Abstract
Introduction
Pancreaticoduodenectomy is one of the more complicated operations that exists in surgery, and is fraught with potential morbidity, the most well-known, and dreaded, of which is the pancreatic leak. While much of the risk associated with pancreatic leak is inherent to the operation, there have been no shortage of techniques employed by surgeons to try to mitigate that risk.
Methods
We focused on four topics of greatest conjecture with regard to reconstruction after pancreaticoduodenectomy: (1) the type of anastomosis, (2) the enteral organ to which the pancreas is sewn, (3) whether to preserve the pylorus and (4) whether or not to use anastomotic silastic stents. We identified the most relevant randomized control trials on each topic, which were appropriately powered.
Results
We identified a total of 15 studies for evaluation, (type of anastomosis: n = 4; enteral organ to which the pancreas is sewn: n = 4; whether to preserve the pylorus, n=3; and whether or not to use anastomotic silastic stents, n = 4). In each group of comparisons, there was no definitive conclusion to be made on superiority of reconstruction.
Conclusion
While clear consensus on how best to reconstruct the anatomy after pancreaticoduodenectomy has not yet been reached, we present the following review in the hope of providing some understanding of the literature for the pancreatic surgeon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are few more hotly debated topics among pancreatic surgeons than the method by which they anastomose the pancreatic remnant after pancreaticoduodenal resection. While there are many retrospective series, meta-analyses, and even some well-conducted randomized control trials (RCTs), most surgeons will favor their chosen anastomosis based on mentorship and anecdotal experience. Regardless of technique, most pancreatic surgeons generally accept a 10–20% rate of pancreatic fistula, of which the minority are clinically significant.
What has become known as the Whipple operation (and we will forgo the debate on its true origins1) is performed for a variety of conditions, both benign and malignant, with varying anatomical and a number of clinical and patient-centered factors that make true comparisons between patients difficult to investigate. Furthermore, to appropriately power a study evaluating a 50% reduction in clinically relevant pancreatic fistula, one would need to enroll several hundred (if not thousand) patients—a feat that even in the highest volume centers would be hard to attain while still minimizing variability, especially in the operating surgeon. However, there have been several well-conducted studies that can inform the pancreatic surgeon on how best to approach their reconstruction.
In this article we focus on three aspects of anastomotic technique. First, we discuss duct-to-mucosa versus invagination pancreaticojejunostomy, followed by pancreaticogastrostomy as it compares to pancreaticojejunostomy. We then follow with comparisons between pylorus preservation technique and more classic Whipple resections, and finally we discuss the use of internal and external anastomotic stents.
Duct-to-Mucosa Versus Invagination Pancreaticojejunostomy
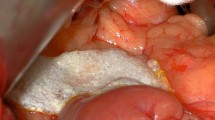
The two most utilized, and most often debated, techniques for pancreaticojejunostomy involve variations of either the duct-to-mucosa pancreaticojejunostomy and the dunking or invagination pancreaticojejunostomy (Table 1). Those who prefer the former (Fig. 1), generally do so because of the direct communication of the duct to the enterostomy, while those who prefer the latter often cite the ability to control leaks from the cut surface of the pancreas (Fig. 2).
Duct-to-mucosa pancreaticojejunostomy: a posterior row of nonabsorbable sutures placed from pancreatic parenchyma to serosal surface of jejunum in an end-to-side fashion, b an inner-layer duct-to-mucosa anastomosis to an enterotomy in full-thickness fashion, and c an anterior layer of nonabsorbable sutures placed from pancreatic parenchyma to serosal surface of jejunum
In 2003, Bassi et al.2 performed an RCT evaluating 144 patients who were randomly assigned to either a two-layer pancreaticojejunal duct-to-mucosa anastomosis (group A) or a single-layer pancreaticojejunal (invagination) anastomosis (group B). The primary endpoint was pancreatic fistula and attained a power of 50% (α = 5%, β = 20%). Indications for the operations and the demographics between groups were similar. Notably, most patients in both groups (90.3% and 86.1%, respectively) underwent a pylorus-preserving operation. Bassi and colleagues found no differences in the outcomes of pancreatic fistula or other significant morbidity or mortality between groups. In their discussion, the authors add that a subgroup analysis evaluating those patients who had anastomotic stenting revealed a higher rate of morbidity and a 50% higher rate of fistula formation for patients who underwent stenting. As this was not a primary or secondary endpoint, and the use of stents was left to the discretion of the operating surgeon, we must allow for inherent operator bias to these data.
Not long after Bassi in 2005, Langrehr and colleagues evaluated a novel single-layer mattress technique for invaginating the pancreas into the jejunum, which minimized shear forces on the pancreas, and performed an RCT to compare with the two-layer, duct-to-mucosa anastomosis.3 In their study of 113 patients, they also had a variety of indications for the operation, although the majority (71.7%) were for malignancy. The authors detected no significant difference in medical or surgical outcomes, including pancreatic fistula and hemorrhage. However, the study was underpowered, and the authors suggest fewer complications occurred with their modified invagination technique.
One of the larger randomized control trials to compare these two anastomotic techniques was done by Berger and colleagues in 2009.4 In their study of 197 patients between two large-volume institutions, they compared a two-layer invaginated pancreaticojejunostomy and a two-layer duct-to-mucosa anastomosis. Most patients underwent pylorus preservation (88%), and 72% of the operations were done for malignant disease. There were no differences in demographics or comorbidities, and the authors demonstrated a near 50% reduction in overall pancreatic fistula rates (24% versus 12%, p = 0.04) and ISGPF5 grade B fistulas (14% versus 5%, p = 0.03).
A more recent randomized trial to evaluate these two anastomotic techniques was done in 2011 by El Nakeeb et al.6 and evaluated a two-layer duct-to-mucosa technique and a two-layer end-to-side technique. In the latter case, the pancreatic parenchyma was sutured to the mucosa as an inner layer and followed with an outer layer suturing the capsule to the serosa. There were 107 patients included, and the authors found no difference in pancreatic fistula rates, morbidity, or mortality between groups. The authors did note a higher rate of steatorrhea in the duct-to-mucosa group at 1 year (42–22%, p = 0.04), but no decrease in the rate of pancreatic fistula.
Pancreaticogastrostomy Versus Pancreaticojejunostomy
Another common anastomotic technique is the pancreaticogastrostomy (PG) (Table 2), in which the pancreatic parenchyma is most commonly sewn in a single or double layer to a gastrotomy created on the posterior aspect of the stomach (Fig. 3). The preference for this over a pancreaticojejunostomy (PJ) is the presumed inactivation of pancreatic enzymes, as there is no enterokinase in the stomach, and the low pH of stomach acid acts to prevent activation of the cascade. Detractors often point out that when a clinically significant leak occurs, feeding the patient orally is problematic, and enteral access distal to the anastomosis or parenteral nutrition is often required. In 1995, the surgeons at Johns Hopkins conducted an RCT evaluating PG to PJ in 145 patients (PJ = 72, PG = 73).7 There was some variability in the type of PJ, with 48 patients undergoing end-to-end anastomosis, and 24 patients undergoing end-to-side anastomosis, but these patients were grouped together for comparison to PG. There was also variability in pylorus preservation, with 119 patients undergoing pyloric-preserving operation and 26 undergoing classic operation, but there was no difference seen between groups. The primary outcome, pancreatic fistula as identified by chemical analysis of the drain fluid or radiographic evidence of pancreatic disruption. The authors found no difference in the rates of pancreatic fistula (PG: 12.3%, PJ: 11.7%, p = NS), nor did they find any difference in any of the secondary endpoints of complication rate (49% versus 43%), length of stay (17.1 versus 17.7 days), or any other of the postoperative parameters measured. The authors concluded that there was no benefit to either approach but did identify two factors which impacted pancreatic fistula development: individual surgeon volume and duodenal or ampullary disease. They found that operations undertaken by less experienced surgeons and those for disease not infiltrating or obstructing the main pancreatic duct were more likely to lead to pancreatic leak.
In addition to their study on pancreaticojejunostomy above, Bassi et al. also compared PG with PJ. In this study, they randomized 151 patients who had what was considered intraoperatively to be a soft pancreatic gland.8 There were 69 patients in the PG group, while there were 82 patients in the PJ group. Most patients in both groups underwent a pylorus-preserving resection, and the authors found no difference in the rates of pancreatic fistula (PG 13% versus PJ 16%). They did note a higher number of complications in the PJ group (39% versus 29%), although this difference did not achieve significance. The authors concluded that, while there was no significant difference in the two anastomotic techniques, PG did limit the number of associated complications, such as biliary fistula and delayed gastric emptying.
In 2008, Fernandez-Cruz et al. used a modified version of the traditional pancreaticogastrostomy by creating a gastric partition along the greater curvature, which acted as a conduit for the pancreaticogastrostomy anastomosis (n = 53), which was fashioned in two layers, with a duct-to-mucosa inner layer.9 This was compared with traditional pancreaticojejunostomy (n = 55), also with a duct-to-mucosa inner layer. There were no significant differences between groups in terms of preoperative or perioperative variables. The authors found a much greater rate of postoperative complications (PJ: 44% versus PG:23%; p = 0.01) as well as pancreatic fistula (18% versus 4%; p = 0.01). The authors concluded that their modified PG anastomosis was superior to traditional PJ in terms of pancreatic fistula rates and overall complications.
The RECOPANC study10 was a multi-institutional RCT with long-term follow-up, which randomized 440 patients (of whom 320 were included in the final analysis) from 14 centers in Germany. Patients were randomized to either pancreatojejunostomy (n = 149) or pancreatogastrostomy (n = 171). Their primary endpoint was clinically relevant (ISGPF B or C) pancreatic fistula and had secondary endpoints of pancreatic function and quality of life (QoL) up to 1 year. The group found no difference in rates of clinically relevant fistula (PG 20% versus PJ 22%, p = 0.62). They did see higher rates of bleeding events and perioperative stroke in PG patients, but with an overall improved QoL in these patients.
Pylorus-Preserving Pancreaticoduodenectomy Versus Classic Whipple
Beyond pancreatic leak, one of the main contributors to extended length of stay and postoperative morbidity is delayed gastric emptying (DGE). Although initially described by Watson in 1944,11 the pylorus-preserving pancreaticoduodenectomy (PPPD) was less commonly employed than the classic Whipple (CW) operation which involves a partial gastrectomy (Table 3). It has been thought that PPPD (Fig. 4) improves weight gain, limits dumping syndrome, and minimizes peptic ulcers.12 However, it has also been associated with increased rates of delayed gastric emptying and anastomotic ulcers,13 as well as the possibility of higher positive margin rates, although this is not borne out in the literature. Several prospective randomized trials, mostly single institution, have evaluated the outcomes of PPPD and classic Whipple.
Tran and colleagues performed a multicenter trial to evaluate PPPD to CW in 2004,14 with primary endpoints including operative time, blood loss, length of stay, and DGE. They evaluated 170 (PPPD: n = 87, CW: n = 83) patients with pancreatic or periampullary tumors who underwent curative intent surgery over an 8-year period. Two patients were converted from PPPD to CW due to intraoperative factors. The authors found no significant differences in any of their primary endpoints, including DGE (PPPD: 22% versus CW: 23%; p = 0.80), and found no difference in margin positivity or long-term survival. The authors concluded that both operations were equally effective for pancreatic cancers.
Seiler and colleagues have also compared PPPD with CW.15 Their study in 2005 compared a final cohort of 130 patients (PPPD: 64, CW: 66) with primary endpoints of perioperative morbidity and secondary endpoints including quality of life and resumption of work and normal activities. They found no significant differences in perioperative morbidity, including DGE (PPPD: 31%, CW: 45%, p = 0.10) and overall morbidity (68.2% versus 54.7% versus 68.2%, p = 0.07), but did note a shorter operating time, less need for blood transfusion, and higher rates of resumption of work at 6 months postoperatively. Still, the authors concluded that both PPPD and CW were equally effective and had no difference in long-term results.
In 2011, Kawai et al published their study comparing PPPD with a pyloric ring resection (PrPD) with preservation of the majority of the stomach with a primary endpoint of DGE.16 They enrolled 130 patients (PPPD: n = 64, PrPD: n = 66) and evaluated all patients with an upper gastrointestinal emptying study using gastrograffin. In addition, the authors evaluated all patients with 13C-acetate breath tests at 1, 3, and 6 months postoperatively. Secondary endpoints included quality of life and mortality, among others. The authors found a significant difference in DGE rates between groups (PPPD: 17.2% versus PrPD: 4.5%; p = 0.02) but did not see a difference in nasogastric tube, initiation of solid foods or length of stay. In addition, there was no difference in QoL, major morbidity, or mortality. The authors conclude that PPPD was associated with a higher rate of DGE.
Use of Pancreatic Anastomotic Stents
As pancreatic fistula is one of the most feared complications of the pancreaticoduodenectomy, some have postulated that pancreatic duct stenting at the time of anastomosis would be of benefit, especially in high-risk glands such as those with a small pancreatic duct or a soft pancreas. There are two common modalities employed for pancreatic duct stenting, both of which generally employ a soft, small caliber, silastic pediatric feeding tube. The first of these is to stent the pancreatic duct and bring out the stent through the abdominal wall to a drainage bag. The second is to cut a smaller piece of the tube and stent across the anastomosis (Fig. 5) with the expectation that the stent will eventually traverse the enteric system and be excreted in the feces.
Stenting of the pancreatic duct with pediatric feeding tube, in either a external or b internal fashion. External stents are brought out through the abdominal wall to a drainage bag, while internal stents are sutured in place with an absorbable suture, allowing enteral passage when the suture has dissolved
One of the most frequently cited studies was done by Winter et al., published in 2006.17 In their study, they enrolled 238 patients, of whom 115 were stented (S) and 119 were nonstented (NS). The anastomotic technique was most commonly end-to-side, two-layer pancreaticojejunostomy, although there was variability in whether invagination technique or a duct-to-mucosa technique were employed. The authors stated that the trial was stopped early due to a real possibility of harm in patients with soft glands where stents were used (S: 21.0% versus NS: 10.7%, p = 0.13). The group found no difference in mortality (1.8% versus 3.5%) or complication rates (57.4% versus 58.0%). The conclusion by the authors was that there was no benefit to pancreatic anastomotic stenting and that there was potential for higher rates of pancreatic fistula in patients with soft glands.
Not long after the Winter et al. study, Poon and colleagues evaluated the role of external pancreatic drainage via stenting (ES) as compared with no stent (NS).18 This study included 120 patients (ES: 60, NS: 60) and all patients underwent a duct-to-mucosa pancreaticojejunostomy. The primary endpoint was postoperative pancreatic fistula and was further classified as either clinical or subclinical in nature. Secondary endpoints included morbidity, mortality, and hospital stay, among others. The authors found a significant reduction in the rate of pancreatic fistula (6.7% versus 20.0%, p = 0.03) and clinically relevant pancreatic fistula (3.3% versus 15.0%; p = 0.03). There was no difference in morbidity or in-hospital mortality, but the authors did report a higher length of stay in intensive care unit (ICU) and hospital. The authors conclude that pancreatic anastomotic stenting with external drainage significantly decreased pancreatic fistula and decreased length of stay for patients undergoing a Whipple operation.
Similar in design to the study by Poon, Pessaux and colleagues evaluated 158 patients who either had an externalized stent (ES, n = 77) or no stent (NS, n = 81).19 Their primary endpoint was also the rate of pancreatic fistula and was defined as a drain amylase higher than three times serum levels and graded according to ISGPF guidelines.20 Similar to Poon, this analysis revealed a far lower rate of pancreatic fistula in the ES group (26% versus 42%; p = 0.03), as well as clinically relevant fistula (24.6% versus 35.8%; p = 0.03) and overall morbidity (41.5% versus 61.7%; p = 0.01). The authors concluded that anastomotic stenting with external drainage reduced pancreatic fistula rates and should be used in patients with soft pancreatic texture and a nondilated pancreatic duct.
One study which compared internal anastomotic stenting (IS) and anastomotic stenting with external drainage (ES) was conducted by Tani et al. 21. In their analysis, all patients underwent duct-to-mucosa anastomosis, although there was variability in whether PPPD or CW as employed during resection. Interestingly, the primary endpoint for this study was length of hospital stay with a secondary endpoint being pancreatic fistula rate. The authors analyzed 99 patients (ES: n = 49, IS, n = 50). They found no difference in pancreatic fistula rates between groups (ES: 20% versus IS: 26%, p = NS). The rate of clinically relevant pancreatic fistula was also similar (6% versus 6%; p = NS). They also found no difference in any of the postoperative outcomes except for hospital stay which was reduced in the IS group (21 days versus 24 days, p = 0.02). The authors concluded that there was no benefit to either type of stent regarding postoperative outcomes, while pointing out that the IS group had a shorter hospital stay.
Conclusions
Despite numerous attempts to elucidate the best reconstruction technique after pancreaticoduodenectomy, there remains no clear consensus regarding the preferred technique to minimize clinically relevant postoperative pancreatic fistula, delayed gastric emptying, or other morbidity. What is important to note from all these studies, we believe, is that there is no “one-way” to anastomose the pancreas after pancreaticoduodenectomy. Instead, what these RCTs show is that, as surgeons, we have in our armamentarium a host of options when it comes to creating the anastomosis between pancreas and the enteric system and we can (and likely should) tailor our approach to the individual patient. Depending on the degree of fibrosis, pancreatitis, size and location of the remnant pancreas, diameter of the remnant pancreatic duct, and volume of pancreas resected, we may use any one of the techniques presented to offer our patients the best possible outcome and minimize the risk of pancreatic fistula.
References
Are C, Dhir M, Ravipati L. History of pancreaticoduodenectomy: early misconceptions, initial milestones and the pioneers. HPB. 2011;13(6):377–84.
Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134(5):766–71.
Langrehr JM, Bahra M, Jacob D, Glanemann M, Neuhaus P. Prospective randomized comparison between a new mattress technique and Cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg. 2005;29(9):1111–9.
Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. 2009;208(5):738–47.
Postoperative pancreatic fistula. an international study group (ISGPF) definition. Surgery. 2005;138:8–13.
El Nakeeb A, El Hemaly M, Askr W, et al. Comparative study between duct to mucosa and invagination pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized study. Int J Surg. 2015;16:1–6.
Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, Lillemoe KD, Pitt HA. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222(4):580–92.
Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, Mantovani W, Pederzoli P. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy results of a comparative study. Ann Surg. 2005;242:767–77.
Fernández-Cruz L, Cosa R, Blanco L, López-Boado MA, Astudillo E. Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy. Ann Surg. 2008;248:930–8.
Wellner UF, Brett S, Bruckner T, et al. Pancreatogastrostomy versus pancreatojejunostomy for RECOnstruction after partial PANCreatoduodenectomy (RECOPANC): study protocol of a randomized controlled trial UTN U1111-1117-9588. Trials. 2012;13:45.
Watson K. Carcinoma of the ampulla of vater successful radical resection. Br J Surg. 1944;31:368–73.
Traverso LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet. 1978;146:959–62.
Traverso LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy: a follow-up evaluation. Ann Surg. 1980;192:306–10.
Tran KT, Smeenk HG, van Eijck CH, Kazemier G, Hop WC, Greve JW, Terpstra OT, Zijlstra JA, Klinkert P, Jeekel H. Pylorus preserving pancreaticoduodenectomy versus standard whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg. 2004;240(5):738–45.
Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W, Friess H, Buchler MW. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection—long term results. Br J Surg. 2005;92:547–56.
Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Uchiyama K, et al. Pylorus ring resection reduces delayed gastric emptying in patients undergoing pancreatoduodenectomy: a prospective, randomized, controlled trial of pylorus-resecting versus pylorus-preserving pancreatoduodenectomy. Ann Surg. 2011;253(3):495–501.
Winter JM, Cameron JL, Campbell KA, Chang DC, Riall TS, Schulick RD, Choti MA, Coleman J, Hodgin MB, Sauter PK, Sonnenday CJ, Wolfgang CL, Marohn MR, Yeo CJ. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2006;10(9):1280–90.
Poon RT, Fan ST, Lo CM, Ng KK, Yuen WK, Yeung C, Wong J. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007;246(3):425–33.
Pessaux P, Sauvanet A, Mariette C, Paye F, Muscari F, Cunha AS, Sastre B, Arnaud JP, Fédération de Recherche en Chirurgie (French). External pancreatic duct stent decreases pancreatic fistula rate after pancreaticoduodenectomy: prospective multicenter randomized trial. Ann Surg. 2011;253(5):879–85.
Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13.
Tani M, Kawai M, Hirono S, Ina S, Miyazawa M, Shimizu A, Yamaue H. A prospective randomized controlled trial of internal versus external drainage with pancreaticojejunostomy for pancreaticoduodenectomy. Am J Surg. 2010;199(6):759–64.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sachs, T.E., Tseng, J.F. Landmark Series in Pancreatic Tumors: Anastomotic Techniques and Route of Reconstruction. Ann Surg Oncol 28, 2227–2234 (2021). https://doi.org/10.1245/s10434-021-09663-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09663-y