analysis of all four groups of patients. Although the difference is not significant, the survival curves separate continuously after a follow-up of about 10 years indicating a possible impact of the mutation on the prognosis of the patients
Discussion
Papillary thyroid carcinomas are predominantly well-differentiated slow-growing malignancies that can be cured or controlled for a long time in the majority of patients leading to a very good overall prognosis. However, the spectrum of aggressiveness within this tumor entity is considerably broad, including neoplasias that demonstrate lymphatic and sometimes distant metastases early in the course of the disease and that may dedifferentiate with time leaving them resistant to radioiodine treatment. The diversity of the biological behavior of PTC triggers an ongoing discussion on the necessary extent of surgical treatment (e. g., lymph node dissection) and post-operative adjuvant therapy. It has frequently been speculated that the increasing knowledge on the genetic background of PTC may enable clinicians to select those patients who need more aggressive treatment from those who do not.
This hypothesis is nourished by molecular studies on the biology and progression of PTCs. Most intriguing is the fact that in contrast to BRAF V600E mutations, RET/PTC1 rearrangements were not detected in undifferentiated thyroid carcinomas indicating that some PTC may slowly dedifferentiate in the course of the disease while others stay stable. Moreover, an association of the BRAF V600E mutation and decreased expression of the sodium–iodine symporter—a key protein for the transmembranous transport of iodine into the cell—has been observed [2, 18, 21].
Most recently, an increasing number of studies have been published evaluating the frequency of the BRAF V600E mutation as well as other genetic changes in PTC and their possible impact on the prognosis [3, 9, 10, 12, 14]. Two reviews also summarized the almost consistent results describing an association of the BRAF mutation with aggressive growth pattern like frequent metastases and capsular invasion as well as decreased tumor-free survival [5, 11]. A single study reported a significant difference in tumor-specific survival when BRAF-positive and BRAF-negative tumors were compared [3]. In contrast to the majority of publications Ito et al. were not able to observe this correlation [8].
Not supporting the current literature results, our data indicate more frequent lymph node metastases in association with RET/PTC1 rearrangements. Nevertheless, the tumor-specific survival of this subgroup of patients is not impaired. Despite a great number of patients and sufficient follow-up time, the present study was also not able to demonstrate significant tumor-specific survival differences when BRAF-positive and BRAF-negative tumors were compared or additional mutations were taken into account as well. Nevertheless, the survival curves imply that the prognostic impact of the mutation may become obvious only after 10 years. It may, therefore, be possible that a significant survival benefit exists that is still obscured by the following facts:
-
1.
The overall good prognosis of PTC—only 32 patients died of disease-specific causes—may demand an increased number of patients and longer follow-up times in order to convey small but significant survival benefits.
-
2.
RET/PTC rearrangements predominantly occur in younger patients especially before the age of 20 while the BRAF mutation is almost exclusively detected in patients beyond the age of 20 years [4]. Patients with BRAF mutation are, therefore, at a greater risk to die of other causes in the follow-up.
-
3.
Aggressive treatment of all patients with PTC—most of the patients included in the study received a meticulous systematic lymph node dissection unless the tumor was less than 1 cm in size—guarantees a high cure rate even for aggressive cancers.
-
4.
Patients without detectable mutation represent a rather inhomogeneous group that includes, in fact, a number of different underlying mutations with unknown impact on the prognosis.
-
5.
Patients with more than one mutation or patients with multifocal diseases and varying expression of mutations in each tumor foci are difficult to categorize. For the analysis in this study, a dominant impact of the BRAF mutation was assumed. Omitting these patients from the analysis did not change the results (data not shown). However, the effect of multiple mutations is unknown.
A small group of patients presented in this study simultaneously expressed a BRAF V600E mutation and rearranged NTRK1 (six patients) or RET (one patient). In four patients with a RET rearrangement, the analysis of BRAF was inconclusive despite several tests of different tumor sites. Moreover, we observed a varying expression of BRAF in some patients with multifocal PTC indicating a multiclonal origin of the malignancy. The latter observation was also described previously [19]. Others observed multiple mutations predominantly in younger patients or in recurrent disease [7, 15]. At this time we are not able to determine in every case if the described simultaneous mutations occurred within one tumor of the same clonal origin or if multifocal tumors are present in these patients. The analysis of laser microdissected tumor tissue is planned to evaluate this problem. However, these groups of tumors indicate that a comprehensive genetic analysis including all known genetic alterations in PTC is necessary for all tumors included in the survival analysis.
Conclusion
The impact of underlying genetic mutation on the prognosis of patients with PTC has to be evaluated in the context of complex interactions of many different influencing factors in order to obtain significant and comprehensive results. Long-term follow-up data on large tumor panels are needed to achieve statistical significance of prognostic predications on PTC. This study provides further evidence that patients harboring BRAF-V600E-positive PTCs may experience an unfavorable course of the disease compared to patients with tumors carrying other genetic alterations. However, at this time the evidence is insufficient to permit changes of well-established treatment strategies.
References
Basolo F, Giannini R, Monaco C et al (2002) Potent mitogenicity of the RET/PTC3 oncogene correlates with its prevalence in tall-cell variant of papillary thyroid carcinoma. Am J Pathol 160:247–254
Durante C, Puxeddu E, Ferretti E et al (2007) BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab 92:2840–2843
Elisei R, Ugolini C, Viola D et al (2008) BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 93:3943–3949
Espadinha C, Santos JR, Sobrinho LG et al (2009) Expression of iodine metabolism genes in human thyroid tissues: evidence for age and BRAFV600E mutation dependency. Clin Endocrinol 70:629–635
Fugazzola L, Puxeddu E, Avenia N et al (2006) Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr-Relat Cancer 13:455–464
Guan H, Ji M, Bao R et al (2009) Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab 94:1612–1617
Henderson YC, Shellenberger TD, Williams MD et al (2009) High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res 15:485–491
Ito Y, Yoshida H, Maruo R et al (2009) BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J 56:89–97
Kebebew E, Weng J, Bauer J et al (2007) The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 246:466–470, discussion 470-461
Kim SK, Song KH, Lim SD et al (2009) Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditis. Thyroid 19:137–141
Lee JH, Lee ES, Kim YS (2007) Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer 110:38–46
Lee X, Gao M, Ji Y et al (2009) Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol 16:240–245
Lima J, Trovisco V, Soares P et al (2004) BRAF mutations are not a major event in post-Chernobyl childhood thyroid carcinomas. J Clin Endocrinol Metab 89:4267–4271
Lupi C, Giannini R, Ugolini C et al (2007) Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab 92:4085–4090
Moses W, Weng J, Khanafshar E et al (2010) Multiple genetic alterations in papillary thyroid cancer are associated with younger age at presentation. J Surg Res 160:179–183
Musholt PB, Imkamp F, Von Wasielewski R et al (2003) RET rearrangements in archival oxyphilic thyroid tumors: new insights in tumorigenesis and classification of Hurthle cell carcinomas? Surgery 134:881–889, discussion 889
Musholt TJ, Musholt PB, Khaladj N et al (2000) Prognostic significance of RET and NTRK1 rearrangements in sporadic papillary thyroid carcinoma. Surgery 128:984–993
Oler G, Cerutti JM (2009) High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer 115:972–980
Park SY, Park YJ, Lee YJ et al (2006) Analysis of differential BRAF(V600E) mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer 107:1831–1838
Pierotti MA, Vigneri P, Bongarzone I (1998) Rearrangements of RET and NTRK1 tyrosine kinase receptors in papillary thyroid carcinomas. Recent Results Cancer Res 154:237–247
Riesco-Eizaguirre G, Rodriguez I, De La Vieja A et al (2009) The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res 69:8317–8325
Salvatore G, Chiappetta G, Nikiforov YE et al (2005) Molecular profile of hyalinizing trabecular tumours of the thyroid: high prevalence of RET/PTC rearrangements and absence of B-raf and N-ras point mutations. Eur J Cancer 41:816–821
Santoro M, Papotti M, Chiappetta G et al (2002) RET activation and clinicopathologic features in poorly differentiated thyroid tumors. J Clin Endocrinol Metab 87:370–379
Sapio MR, Posca D, Raggioli A et al (2007) Detection of RET/PTC, TRK and BRAF mutations in preoperative diagnosis of thyroid nodules with indeterminate cytological findings. Clin Endocrinol 66:678–683
Sapio MR, Posca D, Troncone G et al (2006) Detection of BRAF mutation in thyroid papillary carcinomas by mutant allele-specific PCR amplification (MASA). Eur J Endocrinol 154:341–348
Tallini G, Santoro M, Helie M et al (1998) RET/PTC oncogene activation defines a subset of papillary thyroid carcinomas lacking evidence of progression to poorly differentiated or undifferentiated tumor phenotypes. Clin Cancer Res 4:287–294
Tang KT, Lee CH (2010) BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc 73:113–128
Author information
Authors and Affiliations
Corresponding author
Additional information
No potential conflicts of interest to be announced
Rights and permissions
About this article
Cite this article
Musholt, T.J., Schönefeld, S., Schwarz, C.H. et al. Impact of pathognomonic genetic alterations on the prognosis of papillary thyroid carcinoma. Langenbecks Arch Surg 395, 877–883 (2010). https://doi.org/10.1007/s00423-010-0682-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-010-0682-6