Abstract
Purpose
The aim of this study was to investigate the effect of an ultra-marathon on heart rate variability (HRV) and psychometric indices in endurance runners. In addition, we aimed to determine the magnitude of change and subsequent recovery for 7 days following the race.
Methods
Recreationally trained runners (n = 13 (8M); age = 36.6 ± 7.6 years; height = 174 ± 9 cm; weight = 70.5 ± 9.3 kg) completed measures of HRV upon waking in the morning for 1 week prior to and 1 week following a 64-km running race. Profile of mood states, wellbeing, and muscular soreness were also measured throughout the study period to further contextualise recovery.
Results
An increase in heart rate accompanied by decreased LnSDNN, LnRMSSD, LnLF, LnHF, and LnLF/HF from baseline were observed 1 day post-race (p < 0.05). Indices of HRV had returned to baseline on day 2 of recovery. Perceptual fatigue and muscle soreness increased post-race (immediately following and on day 1 of recovery) (p < 0.05) and took until day 5 of recovery to return to baseline.
Conclusion
The results indicate that cardiac autonomic control is significantly altered in response to a 64 km ultra-marathon. Specifically, parasympathetic activity is suppressed. The change in autonomic control was relatively short-lived, and parasympathetic-related indices had returned to baseline 2 days after the event. Subjective measures of fatigue and wellbeing suggest that athletes were not completely recovered until day 5 post-event, with muscular soreness remaining prominent during this period. A combination of physiological and psychological parameters is important to contextualise recovery in ultra-endurance runners.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 2 decades, ultra-endurance events, defined as endurance performance exceeding 6 h (Zaryski and Smith 2005), have become increasingly popular (Da Fonseca-Engelhardt et al. 2013). The unique characteristics of ultra-endurance events have enticed endurance enthusiasts to challenge themselves both physiologically and psychologically (Whyte 2014). Optimising training load for ultra-endurance athletes who compete in multiple events throughout the year requires a delicate balance between training stress and recovery and as such monitoring tools that may reflect readiness to train are of considerable value. One tool that may be utilised to reflect the functional state of physiological control systems, informing measures of training stress or fatigue, is heart rate variability (HRV). While changes in HRV following chronic training may not reflect cardiac autonomic alterations (Herzig et al. 2018), there is good evidence that short-term alterations in HRV reflect changes in the autonomic nervous system (ANS) that provide valuable information of acute training loads (Plews et al. 2013a, b). While fatigue responses and recovery kinetics in response to short- and medium-duration endurance exercise have been characterised (Michael et al. 2017), the magnitude of disruption to homeostasis, in particular autonomic function, in response to ultra-endurance exercise requires greater description.
As vagal tone has been considered a novel index of stress vulnerability and reactivity, monitoring the parasympathetic nervous system provides a mechanism to assess exercise-induced stress (Porges 1992). For a single aerobic exercise session at threshold intensity but less than 2 h, complete cardiac autonomic recovery takes approximately 24–48 h (Stanley et al. 2013). In the few studies that have monitored HRV during defined recovery periods following ultra-endurance exercise, findings have been heterogeneous with complete autonomic recovery occurring at 24 h (Bernardi et al. 1997), 30 h (Mertová et al. 2017) and 72 h (Gratze et al. 2005) post-event. A consideration in the interpretation of the findings of these ultra-endurance studies is that baseline data were measured either 24 h pre-race or the morning of the event. Pre-race anxiety and other exercise-related stressors related to associated travel and sleep disturbances may have likely reduced parasympathetic activity (Meerlo et al. 2008). Consequently, the magnitude of cardiac parasympathetic suppression following the ultra-endurance exercise may have been underestimated.
Literature suggests that during periods of high-training stress, subjective measures of exercise stress, fatigue, and muscle soreness increase (Meeusen et al. 2013). In combination with indices of cardiac autonomic function, psychometric measures and perceived muscle soreness and wellbeing may provide insight into the athlete’s ability to participate in training that day (Saw et al. 2016). Assessment of these measures of fatigue and recovery in response to an ultra-endurance event may inform the optimal time to return to training and aid in the prescription of training intensity, to optimise physiological adaptation in athletes as they prepare for their next event. With a view to better understand competition stress and recovery for ultra-endurance athletes, this study aimed to (1) investigate HRV and psychometric indices following a 64 km ultra-marathon, and (2) determine the temporal dynamics of recovery in HRV and psychometric indices over the 7 days post-race. It is hypothesised that cardiac autonomic function and psychometric indices would be altered from baseline following the race, with a reduction in parasympathetic activity, and an increase in mood disturbance, fatigue, and muscle soreness. It is also hypothesised that such indices will follow a similar time course to return to baseline within the week post-race.
Methodology
Participants
Thirteen ultra-endurance runners volunteered for this study (n = 13 (8 male); age = 36.6 ± 7.6 years; height = 174.7 ± 9.3 cm; weight = 70.5 ± 9.3 kg). Participants were recruited by social media with the following inclusion criteria: competing in the 2017 Bruny Island Ultra-Marathon, had access to the required smartphone application and had previous ultra-endurance experience. Prior to participation in the study, all participants provided written informed consent. Ethics approval for this study was granted by the Human Research Ethics Committee of The University of Tasmania.
Study design
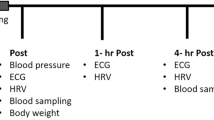
Physiological and psychological variables were measured in participants over 13 days between the 27th of November and the 9th of December 2017, a period inclusive of the Bruny Island Ultra-Marathon on the 2nd of December (day 6). The event required all participants to have at least one support crew and each runner was responsible for their food and water provisions. Participants in this study had previously competed in ultra-endurance events and had their own nutrition plan for the race, completing the race without illness or injury. Participants had staggered starting times between 0500 and 0730 h, and covered 64 km with 1572 m of accumulative altitude change (836 m ascending and 736 m descending) (Suunto Spartan Ultra GPS watch, Suunto, Vantaa, Finland). Temperature ranged from 10.8 to 13.0 °C with relative humidity ranging from 82 to 85%, with scattered rain showers throughout the day.
HRV data were recorded upon waking on days 1–5, and days 7–13. Heart rate measures were not recorded on the day of the event as literature suggests that HRV data may be affected by pre-race anxiety (Stanley et al. 2013). In addition, the early start time may have disturbed sleep, and could be a factor affecting HRV data (Meerlo et al. 2008). Perceived fatigue and wellbeing data were recorded on days 1 through 13, with a profile of mood state (POMS) questionnaire completed in the evening of days 2, 4, 6, 8, and 11. Throughout the study period, participants completed a training diary in which they recorded the duration (minutes) and rating of perceived exertion (RPE) of each training session completed. RPE was measured using a modified CR-10 scale (Foster et al. 2001). A single arbitrary unit (AU) represents training load, calculated by multiplying duration with RPE. Training load was not controlled during the period of this study. All runners tapered their training in the preparation period and resumed training with a gradual increase in training load, despite variance in absolute training load.
Heart rate variability measurement
Upon waking at home, participants attached a Polar H7 heart rate sensor (Polar Electro, Kempele, Finland), and recorded data for 5 min while lying in a supine position, using a smartphone application (Heart Rate Variability Logger; Marco Altini 2013). Currently, these conditions reflect the best practice for athletes in field settings (Buchheit 2014). The application recorded R–R interval length and was validated prior to the data collection period against the Polar RS800cx (Polar Electro, Kempele, Finland). Interclass correlation coefficient was 0.999–1.000 for time-domain indices and 0.985–1.000 for frequency-domain indices. Following HRV recording, the data were uploaded from the smartphone application directly to a secure online Dropbox. Participants were asked to leave the chest strap within arm’s reach to minimise disturbances when applying the apparatus each morning. Breathing was not controlled during the recording. Conscious involvement in controlled breathing may result in HRV measures not being truly reflective of ANS activity and spontaneous breathing is, therefore, considered appropriate for resting measures (Sasaki and Maruyama 2014). Prior to the start date, all participants completed a familiarisation exercise to confirm data which were being recorded and uploaded correctly.
HRV data were analysed using Kubios HRV software (version 2.2, Biomedical Signal Analysis Group, Department of Applied Physics, University of Eastern Finland, Finland). The first and final 30 s of each recording were removed to minimise the impact of noise. Each data file were visually inspected for artefacts which were manually corrected by interpolation from surrounding R–R intervals. In the time-domain, mean R–R intervals, standard deviation of the normal-to-normal sinus-initiated inter-beat intervals (SDNN), and the root mean square of successive differences between R-R intervals (RMSSD) were used as key indices. Literature suggests that they are the most accurate marker of parasympathetic activity, specifically RMSSD, particularly under resting conditions (Buchheit 2014). Frequency-domain data were quantified with the Fast Fourier Transformed method. The power spectral density was measured by frequency bands in ms2 Hz−1 and the spectral power was expressed in ms2. For spectral analysis bands were 0.04–0.15 Hz for low frequency (LF) and 0.15–0.4 Hz for high frequency (HF). HF activity is considered representative of parasympathetic activity, whereas that of the LF band reflects both sympathetic and parasympathetic control. Consequently, the LF/HF ratio is an index of the sympatho-vagal balance (Bosquet et al. 2008).
Psychological measures
Immediately after heart rate variability measurement, a perceptual fatigue and wellbeing questionnaire was completed. The only exception to this was on day 6, where only subjective measures were taken upon waking. The fatigue and wellbeing questionnaire (McLean et al. 2010) assessed the participants’ perceived fatigue, sleep quality, stress levels, and mood on a five-point Likert scale ranging from one to five (e.g., for perceived fatigue: one = always tired, and five = very fresh). A total score out of 20 was then calculated. In addition, a 10 cm visual analogue scale was used to assess muscular soreness. The participants were to place a dash along a 10 cm line (left = no soreness and right = extremely sore) that was then converted to a score out of 10. An abbreviated POMS questionnaire (Grove and Prapavessis 1992) was completed that assessed 40 wellbeing variables on a scale between 0 (not at all) and 4 (extremely) to calculate total mood disturbance. Fatigue and vigour subcomponents of the POMS questionnaire were extracted, as they appear the most sensitive marker of training status (Meeusen et al. 2013).
Statistical analysis
Prior to analysis, a Shapiro–Wilk test was used to examine the distribution of each variable. When HRV data were skewed, data were transformed by taking the natural logarithm (Ln), and once normal distribution was confirmed, parametric statistical comparison was performed. Consequently, SDNN, RMSSD, LF, HF, and LF/HF were log transformed. Parametric variables were training load, resting heart rate, LnSDNN, LnRMSSD, LnLF, LnHF, LnLF/HF, fatigue (POMS), and vigour (POMS). Non-parametric variables were mean R-R, fatigue, sleep, stress, mood, total wellbeing score, muscle soreness, and total mood disturbance (POMS). A post priori power analysis was performed for key outcomes variables of heart rate, mean R–R, and SDNN. Days 1–5 of baseline data collection (pre-race) are presented as days − 5 to − 1. Days 7–13 following the race are presented as day 1 to day 7. Baseline for HRV indices was calculated using the average of day − 4, day − 3, and day − 2. The first day of measuring was excluded as this was considered another familiarisation with the measuring and uploading process (Plews et al. 2013a, b). Subjective measures were averaged across the same period with the exception of POMS, where baseline was calculated using the average of day − 4 and day − 2.
To assess the acute change in HRV and psychometric variables from baseline to post-race (day 1), a paired t test or Wilcoxon signed-rank test was used. Relationships between the magnitude of change (%) in R–R, LnSDNN, LnRMSSD. LnLF and LnHF from baseline to day 1 and race time were determined using a Pearson correlation. To assess recovery of variables during the post-race period (day 1–7), a one-way ANOVA or Friedman test was used with a Tukey’s or Dunn’s post hoc test. Magnitude-based inferences were used to determine the magnitude of effect (Batterham and Hopkins 2006), which involved calculating 90% confidence intervals that defined a range representing the uncertainty in the true value. A three-level scale of substantially positive, trivial, substantially negative was defined by the smallest worthwhile change. Chances that the true value were substantial were calculated by comparing the confidence interval to the three-level scale. If the chance of substantially positive or substantially negative were both > 5%, the true value was deemed as unclear. Otherwise, the chances were labelled quantitatively as follows: 25–75%, possibly; 75–95%, likely; 95–99.5%, very likely; and > 99.5%, most likely (Hopkins et al. 2009). For each participant, intra-individual standard deviation (SD) was calculated for each HRV variable over days − 4, − 3, and − 2. The smallest worthwhile change for difference was set as the pooled SD calculated from all intra-individual SD (Buchheit 2014). Data are presented as mean ± SD for parametric variables, and median and 90% confidence interval (lower to upper range) for non-parametric variables. Statistical analyses were performed using GraphPad Prism 7 for Mac OS X (version 7.0d, GraphPad Software, Inc.) and significance was set at p < 0.05.
Results
Training load
Average training load (and number of athletes training each day) throughout the baseline period (days − 5 to − 1) was 382 ± 525 AU (4), 297 ± 225 AU (8), 306 ± 155 AU (8), 123 ± 73 AU (3), and 130 ± 45 AU (3), respectively. Participants completed the race in 6 h and 32 min (± 42 min) and reported an RPE of 8.4 ± 1.3 with a race load of 3293 ± 435 AU. During the recovery period of days 1–7, average training load (and participating athletes) was 60 AU (1), 145 ± 77 AU (2), 440 ± 228 AU (4), 289 ± 127 AU (7), 457 ± 388 AU (5), 165 ± 73 AU (6), and 441 ± 315 AU (9), respectively. During baseline and the recovery, period modes of exercise included walking, running and cycling.
Heart rate variability indices
Table 1 presents indices of heart rate variability during baseline and the recovery period, and Fig. 1 presents the magnitude of change in these values. In the time-domain, there was a very likely increase in resting heart rate from baseline to post-race (day 1) (p = 0.015). There was a significant main effect of day (baseline and recovery period) on resting heart rate (p = 0.013); however, no days were statistically significantly different from one another. There was a very likely decrease in mean R–R interval from baseline to post-race (day 1) (p = 0.014) and a significant main effect of day (baseline and recovery period) on R-R (p = 0.015), although there were no significant post hoc tests. A very likely decrease in LnSDNN was observed from baseline to post-race (day 1) (p = 0.007). There was a significant main effect of day (baseline and the recovery period) on LnSDNN (p = 0.012), with post hoc testing revealing a significant increase from day 1 to day 2 (p = 0.009). Finally, there was a likely decrease in LnRMSSD from baseline to post-race (day 1) (p = 0.006). There was a significant main effect of day (baseline and the recovery period) on LnRMSSD (p = 0.002), with post hoc testing revealing significant increases from day 1 to day 3 (p = 0.001), day 1 to day 5 (p = 0.022), and day 1–6 (p = 0.007). For the outcome variables of heart rate, mean R–R and LnSDNN power were 0.42, 0.48, and 0.49, respectively.
Changes in resting heart rate and heart rate variability indices from baseline during the recovery period (days 1–7). Δ = difference between actual value and the baseline average. Shaded area denotes trivial changes and was based on the smallest worthwhile change. A three-level scale of substantially positive, trivial, substantially negative was defined by the smallest worthwhile change. ↑ = substantial increase, ↓ = substantial decrease. Values are presented as mean and 90% confidence interval
In the frequency domain, there was a likely decrease in LnLF from baseline to post-race (day 1) (p = 0.027) (Table 1 and Fig. 1) but no main effect of day (baseline and the recovery period) (p = 0.127). LnHF decreased (very likely) from baseline to post-race (day 1) (p = 0.011), with a significant main effect of day (baseline and the recovery period) (p = 0.025). Post hoc testing revealed significant increases from day 1 to day 2 (p = 0.041), day 1 to day 3 (p = 0.003), and day 1 to day 6 (p = 0.034). Together, there was a possible decrease in LnLF/HF from baseline to post-race (day 1) (p = 0.028); however, there was no main effect of day (baseline and the recovery period) on LnLF/HF (p = 0.093).
Race time was significantly negatively correlated with the percentage change from baseline to day 1 of LnSDNN (r = − 0.68, p = 0.01), while there were moderate, non-significant correlations between race time and the change in LnRMSSD (r = − 0.51, p = 0.075) and LnHF (r = − 0.55, p = 0.052). Only weak relationships were observed between the change in R–R and LnLF with race time (both r < − 0.29, p > 0.34).
There was no significant correlation between race time and change in LnSDNN from baseline to day 2–7 (r = − 0.38 to 0.09, p > 0.20) or LnRMSSD on days 2–5 and day 7 (r = − 0.46 to 0.49, p > 0.088). There was a moderate positive correlation on day 6, although this was not significant (r = 0.54, p = 0.057).
Muscle soreness
There was a significant increase in muscle soreness from baseline to post-race (day 1) (p = 0.0002) (Table 2). There was a significant main effect of day (baseline and recovery period) on muscle soreness (p < 0.0001), with post hoc testing revealing a significant increase in muscle soreness from baseline to day 1 (p < 0.0001), baseline to day 2 (p = 0.002), and baseline to day 3 (p = 0.036).
Profile of mood states
There was no change from baseline to post-race in total mood disturbance (p = 0.123) and components of vigour (p = 0.199); however, there was a significant increase in fatigue components (p = 0.031) (Table 3). There was no main effect of day (baseline and recovery) on total mood disturbance (p = 0.313) or vigour (p = 0.1038). There was a main effect of day (baseline and recovery) on fatigue (p = 0.047); however, post hoc testing revealed no days were statistically significantly different from one another.
Fatigue and wellbeing
There was no change from baseline to post-race (day 1) in fatigue (p = 0.2271), sleep (p > 0.999), stress (p = 0.0625), mood (p = 0.234), or total score (p = 0.3301) (Table 2). There was no significant main effect of day (baseline and recovery period) on fatigue (p = 0.0703) and stress (p = 0.2651). There was a significant main effect of day (baseline and recovery period) on sleep (p = 0.0162), mood (p = 0.004) and total score (p = 0.0253); however, post hoc testing revealed that no days were statistically significantly different from one another.
Discussion
This study aimed to contribute to the understanding of cardiac autonomic adjustments and changes in psychometric indices following an ultra-endurance running event. As hypothesised, the race was associated with a significant reduction in parasympathetic activity accompanied by significant increases in fatigue and muscle soreness. While cardiac autonomic function was disturbed, this returned to baseline values within 2 days following the race, with a longer time course for recovery identified for fatigue and muscular soreness which took until 5 days post-race to return to baseline. The temporal dynamics of recovery oppose our hypothesis, where our findings support contextualising cardiac autonomic recovery following an ultra-endurance event with perceptual measures to determine optimal training stress-recovery balance.
Disturbances in autonomic function were evident approximately 20 h following the 64 km run with resting heart rate significantly increased from baseline and reductions in vagally mediated indices of HRV. Reductions in STD-RR have been reported immediately following a competitive 46 km high-altitude trail run (Bernardi et al. 1997) (44.3 ± 4.7 to 19.0 ± 3.6 ms; p < 0.001) in a similar endurance trained population (13 male and 4 female), with values returning to baseline within 24 h post-race. However, as baseline values were taken in a laboratory the evening (14:00–19:00) prior to the altitude run (Bernardi et al. 1997), it is possible that the true magnitude of suppression may have been underestimated. Research in competitive swimmers has shown STD-RR is significantly supressed in athletes presenting high competition associated anxiety (57.9 ± 25.2 ms) when compared to athletes with low competition anxiety (66.1 ± 22.3 ms) (p < 0.01; effect size = 0.6) (Fortes et al. 2017). HRV measures taken close (within 24 h) to the start of a competitive event may be influenced by anxiety or other pre-race stressors (including travel and sleep disturbances) which would affect baseline parasympathetic activity (Stanley et al. 2013). Ideally, standardised timing of measures should be employed to reduce the influence of diurnal fluctuations in HRV (Kim et al. 2014). Mertová et al. (2017) which have reported post-race vagal activity to be recovered 30 h (21:00–22:00) following a high-altitude marathon of similar duration to the present study (338 min vs 392 min, respectively); however, measures were compared to a baseline measure taken 06:00–08:00 the morning of the race. In the present study, we implemented consistency in the timing of HRV measures (upon waking each morning), and as such, HRV was monitored in 24 h intervals. The exercise stress elevated resting heart rate and impacted time-domain indices of HRV, suggesting that cardiac autonomic balance was not restored 1 day following the race.
The 64 km ultra-marathon competitors in the present study also experienced a reduction in HRV frequency-domain indices of low- and high-frequency power that led to a possible decrease in LnLF/HF ratio. Limited research has monitored the response of high-frequency HRV indices during defined recovery periods following ultra-endurance competition. Immediately following a 46 km high-altitude trail run (Bernardi et al. 1997) and a high-altitude marathon (Mertová et al. 2017), reductions in LF and HF power were observed, conversely, there was an increase in LF/HF ratio. Frequency-domain measures had returned to baseline 24 h post-race following the 46 km high-altitude trail run (Bernardi et al. 1997), and 30 h following the high-altitude marathon (Mertová et al. 2017). The inconsistencies between post-race HRV timings may explain the disagreement in recovery kinetics between the studies. It is interesting to note the opposing directions of LF/HF ratio between the studies. However, care must be taken when interpreting these results as the LF component of the ANS reflect some unknown interactions between both sympathetic and parasympathetic components (Billman 2006), and the use of LF/HF as a measure of sympatho-vagal balance is based on four interrelated assumptions (Billman 2013). However, the response of time- and frequency-domain indices in our study suggest that the post-event increase in resting heart rate on day 1 of recovery was associated with continued withdrawal of vagal activity and a possible attenuation of sympathetic modulation. The results establish that cardiac autonomic function is significantly altered in response to a 64 km ultra-marathon in endurance trained athletes.
By day 2 of recovery from the 64 km race, changes in time-domain indices from baseline were trivial. A slight increase in resting heart rate and a reduction in mean R–R were observed; however, these changes were not significant. Given these findings, it appears that parasympathetic-related indices of HRV recovered between day 1 and day 2 following completion of the event. However, the frequency-domain indices of LnLF and LnLF/HF ratio remained unstable during the recovery period. Of note, the LnLF/HF ratio increased on day 6 of recovery and may be attributable to the large increase in training load from day 4 (289 ± 127 AU) to day 5 (457 ± 388 AU). The fluctuations suggest that the sympatho-vagal balance may have been sensitive to perturbation from baseline during the defined recovery period, and despite HRV indices returning to baseline on day 2 of recovery, these results suggest athletes may have been vulnerable to greater training-induced cardiac autonomic imbalances, potentially influenced by only partial perceptual recovery and persisting muscle soreness.
There is evidence that the magnitude of reduction in HRV post-race is associated with training status, and indeed, our findings of the acute post-race HRV response lend support to this as runners with longer race times experienced the greatest reductions in LnSDNN and LnRMSSD. In older (> 45 years) first-time long-distance runners completing a 30 km run, SDNN measured continuously for 4 days following the race was reduced from baseline (obtained from 48 to 24 h prior to the race) for 64 h post-race (Aagaard et al. 2014). A greater magnitude of disturbance in autonomic function was associated with higher levels of cardiac damage marker troponin, with less fit runners experiencing greater post-race decreases in HRV (Aagaard et al. 2014). An impact of training status on the time course of HRV recovery is also supported by Hautala et al. (2001) report of more rapid recovery of altered autonomic function following a 75 km cross country skiing race in those with higher VO2max compared to those of lower aerobic fitness. The magnitude of HRV response of an individual to an ultra-endurance race may provide a valuable benchmark for determining exercise-induced perturbations and to inform subsequent training load in the days following.
Literature is scarce on the response of subjective measures of fatigue and wellbeing following an ultra-endurance event, so these were included in our study to further contextualise recovery status (Bellenger et al. 2016). A small decrease in fatigue determined from the fatigue and wellbeing questionnaire was observed on day 1 of recovery, which was balanced by small improvements in sleep and stress components to maintain a relatively stable total score (Table 3). The more comprehensive POMS questionnaire appeared sensitive to change in athlete’s wellbeing. Literature suggests that during periods of high-training stress, subjective measures of exercise stress, fatigue, and muscle soreness increase (Meeusen et al. 2013). For 1–4 days post-race, muscle soreness was elevated from baseline, with similar post-exercise values to those reported by male and female triathletes competing in an Ironman World Championship (Stearns et al. 2018). Despite HRV returning to baseline on day 2 of recovery, it is evident that subjective measures of fatigue and wellbeing took considerably longer to return to baseline levels than HRV indices. Future research may benefit from investigating a multifactorial model, consisting of HRV and perceptual measures of fatigue and muscle soreness, to enhance the transition from ultra-endurance competition to training. In addition, other physiological parameters, such as neuromuscular and hormonal measures, may also help to contextualise recovery status of athletes.
Whilst the methodology of this study reflects the current recommendations of monitoring HRV in field settings (Buchheit 2014), there are inherent limitations including disturbances which may have included unpredicted noises, quality and quantity of sleep (Hynynen et al. 2006), psychological stress (Lehmann et al. 1992), and temperature (Bosquet et al. 2008). However, post-race changes in HRV were greater than baseline variability indicative of a true change. It is worth noting that the restoration of blood plasma via hydration following the event may have played a key role in parasympathetic recovery. Reductions in blood plasma stimulate the arterial-baroreflex and Bainbridge reflex, resulting in decreased parasympathetic activity (Buchheit et al. 2009), and subsequently, blood volume expansion is likely to inhibit response of baroreceptor reflex activation (Buchheit et al. 2009). Monitoring body mass changes and post-event hydration would be a valuable addition to future studies to contextualise the recovery dynamics of HRV.
Conclusion
In conclusion, the findings of this study suggest that cardiac autonomic control is significantly altered in response to a 64 km ultra-marathon. However, the change in autonomic control was relatively short-lived, and returned to baseline 2 days following the race, suggesting parasympathetic activity completely recovers between 20 and 44 h following ultra-endurance exercise of this nature. Whilst autonomic function is recovered within 2 days following a 64-km run, subjective measures of fatigue and wellbeing suggest that athletes were not completely recovered until day 5 post-event, with muscular soreness remaining prominent during this period. Future research may benefit from investigating a multifactorial model, consisting of HRV and perceptual measures of fatigue and muscle soreness, to enhance the transition from ultra-endurance competition to training. In addition, other physiological parameters, such as neuromuscular and hormonal measures, may also help to contextualise the recovery status of athletes. This may allow informed decisions regarding the optimal time to return to training, and aid in intensity and volume prescription to optimise physiological adaptations in athletes as they prepare for their next event.
Abbreviations
- ANOVA:
-
Analysis of variance
- ANS:
-
Autonomic nervous system
- AU:
-
Arbitrary unit
- HF:
-
High frequency
- HRV:
-
Heart rate variability
- LF:
-
Low frequency
- Ln:
-
Natural logarithm
- POMS:
-
Profile of mood states
- RMSSD:
-
Root mean square of successive differences between R–R intervals
- RPE:
-
Rating of perceived exertion
- SDNN:
-
Standard deviation of the normal-to-normal sinus-initiated inter-beat intervals
References
Aagaard P, Sahlen A, Bergfeldt L, Braunschweig F (2014) Heart rate and its variability in response to running-associations with troponin. Med Sci Sports Exerc 46:1624–1630
Batterham AM, Hopkins WG (2006) Making meaningful inferences about magnitudes. Int J Sports Physiol Perform 1:50–57
Bellenger CR, Karavirta L, Thomson RL, Robertson EY, Davison K, Buckley JD (2016) Contextualizing parasympathetic hyperactivity in functionally overreached athletes with perceptions of training tolerance. Int J Sports Physiol Perform 11:685–692
Bernardi L, Passino C, Robergs R, Appenzeller O (1997) Acute and persistent effects of a 46-kilometer wilderness trail run at altitude: cardiovascular autonomic modulation and baroreflexes. Cardiovasc Res 34:273–280
Billman GE (2006) Cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol 101:684–685
Billman GE (2013) The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 4:26
Bosquet L, Merkari S, Arvisais D, Aubert AE (2008) Is heart rate a convenient tool to monitor over-reaching? A systematic review of the literature. Br J Sports Med 42:709–714
Buchheit M (2014) Monitoring training status with HR measures: do all roads lead to Rome? Front Physiol 5:1–19
Buchheit M, Laursen PB, Al Haddad H, Ahmaidi S (2009) Exercise-induced plasma volume expansion and post-exercise parasympathetic reactivation. Eur J Appl Physiol 105:471–481
Da Fonseca-Engelhardt K, Knechtle B, Rust CA, Knechtle P, Lepers R, Rosemann T (2013) Participation and performance trends in ultra-endurance running races under extreme conditions—'Spartathlon' versus 'Badwater'. Extrem Physiol Med 2:15
Fortes LS, da Costa BDV, Paes PP, do Nascimento Júnior JRA, Fiorese L, Ferreira MEC (2017) Influence of competitive-anxiety on heart rate variability in swimmers. J Sports Sci Med 16:498–504
Foster C, Florhaug JA, Franklin J et al (2001) A new approach to monitoring exercise training. J Strength Cond Res 15:109–115
Gratze G, Rudnicki R, Urban W, Mayer H, Schlogl A, Skrabal F (2005) Hemodynamic and autonomic changes induced by Ironman: prediction of competition time by blood pressure variability. J Appl Physiol 99:1728–1735
Grove B, Prapavessis H (1992) Preliminary evidence for the reliability and validity of an abbreviated profile of mood states. Int J Sport Psychol 23:93–109
Hautala A, Tulppo MP, Makikallio TH, Laukkanen R, Nissila S, Huikuri HV (2001) Changes in cardiac autonomic regulation after prolonged maximal exercise. Clin Physiol 21:238–245
Herzig D, Asatryan B, Brugger N, Eser P, Wilhelm M (2018) The association between endurance training and heart rate variability: the confounding role of heart rate. Front Physiol 9:756
Hopkins WG, Marshall SW, Batterham AM, Hanin J (2009) Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41:3–13
Hynynen E, Uusitalo A, Konttinen N, Rusko H (2006) Heart rate variability during night sleep and after awakening in overtrained athletes. Med Sci Sports Exerc 38:313–317
Kim HS, Yoon KH, Cho JH (2014) Diurnal heart rate variability fluctuations in normal volunteers. J Diabetes Sci Technol 8:431–433
Lehmann M, Gastmann U, Petersen KG et al (1992) Training-overtraining: performance, and hormone levels, after a defined increase in training volume versus intensity in experienced middle- and long-distance runners. Br J Sports Med 26:233–242
McLean BD, Coutts AJ, Kelly V, McGuigan MR, Cormack SJ (2010) Neuromuscular, endocrine, and perceptual fatigue responses during different length between-match microcycles in professional rugby league players. Int J Sports Physiol Perform 5:367–383
Meerlo P, Sgoifo A, Suchecki D (2008) Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev 12:197–210
Meeusen R, Duclos M, Foster C et al (2013) Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc 45:186–205
Mertová M, Botek M, Krejčí J, McKune A (2017) Heart rate variability recovery after a skyrunning marathon and correlates of performance. Acta Gymnica 47:161–170
Michael S, Graham KS, Davis GM (2017) Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—a review. Front Physiol 8:301
Plews DJ, Laursen PB, Kilding AE, Buchheit M (2013a) Evaluating training adaptation with heart-rate measures: a methodological comparison. Int J Sports Physiol Perform 8:688–691
Plews DJ, Laursen PB, Stanley J, Kilding AE, Buchheit M (2013b) Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Sports Med 43:773–781
Porges SW (1992) Vagal tone: a physiologic marker of stress vulnerability. Pediatrics 90:498–504
Sasaki K, Maruyama R (2014) Consciously controlled breathing decreases the high-frequency component of heart rate variability by inhibiting cardiac parasympathetic nerve activity. Tohoku J Exp Med 233:155–163
Saw AE, Main LC, Gastin PB (2016) Monitoring the athlete training response: subjective self-reported measures trump commonly used objective measures: a systematic review. Br J Sports Med 50:281–291
Stanley J, Peake JM, Buchheit M (2013) Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Med 43:1259–1277
Stearns RL, Nolan JK, Huggins RA et al (2018) Influence of cold-water immersion on recovery of elite triathletes following the ironman world championship. J Sci Med Sport 21:846–851
Whyte G (2014) Age, sex and (the) race: gender and geriatrics in the ultra-endurance age. Extrem Physiol Med 3:1
Zaryski C, Smith DJ (2005) Training principles and issues for ultra-endurance athletes. Curr Sports Med Rep 4:165–170
Author information
Authors and Affiliations
Contributions
The study was designed by LF, CK, and JF. Data were collected by LF. Data were analysed by LF, CK, and JF. The manuscript was written by LF and CK. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Fabio Fischetti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fazackerley, L.A., Fell, J.W. & Kitic, C.M. The effect of an ultra-endurance running race on heart rate variability. Eur J Appl Physiol 119, 2001–2009 (2019). https://doi.org/10.1007/s00421-019-04187-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04187-6