Abstract
Rearranged during transfection (RET) is a proto oncogene implicated in thyroid carcinogenesis of papillary type (PTC). The RET proto-oncogene in PTC is constitutively activated by fusion of its tyrosine kinase domain with the 5 ´region of another gene thereby generating chimeric products collectively named RET/PTCs. RET/PTC1 and RET/PTC3 are best characterized among all RET/PTC rearrangements. Kashmir valley has witnessed an alarming increase in thyroid cancer incidence in young women. Therefore, we investigated the occurrence of RET/PTC 1 & 3 rearrangements by semi quantitative and qPCR in thyroid cancer patients (n = 48) of Kashmiri population and interrelated results with various clinicopathological characteristics. We observed that all the RET/PTC rearrangements were confined to PTC cases (10/40). Presence of RET/PTC rearrangement significantly correlated with gender, elevated TSH levels and lymph node metastasis. Overall, our study advocates that RET/PTC3 rearrangement is a frequent event in the carcinogenesis of thyroid gland in Kashmiri population although a study with a larger sample size is needed to get a clear scenario.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is a very common malignancy of endocrine system with increasing incidence rate over the previous two decades. However, the mortality rates are not too high due to good screening techniques and early diagnosis [1]. The disease which is more common in women than in men could become the fourth most common cancer by 2030 in the United States [2]. Several risk factors have been attributed to the development of thyroid carcinomas such as exposure to ionizing radiations, iodine uptake, Hashimoto’s thyroiditis, diabetes, reproductive factors [3]. Main histotypes of thyroid cancers include Papillary thyroid cancer (PTC), Follicular thyroid cancer (FTC) and Anaplastic thyroid cancer (ATC). of which PTC accounts for 80% of all the cases worldwide [4]. In thyroid tumours the difference between benign and malignant disease is largely based on a cytological assessment of thyroid cells obtained by fine needle aspiration cytology (FNAC). However many benign thyroid nodules show morphological features of PTC making FNAC inconclusive. Genetic markers especially pathogenic oncogenes known for thyroid cell transformation improve FNAC precision [5].
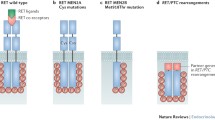
One of the first oncogenes revealed to have a role in PTC was RET/PTC. It is one of the established genetic markers exclusively of PTC. The RET (rearranged during transfection) is a proto-oncogene which encodes a cell membrane receptor tyrosine kinase. The RET protein encompasses an ligand-binding extracellular domain followed by a transmembrane region (a cysteine-rich loop) and an intracellular domain (containing the juxtamembrane domain and the tyrosine kinase domain) [6]. Activation of RET stimulates mitogen-activated protein kinase (MAPK) and PI3K pathways that promote cell growth, differentiation, proliferation and cellular survival [7]. In PTC, the fusion of the Receptor tyrosine kinase domain with the 5′ end sequence of one of the different heterologous genes via rearrangements creates a series of at least 12 chimeric oncogenes jointly branded as RET/PTC. RET/PTC1 and RET/PTC3 being most common variants result from the fusion of tyrosine kinase domain of RET with H4 (D10S170) and ELE1 gene respectively [8].
Kashmir valley has witnessed an alarming increase in PTC over the last few years with 75% of the patients being young females. In our previous studies we have elucidated the downstream gladiators of RET such as BRAF and RAS genes in our population, wherein, the BRAF mutation accumulated to 25% with absence of any RAS Mutation [9, 10]. Therefore, in this study, we determined the prevalence of RET/PTC1 and PET/PTC3 rearrangements as a possible cause for PTC in Kashmiri population and interrelated results with various clinicopathological factors.
Materials and Methods
Sample Collection
Forty-eight (n = 48) thyroid tumour and their adjacent normal tissues were collected from the Department of General and Minimal Invasive Surgery, SKIMS, Srinagar and Department of ENT, SMHS, Govt. Superspeciality Hospital, Srinagar. All the samples were histopathologically confirmed. Tissue samples were collected and immediately stored at −80 °C till further use. Chemo or radiotherapy was not received by any patient.
RNA Extraction, cDNA Synthesis and Semiquantitative PCR
RNA was extracted using trizol reagent (Invitrogen Inc.) (Fig. 1) and cDNA was synthesized using single strand cDNA synthesis kit (Thermo Scientific Ltd.) according to manufacturer’s guidelines. Briefly, 1 μg RNA was reverse-transcribed using AMV Reverse transcriptase and oligo dT primers in a final volume of 20 μl. For semi-quantitative PCR the cDNA synthesized was subjected to routine endpoint PCR. The amplification reaction was carried out in 25 μl reaction volume which included 50 mM Mgcl2, 10 mM dNTP mix, 1 U Taq Polymerase, 10 mM primers specific for RET/PTC1, RET/PTC3 and β-actin. Primer sequences were; RET/PTC1 Forward: 5’-ATTGTCATCTCGCCGTTC-3′, Reverse 5’-TGCTTCAGGACGTTGAAC-3′; RET/PTC3 Forward: 5’-TGGAGAAGAGAGGCTGTATC-3′, Reverse 5’-CGTTGCCTTGACTTTTC-3′; β-actin Forward: 5’-TGCGTGACATTAAGGAGAAG-3′, Reverse: 5’-GCTCGTAGCTCTTCTCCA-3′. PCR Conditions were: 95 °C for 10 min, 35 cycles of 94 °C for 30 s, 60 °C for 45 s, 72 °C for 30 s, and 72 °C for 10 min. The amplicons were resolved using 2% agarose gels containing ethidium bromide and finally visualized by gel documentation system (Fig. 2).
Quantitative Real-Time PCR (qRT-PCR)
qRT-PCR was performed on samples which were positive for RET/PTC rearrangements by semi quantitative PCR. qRT-PCR assay was performed by SYBR Green master mix qRT-PCR kit (Thermo Scientific Ltd.) as per maker’s instruction. Briefly 02 μl of cDNA was mixed with distilled water, SYBR Green Master Mix and primers and reaction mixture brought to a final total volume of 25 μl. PCR and data acquisition was performed by Piko Real Real-Time PCR (Thermo Scientific Ltd.). qRT-PCR data were quantitated using the relative quantification by ΔΔCT method. One positive sample of RET/PTC3 rearrangements identified by semi-quantitative PCR served as a reference standard (calibrator). β-Actin mRNA was used as an internal control. qRT-PCR reactions were carried out in triplicates, and no template control (NTC) was included each time. For ensuring single product formation Melt curve analysis was performed (Fig. 3).
Statistical Analysis
Pearson’s χ2 test, Fisher’s exact test or χ2 test (trend) were used for discrete variables; independent t-test and paired t-test for continuous variables. Bivariate logistic regression analysis was used to obtain odds ratios (ORs) and 95% confidence intervals (CIs). P ≤ 0.05 was marker of significance. Statistical tests were performed using the software SPSS 16.0 (SPSS Inc., Chicago, Illinois).
Results
Patient Features
In this study thyroid cancer cases (n = 48) which histologically confirmed and their adjacent normal tissues were analyzed for RET/PTC rearrangements. The cases included 40 females and 08 males. 40 of 48 (83.3%) subjects were < 45 years and 8 of 48 (16.7%) were ≥ 45 years had a mean age of 24 ± 38. No patient had a history of exposure to any kind of ionizing radiations.at the time of diagnosis. Table 1 contains the clinicopathological characteristics of the study subjects.
RET/PTC1 & 3 Detection
We investigated RET/PTC rearrangements in 40 PTC, 03 FTC and 05 other types of thyroid cancer cases and their histologically verified adjacent normal tissues. RET/PTC1 rearrangement was absent in our population. Only RET/PTC3 rearrangements were observed confined to 20.83% (10/48) of the cases (Fig. 1). RET/PTC3 rearrangement was not observed in any of the normal tissues. qPCR analysis was performed for all the samples which were positive for RET/PTC3 rearrangements by semi-quantitative PCR. All the samples found positive for rearrangements by semi quantitative PCR gave positive signal for qRT-PCT. Further, the relative quantification of the data showed that there was no significant difference between the expression levels of RET/PTC3-positive samples and positive control (calibrator). Melt curve analysis showed that there was no production of any nonspecific template (Fig. 2).
RET/PTC3 Rearrangement and Correlation with Clinicopathological Characteristics
The relationship between RET/PTC3 rearrangements and clinicopathological characteristics is given in Table 2. A statistical significance was observed with gender [Adj OR = 5.6, 95% CI (1.1–19.1) (P = 0.04)], elevated TSH levels [Adj OR = 0.09, 95% CI (0.01–0.5) (P = 0.003)] and lymph node metastasis [Adj OR = 0.2, 95% CI (0.03–1.08) (P = 0.049)]. But, the association of RET/PTC3 rearrangements with age, dwelling, smoking status, the presence of benign thyroid disease, histological types, grade, stage and vascular invasion was not statistically significant.0.
Discussion
The identification of various molecular mechanisms in the thyroid cell transformation is critical for understanding the pathogenesis of this disease. Several genetic alterations have been reported in thyroid cancers such as mutations in RAS, BRAF, P53, TSHR genes [4, 11]. However, RET/PTC oncogene is particularly important for its specificity in thyroid neoplasms. RET/PTC rearrangements have been hallmarks of PTCs [12, 13]. The RET/PTC oncoproteins are constitutively phosphorylated receptors produced by rearrangements of RET with a range of activating genes. These rearrangements in PTC have been strongly associated with ionizing radiations as seen in atomic bomb children survivors of post-Chernobyl nuclear disaster [14,15,16].
In the present study we investigated the prevalence of RET/PTC1 and RET/PTC3 rearrangements in thyroid cancers in northern India (Kashmir) and interrelated the results with their various clinicopathological features. To our knowledge this is the first study conducted in Kashmir valley regarding the occurrence of RET/PTC rearrangements in thyroid carcinogenesis. Out of 48 thyroid cancer cases RET/PTC1 rearrangement was absent but RET/PTC3 rearrangement was found in 20.83% (10/48) of cases which were confined to PTC only. The study conducted on German population showed low frequency of RET/PTC rearrangements in PTC with only 7% RET/PTC1 and 1% RET/PTC3 positivity [17]. Similar low frequency results have been observed in France (11%) and Saudi Arabia (3%) [6, 18]. Studies conducted from Taiwan showed a high frequency of RET/PTC1 & 2 rearrangements in PTC (55%) [19]. Similar high frequency results have been observed in New Caledonian (70%) and Australian population (85%) and Italy (33–35%) [6, 20]. Studies done on Korean population found RET/PTC1, RET/PTC2 and RET/PTC3 rearrangements to be 6.5%, 6.5% and 0% respectively whereas studies from Japanese population showed prevalence of RET/PTC rearrangements to be 30% in PTC [21, 22]. RET/PTC rearrangements have been reported to be very high (69% to 83%) in areas exposed to radiations [16, 23]. This heterogeneity in RET/PTC rearrangements may be due to ethnicity, different geographical location, environmental exposure and techniques employed to detect RET/PTC rearrangements. Although majority of studies support the fact that RET/PTC rearrangements occurs due to double-stranded break in each gene participating in rearrangement but there are studies who have shown that in addition to ionizing radiations, other recognized carcinogens, such as caffeine, ethanol, hypoxia, can also induce double-stranded DNA breaks and generate RET/PTC rearrangements [24] which are in consistency with our study where all the patients with RET/PTC3 rearrangements had no history of exposure to any kind of radiations.
In our study we also correlated RET/PTC3 positivity with various clinicopathological characteristics. RET/PTC3 rearrangements were significantly associated with gender, lymph node metastasis and elevated TSH levels (P ≤ 0.05). Our results are in coherence with Adeniran et al. who reported that lymph node metastasis is significantly associated with RET/PTC rearrangements [25]. Su et al. reported that high prevalence of RET/PTC rearrangements is associated with female gender and younger age [26]. Although statistically insignificant, the RET/PTC3 rearrangements were restricted only to PTC (P > 0.05) in uniformity with the majority of studies [14, 15, 27] but not in full agreement with few studies who established that RET/PTC rearrangements are present in other variants such as FTC, MTC, hoshimotos thyroiditis, adenomas and goiter in addition to PTC [28,29,30,31]. In agreement with our observation several other studies did not show any link between age, tumor size, staging, history of benign thyroid disease histological subtype smoking, alcohol consumption and RET/PTC rearrangements [32, 33].
In summary, this is the first study regarding the quantitative analysis of rearranged forms of RET in thyroid carcinoma from this part of the world which is very imperative for understanding the role of RET activation in thyroid carcinogenesis. Our results indicate that RET/PTC3 rearrangements were restricted to PTC. There was a significant association between RET/PTC3 rearrangements and the clinicopathological parameters like, female gender, elevated TSH levels and lymph node metastasis. Although the observations are quite promising but a study with a larger sample size is needed to validate our results.
References
Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R (2013) Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013:1–10. https://doi.org/10.1155/2013/965212
La Vecchia C, Malvezzi M, Bosetti C et al (2015) Thyroid cancer mortality and incidence: a global overview. Int J Cancer 136:2187–2195. https://doi.org/10.1002/ijc.29251
Liu Y, Su L, Xiao H (2017) Review of factors related to the thyroid Cancer epidemic. Int J Endocrinol 2017:1–9. https://doi.org/10.1155/2017/5308635
Xing M (2013) Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13:184–199. https://doi.org/10.1038/nrc3431
Marotta V, Guerra A, Sapio MR, Vitale M (2011) RET/PTC rearrangement in benign and malignant thyroid diseases: a clinical standpoint. Eur J Endocrinol 165:499–507 https://doi.org/10.1530/EJE-11-0499
Santoro M, Carlomagno F (2013) Central role of RET in thyroid cancer. Cold Spring Harb Perspect Biol 5:1–17. https://doi.org/10.1101/cshperspect.a009233
Kurokawa K, Kawai K, Hashimoto M, Ito Y, Takahashi M (2003) Cell signalling and gene expression mediated by RET tyrosine kinase. J Intern Med 253:627–633
Nikiforov YE (2002) RET/PTC rearrangement in thyroid tumors. Endocr Pathol 13:3–16. https://doi.org/10.1385/EP:13:1:03
Tong G-X, Mody K, Wang Z, Hamele-Bena D, Nikiforova MN, Nikiforov YE (2015) Mutations of TSHR and TP53 genes in an aggressive clear cell follicular carcinoma of the thyroid. Endocr Pathol 26:315–319 https://doi.org/10.1007/s12022-015-9388-1
Khan MS, Pandith AA, Azad N, Hussain MU, Masoodi SR, Wani KA, Andrabi KI, Mudassar S (2014) Impact of molecular alterations of BRAF in the pathogenesis of thyroid cancer. Mutagenesis 29:131–137
Khan MS, Pandith AA, Hussain MU, Iqbal M, Khan NP, Wani KA, Masoodi SR, Mudassar S (2013) Lack of mutational events of RAS genes in sporadic thyroid cancer but high risk associated with HRAS T81C singlenucleotide polymorphism (case–control study). Tumor Biol 34:521–529
Jhiang SM (2000) The RET proto-oncogene in human cancers. Oncogene 19:5590–5597. https://doi.org/10.1038/sj.onc.1203857
Romei C, Ciampi R, Elisei R (2016) A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol 12:192–202 https://doi.org/10.1038/nrendo.2016.11
Thomas GA, Bunnell H, Cook HA et al (1999) High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab 84:4232–4238. https://doi.org/10.1210/jcem.84.11.6129
Hamatani K, Eguchi H, Ito R, Mukai M, Takahashi K, Taga M, Imai K, Cologne J, Soda M, Arihiro K, Fujihara M, Abe K, Hayashi T, Nakashima M, Sekine I, Yasui W, Hayashi Y, Nakachi K (2008) RET/PTC rearrangements preferentially occurred in papillary thyroid Cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res 68:7176–7182 https://doi.org/10.1158/0008-5472.CAN-08-0293
Bounacer A, Wicker R, Schlumberger M, Sarasin A, Suárez HG (1997) Oncogenic rearrangements of the ret proto-oncogene in thyroid tumors induced after exposure to ionizing radiation. Biochimie 79:619–623. https://doi.org/10.1016/S0300-9084(97)82012-3
Mayr B, Pötter E, Goretzki P, Rüschoff J, Dietmaier W, Hoang-Vu C, Dralle H, Brabant G (1998) Expression of ret/PTC1, −2, −3, −delta3 and −4 in German papillary thyroid carcinoma. Br J Cancer 77:903–906
Zou M, Shi Y, Farid NR (1994) Low rate of ret proto-oncogene activation (PTC/retTPC) in papillary thyroid carcinomas from Saudi Arabia. Cancer 73:176–180
Lee CH, Hsu LS, Chi CW et al (1998) High frequency of rearrangement of the RET protooncogene (RET/PTC) in Chinese papillary thyroid carcinomas. J Clin Endocrinol Metab 83:1629–1632. https://doi.org/10.1210/jcem.83.5.4774
Chua EL, Wu WM, Tran KT et al (2000) Prevalence and distribution of ret/ptc 1, 2, and 3 in papillary thyroid carcinoma in New Caledonia and Australia. J Clin Endocrinol Metab 85:2733–2739. https://doi.org/10.1210/jcem.85.8.6722
Detection of RET/PTC oncogene rearrangements in Korean papillary thyroid carcinomas. - PubMed - NCBI. https://www.ncbi.nlm.nih.gov/pubmed/10646664. Accessed 5 Apr 2018
Wajjwalku W, Nakamura S, Hasegawa Y, Miyazaki K, Satoh Y, Funahashi H, Matsuyama M, Takahashi M (1992) Low frequency of rearrangements of the ret and trk proto-oncogenes in Japanese thyroid papillary carcinomas. Jpn J Cancer Res Gann 83:671–675
Smida J, Salassidis K, Hieber L, Zitzelsberger H, Kellerer AM, Demidchik EP, Negele T, Spelsberg F, Lengfelder E, Werner M, Bauchinger M (1999) Distinct frequency of ret rearrangements in papillary thyroid carcinomas of children and adults from Belarus. Int J Cancer 80:32–38
Gandhi M, Dillon LW, Pramanik S, Nikiforov YE, Wang YH (2010) DNA breaks at fragile sites generate oncogenic RET/PTC rearrangements in human thyroid cells. Oncogene 29:2272–2280 https://doi.org/10.1038/onc.2009.502
Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, Biddinger PW, Nikiforov YE (2006) Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol 30:216–222
Su X, Li Z, He C, Chen W, Fu X, Yang A (2016) Radiation exposure, young age, and female gender are associated with high prevalence of RET/PTC1 and RET/PTC3 in papillary thyroid cancer: a meta-analysis. Oncotarget 7:16716–16730. https://doi.org/10.18632/oncotarget.7574
Tallini G, Asa SL (2001) RET oncogene activation in papillary thyroid carcinoma. Adv Anat Pathol 8:345–354
Grubbs EG, Ng PK-S, Bui J, Busaidy NL, Chen K, Lee JE, Lu X, Lu H, Meric-Bernstam F, Mills GB, Palmer G, Perrier ND, Scott KL, Shaw KR, Waguespack SG, Williams MD, Yelensky R, Cote GJ (2015) RET fusion as a novel driver of medullary thyroid carcinoma. J Clin Endocrinol Metab 100:788–793. https://doi.org/10.1210/jc.2014-4153
Guerra A, Sapio MR, Marotta V et al (2011) Prevalence of RET/PTC rearrangement in benign and malignant thyroid nodules and its clinical application. Endocr J 58:31–38
Wirtschafter A, Schmidt R, Rosen D, Kundu N, Santoro M, Fusco A, Multhaupt H, Atkins JP, Rosen MR, Keane WM, Rothstein JL (1997) Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto’s thyroiditis. Laryngoscope 107:95–100
Sheils OM, O’Leary JJ, Uhlmann V et al (2000) Ret/PTC-1 activation in Hashimoto thyroiditis. Int J Surg Pathol 8:185–189. https://doi.org/10.1177/106689690000800305
Basolo F, Molinaro E, Agate L, Pinchera A, Pollina L, Chiappetta G, Monaco C, Santoro M, Fusco A, Miccoli P, Elisei R, Capezzone M, Pacini F (2001) RET protein expression has no prognostic impact on the long-term outcome of papillary thyroid carcinoma. Eur J Endocrinol 145:599–604
Puxeddu E, Moretti S, Giannico A, Martinelli M, Marino C, Avenia N, Cristofani R, Farabi R, Reboldi G, Ribacchi R, Pontecorvi A, Santeusanio F (2003) Ret/PTC activation does not influence clinical and pathological features of adult papillary thyroid carcinomas. Eur J Endocrinol 148:505–513
Acknowledgments
The authors acknowledge the surgical staff of the Department of General Surgery, SKIMS and ENT, SMHS hospital who facilitated the tissue sampling and the scientific staff of Department of Biotechnology, University of Kashmir who helped in standardizing the qPCR conditions.
Funding
The Study was supported by the Department of Biotechnology, Ministry of Science and Technology, Govt. of India (BT/PR4626/MED/30/702/2012 Dated: 07.08.2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There is no conflict of interest.
Ethical Approval
The study was approved by the Ethical Clearance Committee of SKIMS.
Informed Consent
All the samples were collected after taking written informed consent from the patients and proper ethical procedures were followed.
Rights and permissions
About this article
Cite this article
Khan, M.S., Qadri, Q., Makhdoomi, M.J. et al. RET/PTC Gene Rearrangements in Thyroid Carcinogenesis: Assessment and Clinico-Pathological Correlations. Pathol. Oncol. Res. 26, 507–513 (2020). https://doi.org/10.1007/s12253-018-0540-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0540-3