Abstract
Purpose
A brief compressive stimulus is known to induce a rapid hyperemia in skeletal muscles, considered to contribute to the initial phase of functional hyperemia. Whether the same mechano-sensitivity characterizes the cutaneous circulation is debated. This study aims to investigate whether a rapid hyperemic response to compressive stimuli is also expressed by skin blood flow in humans.
Methods
In 12 subjects, brief compressive stimuli were delivered to the forearm at varying pressures/durations (50/2, 100/2, 200/2, 200/1, 200/5 mmHg/s); the sequence was randomized and repeated with the arm above and below heart level. Laser Doppler flowmetry technique was used to monitor skin blood flow. The response was described in terms of peak skin blood flow normalized to baseline (nSBFpeak), time-to-peak from the release of compression, and excess blood volume (EBV, expressed in terms of seconds of basal flow, s-bf) received during the response.
Results
The results consistently evidenced the occurrence of a compression-induced hyperemic response, with nSBFpeak = 2.9 ± 1.1, EBV = 17.0 ± 6.6 s-bf, time-to-peak = 7.0 ± 0.7 s (200 mmHg, 2 s, below heart level). Both nSBFpeak and EBV were significantly reduced (by about 50%) above compared to below heart level (p < 0.01). In addition, EBV slightly increased with increasing pressure (p < 0.05) and duration (p < 0.01) of the stimulus.
Conclusions
For the first time, the rapid dilatator response to compressive stimuli was demonstrated in human cutaneous circulation. The functional meaning of this response remains to be elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is now generally accepted that rapid dilatations and hyperemic responses can be elicited by mechanical stimulation of blood vessel such as rapid changes in transmural pressure (Clifford et al. 2006).
For this reason, the myogenic effect has often been considered to have a role in this response (Mohrman and Sparks 1974; Tschakovsky and Sheriff 2004; Turturici et al. 2012) although the possible underlying mechanisms are still object of investigation (Turturici and Roatta 2014; Crecelius et al. 2013a,b; Clifford and Tschakovsky 2008).
This phenomenon has received considerable attention in recent years, because it has been suggested to have a role in the rapid hyperemia at the beginning of exercise (Clifford 2007; Corcondilas et al. 1964; Kindig et al. 2002; Kirby et al. 2007). In addition, these active dilatory mechanisms have been considered to be relevant for treatments based on intermittent pneumatic compressions (IPC) (van Bemmelen et al. 1994; Morgan et al. 1991; Oresanya et al. 2018). For these reasons, most studies focused on the vascular responses in skeletal muscles while very few studies investigated the response of cutaneous circulation.
However, understanding the mechanosensitivity of cutaneous blood vessels is important for different reasons: (1) to correctly interpret the relative contribution of cutaneous and vascular beds when mechanical stimuli (e.g., limb compressions) are delivered to both muscular and cutaneous tissues and hemodynamic responses are recorded at the level of large arteries supplying both tissues (Messere et al. 2017,2018b; Kirby et al. 2007; Credeur et al. 2015; Sheldon et al. 2012); (2) to address therapeutic treatments aimed at improving cutaneous circulation (van Bemmelen et al. 1994; Morgan et al. 1991; Eze et al. 1996; Sheldon et al. 2013), (3) to develop new diagnostic test that can assess microvascular function and that are not uncomfortable for the patients (Henni et al. 2019).
In general, the myogenic response is not considered to be particularly prominent in cutaneous circulation (Mellander 1989; Schubert and Mulvany 1999), although some responsiveness of cutaneous blood vessels to transmural pressure changes has been reported in some animal models such as cat (Folkow 1949), rabbit (Speden 1984) and humans (Jepsen and Gaehtgens 1993).
It was however pointed out that classical myogenic responses are generally rather slow compared to the immediate blood flow changes arising in response to compressive stimuli (Jasperse et al. 2015; Tschakovsky and Sheriff 2004; Turturici et al. 2012). The occurrence of a mechanically induced rapid hyperemia was specifically investigated in the cutaneous vascular bed of the rabbit ear: in response to a short-lasting occlusion of the suppling ear artery a very small hyperemic response was observed compared to the fourfold increase in muscle blood flow observed in response to occlusion of the masseteric artery (Turturici et al. 2012). Although several human studies employed compressive stimuli for assessing cutaneous microvascular function, e.g., in post-occlusive reactive hyperemia (PORH) (Farnebo et al. 2010) and, more recently, post-compressive reactive hyperemia (PCRH) (Henni et al. 2019), none has previously investigated the occurrence of a rapid hyperemic response to short-lasting, non-ischemic compressive stimuli. Interestingly, van Bemmelen et al. (1994) reported a rapid increase in cutaneous blood flow in response to an IPC treatment, in the chronically ischemic leg of diabetic patients, but the effect was observed outside of the pneumatic cuff, i.e., over a cutaneous area not directly subjected to the compressive stimulus. The effect was thus attributed to the increase in the arteriovenous gradient (due to the IPC-induced decrease in venous pressure) rather than to active dilatory mechanisms (van Bemmelen et al. 2000).
The aim of the present study was to verify the presence of a rapid cutaneous hyperemic response to short-lasting compressive stimuli in healthy humans and to assess its dependence on (i) duration and magnitude of the stimulus and (ii) position of the stimulated area with respect to heart level, since positional differences in hemodynamic responses have been evidenced in other studies and conditions (Jasperse et al. 2015; Credeur et al. 2015; Tschakovsky et al. 1996). To achieve this aim, a particular setup was devised to measure cutaneous blood flow through laser Doppler flowmetry beneath the compressive cuff so as to obtain a direct measure from the stimulated area of the forearm.
Methods
Subjects
The sample group consisted of 12 healthy subjects (9 men and 3 women; age: 30 ± 9 year; weight: 70 ± 12 kg; height: 175 ± 12 cm). All the participants were informed about the aim and the procedures of the study, and none of them were overweight/obese or reported any diagnosed diseases. The study was approved by the Bioethics Committee of the University of Torino and all the subjects had signed a written informed consent form.
Experimental setup
Forearm compressions
Repeatable compressive stimuli were delivered to the forearm by controlled rapid inflation of a pneumatic cuff (Gima, Gessate, IT; width: 5 cm) performed by means of a controlled pneumatic system, recently developed (Messere et al. 2018b; Ferraresi et al. 2019). Briefly, the system controls the action of two 2/2 electrical solenoid valves (VXE2330-02F-6D01, SMC, Tokyo, Japan) and of a proportional pressure valve (ITV0010, SMC, Tokyo, Japan) connected to an air compressor to inflate and deflate the blood pressure cuff up to the desired pressure level. A pressure monitor (BP-1, WPI, Sarasota, USA), placed at the outlet of the blood pressure cuff, is used as a feedback signal of the actual pressure reached. A pediatric-sized cuff (width: 5 cm) was adopted, as it was found to produce smaller movement artifacts as compared to normal-sized cuffs (14 cm). The cuff was placed on the proximal third of the left forearm. It was rapidly inflated and maintained at a pre-determined pressure level (in the range 50–200 mmHg) for a short duration (in the range 1–5 s) and then rapidly deflated.
Laser Doppler flowmetry (LDF)
Continuous measurement of skin blood flow (SBF) was performed at the proximal third of the left forearm, over the brachioradialis muscle, underneath the pneumatic cuff by laser Doppler flowmetry (Periflux PF2, Periflux Järfälla, Sweden) (Fig. 1). This was made possible by inserting the LDF probe holder through the sac accommodating the cuff. To this aim a hole (diameter: 8 mm) was made through the sac, in a region where the cuff was not present. The LDF probe holder was also stuck with medical tape on the skin to limit displacements during compression.
Experimental setup and forearm cross section: a Below heart level position. b Above heart level position. The arm was kept in position in both postural changes, thanks to the support. In both cases, the LDF probe holder was placed into a hole through the cuff and stuck to the skin by means of adhesive tape. LDF laser Doppler flowmetry
Experimental protocol
The experimental protocol was performed in a quiet room with a constant ambient temperature (21–23 °C). The subject sat on an adjustable straight-backed chair, with their left arm laying on a rigid support, their left shoulder slightly flexed and extra-rotated (approximately 45° and 20°) and the elbow flexed at about 20°. In this position, the forearm was below heart level (position A in Fig. 1). The rigid arm support could rotate around an axis aligned with the shoulder joint. In this way, with negligible movement of the elbow joint, the forearm could be easily moved above heart level, with the shoulder flexed and extra-rotated above the heart level (by 135° and 20°, respectively, position B).
The protocol consisted of a sequence of five compressive stimuli aimed to explore the effect of magnitude and duration of the compression: 50 mmHg, 2 s; 100 mmHg, 2 s; 200 mmHg, 2 s; 200 mmHg, 1 s; 200 mmHg, 5 s. Resting intervals of about 2 min were allowed between subsequent stimuli, or longer in a few instances where SBF was not at the prior baseline after 2 min. The sequence was performed twice, with the arm positioned below and above heart level. The sequence order, as well as the order of the stimuli within each sequence, was randomized.
Data acquisition and processing
The LDF analogic output was digitally sampled (at 100 Hz by means of CED Micro 1041, Cambridge Electronic Design, Cambridge, UK) along with the pneumatic pressure in the cuff and stored on the computer for later analysis with Spike2 software (version 6.10, Cambridge Electronic Design, UK).
The following parameters were considered: (SBFbsl), calculated as the time average of SBF over the 5-s interval preceding the inflation of the cuff; peak blood flow (SBFpeak) was computed as the maximum value observed in a time window of 15 s following deflation of the cuff; time-to-peak (Tpeak), which refers to time distance between cuff deflation and SBFpeak. The hyperemic response was characterized in terms of normalized peak blood flow, nSBFpeak = SBFpeak/SBFbsl, and of the excess of blood volume (EBV) received by the tissue during the hyperemic response: EBV = time integral of ((SBF(t)-SBFbsl)/SBFbsl) computed over a fixed time interval of 15 s starting after cuff deflation. To exclude the artifacts produced by cuff deflation, this interval started at the first minimum flow value after cuff deflation. Since the time integral is calculated on the normalized (adimensional) flow curve, it is expressed in s and represents the “seconds of basal-flow” (s-bf), 1 s-bf being equivalent to the blood volume that reaches the tissue in 1 s in resting conditions.
Statistics
A repeated-measures two-way ANOVA was implemented to examine the dependence of baseline SBF, tEBV, nSBFpeak and Tpeak on cuff pressure (three levels: 50, 100, 200 mmHg) and on arm position (above, below heart level). A repeated-measures two-way ANOVA was also implemented to assess the effects on the same variables on compression duration (three levels: 1, 2, 5 s) and position (above, below heart level). Significance was inferred at p < 0.05. When significant main effects were found, Bonferroni post hoc test was performed. All data are expressed as means ± standard deviation in the text to represent variability of the data sampled and means ± standard error in figures to represent the precision of the mean.
Results
The probe-cuff arrangement proved effective in recording SBF before and after the compressive maneuver, except for the occurrence of two movement artifacts at inflation and at deflation of the cuff. These artifacts were however very consistently reproduced in the responses to subsequent compressive stimuli as well as across different subjects and were also rather short lasting (1–2 s), thus leaving the ensuing hyperemic response largely unaffected.
The time course of skin blood flow following a 2-s compression delivered at 200 mmHg below heart level is shown in Fig. 2. On average in this experimental condition, normalized blood flow peaked at 2.9 ± 1.1 in 4.9 ± 0.6 s after the deflation of the cuff resulting in a hyperemic response of EBV = 17.0 ± 6.6 s-bf (Fig. 3), while above heart level the same compressive stimulus produced a response characterized by: peak blood flow of 2.6 ± 1.2 reached in 5.5 ± 1.6 s and EBV = 8.8 ± 8.2 s-bf.
Experimental series. Original skin blood flow (SBF) recording from a representative subject illustrating the response to 2 s, 200 mmHg forearm compression performed below heart level. Black square: compressive stimulus, Gray–white striped area: excess blood volume. Dotted line: basal level, EBV excess blood volume, Tpeak time-to-peak, SBFpeak skin blood flow peak
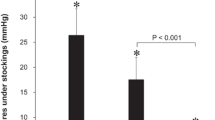
Dependence of the hyperemic response on stimulus duration. Average normalized cutaneous blood flow responses to 200 mmHg of applied pressure at different duration: a 1 s; b 2 s; c 5 s. d Average EBV responses, e average LDF time-to-peak responses and f average normalized to baseline cutaneous blood flow responses. Black boxes represent below heart level responses, gray boxes above heart level responses. Asterisks indicate p < 0.01. Error bars indicate the standard error, n = 12
Effect of compression duration and position
Figure 3 summarizes blood flow responses to 200 mmHg compressions of different duration (1, 2, and 5 s). There was a main effect of both duration (p < 0.01) and position (p < 0.01), with no significant interaction.
Pairwise comparisons indicated a significantly different EBV responses to 5-s compressions (18.3 ± 2.4 s-bf, average over both positions) compared to 2-s (12.9 ± 1.6 s-bf, p < 0.01) and 1-s compressions (11.6 ± 1.9 s-bf, p < 0.01) (Fig. 3d) and different EBV responses below (19.8 ± 11.5 s-bf, average over all durations) compared to above heart level (9.1 ± 8.3 s-bf).
Baseline SBF was not affected by duration or by position; however, it was observed that raising the arm above heart level tended to increase basal skin blood flow (by 19.7 ± 50.3% on average, n.s.), This effect could have contributed to the decrease in normalized EBV (above compared to below). To exclude this possible influence, the significance of the comparisons was verified also on EBV responses all normalized to the basal blood flow in the dependent position. The two-way ANOVA for repeated measures still evidenced a significant main effect for position (p < 0.01), as well as for duration (p < 0.01).
Time-to-peak was not dependent on duration or on position, and no significant interaction was observed between these factors (Fig. 3e), while nSBFpeak was only affected by position (p < 0.05) with no significant effect of duration and no interaction between the two, average values ranging between 2 and 3.3 (Fig. 3f).
Effect of compression pressure
Changes in blood flow induced by compressions at different pressure levels (50, 100, 200 mmHg) are reported in Fig. 4.
Dependence of the hyperemic response on applied pressure. Average normalized cutaneous blood flow responses to 2 s of compressive stimulus duration: a 50 mmHg; b100 mmHg; c 200 mmHg. d Average EBV responses, e average LDF time-to-peak responses and f average normalized to baseline cutaneous blood flow responses. Black boxes represent below heart level responses, gray boxes represent above heart level responses. Asterisks indicate p < 0.01. Error bars indicate the standard error, n = 12
There was a significant main effect of pressure (p < 0.05) and position (p < 0.01) on EBV responses, with no significant interaction. Pairwise comparisons indicated significant differences responses to between 200 and 50 mmHg corresponding to 12.9 ± 1.6 s-bf and 8.1 ± 1.2 s-bf (averaging over both positions, Fig. 4d.
There was no main effect of cuff pressure and arm position on time-to-peak and no significant interaction, average values ranging between 5 and 6 s in the different conditions (Fig. 4e).
No significant main effect for pressure and position on nSBFpeak and no significant interaction between the two factors were observed.
Discussion
For the first time, the occurrence of a compression-induced rapid hyperemia in cutaneous circulation has been described in healthy human subjects. This response, elicited by pneumatic compression of the forearm, is evoked by pressures as low as 50 mmHg and 2-s duration, exhibits a slight dependence on pressure and duration of the stimulus and a marked dependence on the limb position relative to heart level.
Experimental approach
Laser Doppler flowmetry is probably the most appropriate technique for investigating skin blood flow in humans given that: (1) it is noninvasive, (2) has a high temporal resolution, and (3) is highly specific for cutaneous microcirculation because of its small sample volume (radius of about 1 mm). However, a main drawback of this technique is its high sensitivity to movement artifacts. Different approaches have been adopted to embed the laser Doppler probe within the compressive tool (Jan et al. 2011; Mayrovitz et al. 2003; Jepsen and Gaehtgens 1993; Henni et al. 2019) however, in all cases compressive stimuli lasted more than 1 min and thus added an ischemic challenge to the tissue, giving rise to a reactive hyperemia upon compression release. Other studies more specifically investigated the rapid blood flow changes associated with the mechanical compressive stimulus, but the skin blood flow (SBF) measurement was not performed at the compressed site but in a neighboring area (van Bemmelen et al. 2000; Husmann et al. 2008; Labropoulos et al. 2005).
In the present study, we managed to record the rapid hemodynamic response directly from the compressed skin. Although movement artifacts were clearly visible in our recordings, we succeeded in limiting their duration and impact on the measurement and in obtaining a clear and consistent description of the time course of the hyperemic response to compression, by the experimental arrangement of Fig. 1.
Cutaneous vs. muscle rapid hyperemia
The rapid cutaneous hyperemia developing upon short-lasting compression described in the present study exhibits a pattern comparable to the rapid hyperemia detected in skeletal muscle or in large arteries supplying the whole limb. In fact, several studies investigated this phenomenon by recording the response to short-lasting compression produced by large pneumatic cuffs wrapped around the forearm (Kirby et al. 2007; Messere et al. 2017a; b; Brock et al. 1998) or the leg (Credeur et al. 2015; Messere et al. 2017a,2018a), or by localized pressure stimuli in animal models (Turturici and Roatta 2013a, b; Turturici et al. 2012). All these responses exhibit a similar duration, ranging between 10 and 25 s in the different setups (Kirby et al. 2007; Messere et al. 2017a; b; Brock et al. 1998; Messere and Roatta 2013), a similar slight dependence on magnitude and duration of the compressive stimulus (Kirby et al. 2007; Credeur et al. 2015; Turturici and Roatta 2013a) and are similarly affected by limb position, i.e., hemodynamic responses are larger when tested below as compared to above heart level (Tschakovsky et al. 1996; Credeur et al. 2015). In addition, the cutaneous hyperemia can be evoked with compressions as low as 50 mmHg, similarly to what was previously observed in whole forearm (Kirby et al. 2007) and in the skeletal muscle (Turturici and Roatta 2013a).
A slight difference is instead observed in the time-to-peak of the blood flow response: it is here observed to be about 5 s from cuff deflation, which is slightly larger than the 2–3 s value reported in the literature for Doppler ultrasound measurement in the brachial artery (Messere et al. 2017b; Brock et al. 1998; Tschakovsky et al. 1996; Kirby et al. 2007). This may be a delay with which the microcirculation follows the blood flow increase in supplying arteries, due to capillary compliance (Slaaf and Egbrink 2002) and capillary recruitment (Fry et al. 2013).
As for the magnitude of the response a quantitative comparison may be attempted in terms of EBV: short-lasting compression of the forearm (1-s duration, 250 mmHg) produced a hyperemic response of EBV = 21 s-bf (calculated from EBV = 17 ml and basal flow of 49.7 ml/min, assessed at the level of the brachial artery) and compression of the calf produced EBV = 18 s-bf at the level of the superficial femoral artery (Messere et al. 2018b), which are comparable to the EBV = 17 s-bf here observed in the SBF response to 1-s, 200-mmHg compressions. However, it is necessary to point out that responses measured at the level of large arteries underestimate the reactivity of the muscle vascular bed, given that the artery also supplies nonstimulated areas in the limb, e.g., the whole hand and wrist, when compressing the forearm.
When blood flow was recorded from purely muscular arteries (Turturici and Roatta 2013a), much higher responses were observed in response to 1-s lasting compression, with peak flow exceeding 500% of baseline (Turturici and Roatta 2013a, b; Turturici et al. 2012), as compared to the blood flow increase of 150–350% observed in whole-limb studies (Kirby et al. 2007; Credeur et al. 2015; Brock et al. 1998) and to the 200–300% increase observed in the present study.
Based on these mostly qualitative comparisons among hemodynamic responses in the different experimental models, we suggest that the cutaneous circulation possesses the same type of mechanosensitivity of muscular circulation to rapid transmural pressure changes, such as those produced by compressive stimuli. From the quantitative point of view, cutaneous vascular reactivity appears to be weaker than the musculovascular, although accurate quantitative studies are necessary to confirm this latter hypothesis.
Surprisingly, these findings are in contrast with the almost complete lack of responsiveness reported for the cutaneous vascular bed of the rabbit ear (Turturici et al. 2012). In fact, this vascular region was shown to be mostly unresponsive to the transmural pressure changes produced by short-lasting occlusion of the central ear artery, as compared to the large hyperemia observed in the muscular masseteric artery in response to the same stimulus. Whether this is due to species or experimental differences (e.g., related to the way the stimulus is provided) remains to be ascertained.
Underlying mechanisms
Although the present study was not meant to directly investigate the physiological mechanisms behind the cutaneous hyperemia, a few potential mechanisms warrant discussion based on data from the literature. It is interesting to observe that a similar hyperemic response of skin blood flow has been recorded in response to cuff compression of limb portions proximal to the investigated cutaneous areas (van Bemmelen et al. 1994; Husmann et al. 2008), i.e., without actual compression of the examined tissue. The effect was attributed to the “muscle pump” mechanism, i.e., the reduction in venous pressure that follows the compressive stimulus (which takes place even if the compression is delivered proximally to the investigated area) and that results in increased perfusion pressure. The involvement of the veno-arteriolar reflex producing arteriolar dilatation in response to a fall in venous pressure was also called into play (Husmann et al. 2008). However, the mechanisms behind the mechanically induced rapid dilatation in response to brief contraction or compressive stimuli have long been debated in muscle circulation (Shoemaker et al. 1998; Clifford 2007; Credeur et al. 2015) and a number of arguments have been brought forward against a relevant role of the muscle pump (Jasperse et al. 2015; Turturici et al. 2012). For instance, to the muscle pump has been attributed the result of having larger responses below vs. above heart level (Leyk et al. 1994; Nadland et al. 2009; Shoemaker et al. 1998); however, Jasperse et al. (2015) recently showed that also the response to reactive hyperemia, governed by metabolic mechanisms, is larger in the dependent than in the independent limb. The authors, thus, concluded that the driving pressure and changes in transmural pressure, which depend on limb position, are a major determinant of the response (Jasperse et al. 2015). This interpretation was recently further emphasized by Lynn et al. (2019) and is in agreement with previous observations that a rapid hyperemic response can be produced by a short-lasting occlusion of the supplying artery (Turturici et al. 2012), producing a transient drop in transmural pressure of downstream territories but no emptying of venous compartments. The ensuing dilatation could then result from a rapid myogenic response (Turturici et al. 2012). Supporting this view are the early observation of Folkow (1949) reporting rapid hyperemic responses to partial occlusions of the supplying artery in both muscular and cutaneous vascular beds. It can be hypothesized that the occlusion of a supplying artery produces the same changes in downstream pressure as a proximal limb compression, and thus that the cutaneous hyperemia produced by foot and leg compression at the level of the metatarsus or of the big toe (van Bemmelen et al. 2000; Husmann et al. 2008) results from the transmural pressure changes in these areas. Although a number of studies investigated the nature and the pathways of this vascular mechanosensitiveness (Crecelius et al. 2012; Turturici and Roatta 2014), the mechanosensitive structures and their localization in the vascular bed remain to be identified.
Functional significance and implications
First of all, it must be underlined that compression-induced hyperemia here observed represents a true increase in perfusion given that, as quantified in terms of seconds of basal flow, it exceeds the blood flow interruption that takes place during the compression. In fact, EBV ranges between 9 and 28 s-bf in the different conditions below heart level and, even when the arm is positioned above heart level, average EBV exceeds the duration of the compression. Furthermore, it must be reminded that EBV underestimates the actual reperfusion, as the first 2–3 s immediately following the release of the compression were not included in the calculation of EBV to exclude the movement artifact.
From the functional point of view, the rapid dilatation in response to compressive stimuli in skeletal muscle was hypothesized to implement a feedforward control of blood flow, granting immediate increase in perfusion at the beginning of exercise (intramuscular blood vessels are compressed by muscle contraction (Clifford 2007; Kirby et al. 2007). This functional hypothesis does not seem directly applicable to the cutaneous circulation, given that it is not compressed by muscle contraction, nor does it need an anticipatory boost in perfusion, since its metabolism remains constant in time. It is possible that other mechanical stimuli (e.g., skin contact with external objects or cutaneous stretch during movements) provoke transient increases in skin perfusion, although the functional meaning of this phenomenon remains unclear.
Intermittent pneumatic compression is an established treatment not only for the prevention of venous thromboembolism, but also for improving blood flow in skin and muscle tissues, e.g., for the treatment of cutaneous ulcers (Comerota 2011) or peripheral artery disease (van Bemmelen et al. 2000; Labropoulos et al. 2005; Husmann et al. 2008). Its effectiveness is considered to be mainly based on displacing venous blood, which empties venous compartments, lowers peripheral venous pressure and increases perfusion pressure (as with the muscle pump). With regard to muscle circulation, it has been largely discussed that in addition to the muscle-pump mechanism, vascular compression engages an active rapid dilatation of resistance vessels, which contributes to the ensuing hyperemia (Tschakovsky and Sheriff 2004; Clifford et al. 2006; Clifford and Tschakovsky 2008; Turturici et al. 2012). The present results indicate that this reactivity to mechanical stimuli is present also in cutaneous blood vessel. Improved understanding of the physiological mechanisms behind compression-induced hyperemia may have consequences on the design of IPC treatments (Sheldon et al. 2013). For example, the present data indicate that even short-lasting (1 s) and weak (50 mmHg) compressions are effective in producing a significant cutaneous hyperemia. Moreover, recent whole limb studies indicate that the effectiveness of repetitive stimulation is affected by the frequency of stimulation, and that the increase in tissue oxygenation has a role in attenuating the hyperemic response to subsequent stimuli (Messere et al. 2018b,2017a; b). Whether the same perfusion control mechanism applies to cutaneous tissue needs to be investigated. Finally, the assessment of the rapid hyperemic response to a short-lasting skin compression may become a means to specifically investigate cutaneous vascular reactivity to mechanical stimulation, in analogy to what has been proposed in whole limb studies based on assessment of the hemodynamic response to passive movement (Broxterman et al. 2017) or to single muscle contractions (Hughes et al. 2015) and in addition to other possible tests of vascular function (Henni et al. 2019; Roustit and Cracowski 2013).
Limitations
In the present study, arterial blood pressure was not monitored. For this reason, we could not express the hemodynamic responses in terms of changes in vascular conductance. While arterial blood pressure is not expected to be affected by passive changes of arm position (Jasperse et al. 2015; Jepsen and Gaehtgens 1993; Credeur et al. 2015), the vascular conductance is slightly decreased above heart level, given the slight decrease in driving pressure and increase in basal flow. Compression-induced relative changes in vascular conductance would have been equal to relative changes in SBF and EBV. However, absolute changes in vascular conductance would have been slightly increased above heart level, thus attenuating the difference with respect to below heart level.
Conclusions
In the present study, we showed that, short-lasting direct skin compression elicits a rapid hyperemic response in skin blood flow. This response is qualitatively and quantitatively similar to the compression-induced rapid hyperemia observed in whole limbs, but slightly slower and of smaller magnitude than that reported for skeletal muscle. Although the functional meaning of this mechanosensitive rapid responsiveness of cutaneous circulation remains unclear, a better understanding of this phenomenon may help to optimize clinical treatments aiming to improve cutaneous circulation.
Abbreviations
- ANOVA:
-
Analysis of variance
- Bsl:
-
Baseline
- EBV:
-
Excess of blood volume
- IPC:
-
Intermittent pneumatic compression
- LDF:
-
Laser Doppler flowmetry
- PCRH:
-
Post-compressive reactive hyperemia
- PORH:
-
Post-occlusive reactive hyperemia
- s-bf:
-
Seconds of basal blood flow
- SBF:
-
Skin blood flow
References
Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL (1998) Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol 85(6):2249–2254
Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Hydren JR, Nelson AD, Morgan DE, Jessop JE, Bledsoe AD, Richardson RS (2017) Single passive leg movement assessment of vascular function: contribution of nitric oxide. J Appl Physiol (1985) 123(6):1468–1476. https://doi.org/10.1152/japplphysiol.00533.2017
Clifford PS (2007) Skeletal muscle vasodilatation at the onset of exercise. J Physiol 583(Pt 3):825–833. https://doi.org/10.1113/jphysiol.2007.135673
Clifford PS, Tschakovsky ME (2008) Rapid vascular responses to muscle contraction. Exerc Sport Sci Rev 36(1):25–29. https://doi.org/10.1097/jes.0b013e31815ddba4
Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL (2006) Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572(Pt 2):561–567. https://doi.org/10.1113/jphysiol.2005.099507
Comerota AJ (2011) Intermittent pneumatic compression: physiologic and clinical basis to improve management of venous leg ulcers. J Vasc Surg 53(4):1121–1129. https://doi.org/10.1016/j.jvs.2010.08.059
Corcondilas A, Koroxenidis GT, Shepherd JT (1964) Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol 19:142–146
Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA (2012) ATP-mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans. J Physiol 590(Pt 21):5349–5359. https://doi.org/10.1113/jphysiol.2012.234245
Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA (2013a) Mechanisms of rapid vasodilation following a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305(1):H29–40. https://doi.org/10.1152/ajpheart.00298.2013
Crecelius AR, Kirby BS, Richards JC, Dinenno FA (2013b) Mechanical effects of muscle contraction increase intravascular ATP draining quiescent and active skeletal muscle in humans. J Appl Physiol 114(8):1085–1093. https://doi.org/10.1152/japplphysiol.01465.2012
Credeur DP, Holwerda SW, Restaino RM, King PM, Crutcher KL, Laughlin MH, Padilla J, Fadel PJ (2015) Characterizing rapid-onset vasodilation to single muscle contractions in the human leg. J Appl Physiol (1985) 118(4):455–464. https://doi.org/10.1152/japplphysiol.00785.2014
Eze AR, Comerota AJ, Cisek PL, Holland BS, Kerr RP, Veeramasuneni R, Comerota A Jr (1996) Intermittent calf and foot compression increases lower extremity blood flow. Am J Surg 172(2):130–135
Farnebo S, Thorfinn J, Henricson J, Tesselaar E (2010) Hyperaemic changes in forearm skin perfusion and RBC concentration after increasing occlusion times. Microvasc Res 80(3):412–416
Ferraresi C, De Benedictis C, Maffiodo D, Franco W, Messere A, Pertusio R, Roatta S (2019) Design and simulation of a novel pneumotronic system aimed to the investigation of vascular phenomena induced by limb compression. J Bionic Eng 16(3):550–562
Folkow B (1949) Intravascular pressure as a factor regulating the tone of the small vessels. Acta Physiol Scand 17(4):289–310
Fry BC, Roy TK, Secomb TW (2013) Capillary recruitment in a theoretical model for blood flow regulation in heterogeneous microvessel networks. Physiol Rep 1(3):e00050
Henni S, Hersant J, Loufrani L, Duval G, Humeau-Heurtier A, Riou J, Abraham P, Group S, Research SGJM ( 2019) Painless local pressure application to test microvascular reactivity to ischemia. Random Control Trial 122:13–21
Hughes WE, Ueda K, Treichler DP, Casey DP (2015) Rapid onset vasodilation with single muscle contractions in the leg: influence of age. Physiol Rep 3(8):e12516
Husmann M, Willenberg T, Keo HH, Spring S, Kalodiki E, Delis KT (2008) Integrity of venoarteriolar reflex determines level of microvascular skin flow enhancement with intermittent pneumatic compression. J Vasc 48(6):1509–1513
Jan Y-K, Brienza D, Boninger M, Brenes G (2011) Comparison of skin perfusion response with alternating and constant pressures in people with spinal cord injury. Spinal Cord 49(1):136
Jasperse JL, Shoemaker JK, Gray EJ, Clifford PS (2015) Positional differences in reactive hyperemia provide insight into initial phase of exercise hyperemia. J Appl Physiol 119(5):569–575. https://doi.org/10.1152/japplphysiol.01253.2013
Jepsen H, Gaehtgens P (1993) Postural vascular response in human skin: passive and active reactions to alteration of transmural pressure. Am J Physiol 265(3 Pt 2):H949–958
Kindig CA, Richardson TE, Poole DC (2002) Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol 92(6):2513–2520. https://doi.org/10.1152/japplphysiol.01222.2001
Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA (2007) Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583(Pt 3):861–874. https://doi.org/10.1113/jphysiol.2007.131250
Labropoulos N, Leon LR Jr, Bhatti A, Melton S, Kang SS, Mansour AM, Borge M (2005) Hemodynamic effects of intermittent pneumatic compression in patients with critical limb ischemia. J Vasc Surg 42(4):710–716. https://doi.org/10.1016/j.jvs.2005.05.051
Leyk D, Eßfeld D, Hoffmann U, Wunderlich H-G, Baum K, Stegemann J (1994) Postural effect on cardiac output, oxygen uptake and lactate during cycle exercise of varying intensity. Eur J Appl Physiol Occup Physiol 68(1):30–35
Lynn MJ, Mew OK, Drouin PJ, Liberman NL, Tschakovsky ME (2019) Greater post-contraction hyperaemia below vs. above heart level: the role of active vasodilatation vs. passive mechanical distension of arterioles. J Physiol 598:85–99
Mayrovitz HN, Sims N, Dribin L (2003) Heel skin hyperaemia: direct compression versus vascular occlusion. Clin Trial 23(6):354–359
Mellander S (1989) Functional aspects of myogenic vascular control. J Hypertens Suppl 7(4):S21–30
Messere A, Roatta S (2013) Influence of cutaneous and muscular circulation on spatially resolved versus standard Beer-Lambert near-infrared spectroscopy. Physiol Rep 1(7):e00179. https://doi.org/10.1002/phy2.179
Messere A, Ceravolo G, Franco W, Maffiodo D, Ferraresi C, Roatta S (2017) Increased tissue oxygenation explains the attenuation of hyperemia upon repetitive pneumatic compression of the lower leg. J Appl Physiol 123(6):1451–1460. https://doi.org/10.1152/japplphysiol.00511.2017
Messere A, Turturici M, Millo G, Roatta S (2017) Repetitive muscle compression reduces vascular mechano-sensitivity and the hyperemic response to muscle contraction. J Physiol Pharmacol 68(3):427–437
Messere A, Pertusio R, Macrì C, Maffiodo D, Franco W, De Benedictis C, Ferraresi C, Roatta S (2018a) Delivery of customizable compressive patterns to human limbs to investigate vascular reactivity. Biomed Phys Eng Express 4(6):067003
Messere A, Tschakovsky ME, Seddone S, Lulli G, Franco W, Maffiodo D, Ferraresi C, Roatta S (2018b) Hyper-oxygenation attenuates the rapid vasodilatory response to muscle contraction and compression. Front Physiol 9:1078
Mohrman DE, Sparks HV (1974) Myogenic hyperemia following brief tetanus of canine skeletal muscle. Am J Physiol 227(3):531–535
Morgan R, Carolan G, Psaila J, Gardner A, Fox R, Woodcock J (1991) Arterial flow enhancement by impulse compression. Vasc Endovasc Surg 25(1):8–16
Nadland IH, Walloe L, Toska K (2009) Effect of the leg muscle pump on the rise in muscle perfusion during muscle work in humans. Eur J Appl Physiol 105(6):829–841. https://doi.org/10.1007/s00421-008-0965-6
Oresanya L, Mazzei M, Bashir R, Farooqui A, Athappan G, Roth S, Choi ET, van Bemmelen P (2018) Systematic review and meta-analysis of high-pressure intermittent limb compression for the treatment of intermittent claudication. J Vasc Surg 67(2):620–628
Roustit M, Cracowski J-L (2013) Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci 34(7):373–384
Schubert R, Mulvany MJ (1999) The myogenic response: established facts and attractive hypotheses. Clin Sci (Lond) 96(4):313–326
Sheldon RD, Roseguini BT, Thyfault JP, Crist BD, Laughlin MH, Newcomer SC (2012) Acute impact of intermittent pneumatic leg compression frequency on limb hemodynamics, vascular function, and skeletal muscle gene expression in humans. J Appl Physiol (1985) 112(12):2099–2109. https://doi.org/10.1152/japplphysiol.00042.2012
Sheldon RD, Roseguini BT, Laughlin MH, Newcomer SC (2013) New insights into the physiologic basis for intermittent pneumatic limb compression as a therapeutic strategy for peripheral artery disease. J Vasc Surg 58(6):1688–1696. https://doi.org/10.1016/j.jvs.2013.08.094
Shoemaker JK, Tschakovsky ME, Hughson RL (1998) Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol 76(4):418–427
Slaaf D, Egbrink M (2002) Capillaries and flow redistribution play an important role in muscle blood flow reserve capacity. J Mal Vasc 27(2):63–68
Speden RN (1984) Active reactions of the rabbit ear artery to distension. J Physiol 351:631–643
Tschakovsky ME, Sheriff DD (2004) Immediate exercise hyperemia: contributions of the muscle pump vs rapid vasodilation. J Appl Physiol 97(2):739–747. https://doi.org/10.1152/japplphysiol.00185.2004
Tschakovsky ME, Shoemaker JK, Hughson RL (1996) Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol 271(4 Pt 2):H1697–1701
Turturici M, Roatta S (2013a) Compression-induced hyperaemia in the rabbit masseter muscle: a model to investigate vascular mechano-sensitivity of skeletal muscle. Physiol Meas 34:307–314
Turturici M, Roatta S (2013b) Inactivation of mechano-sensitive dilatation upon repetitive mechanical stimulation of the musculo-vascular network in the rabbit. J Physiol Pharmacol 64(3):299–308
Turturici M, Roatta S (2014) Effects of gadolinium chloride on basal flow and compression-induced rapid hyperemia in the rabbit masseter muscle. J Physiol Pharmacol 65(3):409–415
Turturici M, Mohammed M, Roatta S (2012) Evidence that the contraction-induced rapid hyperemia in rabbit masseter muscle is based on a mechanosensitive mechanism, not shared by cutaneous vascular beds. J Appl Physiol 113(4):524–531. https://doi.org/10.1152/japplphysiol.00237.2012
van Bemmelen PS, Mattos MA, Faught WE, Mansour MA, Barkmeier LD, Hodgson KJ, Ramsey DE, Sumner D (1994) Augmentation of blood flow in limbs with occlusive arterial disease by intermittent calf compression. J Vasc Surg 19(6):1052–1058
van Bemmelen PS, Weiss-Olmanni J, Ricotta JJ (2000) Rapid intermittent compression increases skin circulation in chronically ischemic legs with infra-popliteal arterial obstruction. VASA 29(1):47–52
Funding
This research was supported by local grants from the University of Torino (ROAS_RILO_17_01).
Author information
Authors and Affiliations
Contributions
SS, AM and SR conceived and designed the research. AM and SS conducted experiments and analysis of the data. All authors contributed to writing the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Additional information
Communicated by I. Mark Olfert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seddone, S., Messere, A. & Roatta, S. Vascular reactivity of cutaneous circulation to brief compressive stimuli, in the human forearm. Eur J Appl Physiol 120, 1041–1050 (2020). https://doi.org/10.1007/s00421-020-04343-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04343-3