Abstract
Purpose
Endurance exercise may cause transient alterations in cardiac tissue. The number of studies evaluating the relationship between changes in heart rate variability (HRV) and cardiac biomarkers following an endurance event is limited. We hypothesized that there would be a time-dependent correlation between biomarkers of cardiac damage and the reduction in parasympathetic indices of HRV within 24 h after 60 min of running in middle-aged recreational runners.
Methods
The trained, middle-aged runners who participated in this study ran 60 min at a half-marathon pace on a treadmill. Blood samples (before and 0, 4, and 24 h after the running test) and HRV data (before and 0, 1, 4, and 24 h after the running test) were obtained.
Results
After running, cardiac biomarkers (total creatine kinase, cardiac isoform of creatine kinase, creatine kinase-index [CK-Index], cardiac troponin [cTnI]) increased significantly, and HRV measures related to parasympathetic nervous system activity decreased significantly; these measures returned to baseline levels within 24 h. Finally, there were significant correlations (all p < 0.05) between the change (4 h post-running vs. pre-running) in the CK-Index and the changes (post- vs pre-running) in time-domain and nonlinear measures of HRV (r − 0.61 to − 0.67). In addition, significant correlations (all p < 0.05) were found between the area under the cTnI curve and change (1-h post- and pre-running) in time-domain and nonlinear measures of HRV (r − 0.48 to − 0.51).
Conclusions
The correlation between HRV and cardiac biomarkers indicates that HRV analysis may be an alternative approach to determine the magnitude of cardiac stress after endurance exercises.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endurance running has become one of the most popular leisure sports activities in recent years. Every year, millions of people compete in races over different distances, from 5 km to ultramarathon length. For many people, the desire for a healthy life is an important source of motivation for regular running. However, there is debate over the various possible risks that vigorous endurance running may pose to athletes’ cardiovascular system [1]. The incidence of sudden cardiac death in endurance events is 1:50,000, predominantly affecting middle-aged men (aged 35–65 years), who generally constitute more than 40% of competitors in such events [2]. Even though many participants complete endurance events without any complaints, numerous investigators have shown transient echocardiographic, electrophysiological, and biochemical alterations after endurance exercise [3].
Some reports have been published regarding an increase in the incidence of atrial fibrillation or arrhythmogenic right ventricular cardiomyopathy among endurance athletes, together with alterations in cardiac biomarkers [4]. The cardiac isoform of creatine kinase (CK-MB) and cardiac troponin (cTn) are elevated after short- and long-distance exercise [5]. Moreover, the creatine kinase index (CK-Index; ratio of CK-MB to CK) may be used to estimate the relative contribution of cardiomyocyte damage versus nonspecific myocyte damage when elevated levels of CK and CK-MB are present [6]. Even though the increase is usually thought to be physiologic rather than pathologic, exercise-induced elevations in cardiac troponin have been shown to correlate with transient right ventricular systolic and biventricular diastolic dysfunction [7, 8]. Moreover, Yeo et al. investigated changes in peripheral blood biomarkers of myocardial injury with right and left ventricular strain in recreational runners completing a 21 km treadmill run. These investigators observed reversible elevation of cardiac biomarkers of myocardial injury, which was thought to be associated with ventricular stunning [9]. Previous studies have shown that prolonged exercise increases cardiac biomarkers (CK, CK-MB, myoglobin, troponin, LDH, AST, and NT-proBNP) and postexercise transient myocardial dysfunction. Regular physical activity is a part of current recommendations for preventing cardiovascular diseases and cardiac rehabilitation. However, endurance athletes over than 35 years of age may have myocardial fibrosis, elevated coronary artery calcium scores, and a greater incidence of atrial fibrillation. In addition, high-volume, high-intensity training regimens may, at least in some individuals, create a substrate for adverse cardiovascular adaptations and the potential for exercise-related acute cardiac events [10, 11]. Moreover, investigators emphasize that repetitive cardiac microtraumas during endurance activities, as indicated by troponin release, may cause long-term cardiac maladaptation [9].
Heart rate variability (HRV) analysis is a widely used, noninvasive, time-efficient, and cost-effective method for assessing cardiovascular autonomic nervous system function [12]. Within a few minutes to hours after exercise, cardiac parasympathetic activity decreases, and sympathetic nerve activity increases. This change in cardiac autonomic modulation is due to reduced baroreceptor activity and accumulation of stress metabolites in the blood and skeletal muscle [13]. The return of parasympathetic activity to baseline levels is defined as cardiac parasympathetic reactivation and is affected by exercise intensity. Parasympathetic reactivation takes less than 24 h following low-intensity exercise, whereas it occurs within 24–48 h following exercises performed at the gas exchange threshold. This duration may exceed 48 h after high-intensity exercises [13].
It is well known that exercise elicits substantial changes in HRV measures. However, the number of studies evaluating the relationship between changes in HRV and cardiac biomarkers following an endurance event is limited. Even though investigators described significant changes in cardiac biomarkers following running events in all these studies, only a few of the post-race HRV measures showed significant relationships with cardiac biomarkers [3, 14, 15]. Accordingly, it is difficult to say that a consistent relationship exists between HRV and cardiac biomarker changes after running. These studies' common feature is that the blood samples were taken from the participants only before and after the competition. This methodological approach may not be sufficient to evaluate changes that occur later after the completion of the endurance event. In a recent study, Huang et al. investigated the heart rate variability and cardiac troponin T (cTnT) kinetic responses to continuous high- and moderate-intensity exercise in young, sedentary, overweight/obese women. These investigators found a significant transient reduction in parasympathetic activity and an elevation in cardiac troponin T following exercise. However, the changes in HRV and cTnT were not temporally associated [16]. In this study, we aimed to investigate a possible time-dependent relationship between HRV measures and cardiac markers following 60 min of running on a treadmill at a half-marathon pace in trained middle-aged athletes, who may be at risk for exercise-related cardiac events. We hypothesized that there might be a time-dependent correlation between biomarkers of cardiac damage and the reduction in parasympathetic indices of HRV within 24 h after a 60-min bout of running in trained middle-aged athletes.
Methods
Participants
Eighteen middle-aged men who were trained long-distance runners participated in this study (age 46.8 ± 7.1 years; height 171.2 ± 5.7 cm; weight 73.3 ± 7.7 kg). The participants were recreationally trained athletes (\(\dot{V}O_{2max}\): 47.6 ± 4.8 ml/min.kg; training history: 11.6 ± 9.0 years; weekly training distance: 72.2 ± 27.8 km) and were members of a local athletic club. The study was explained to all participants in detail, and the participants signed informed consent forms. The study was performed in accordance with the Declaration of Helsinki, with the approval of the Çukurova University Faculty of Medicine Clinical Research Ethics Committee no. 2018/83. All tests were conducted at the Sports Physiology Research and Analysis Laboratory of the Physiology Department of Çukurova University Medical Faculty before noon at a constant temperature of 23 ± 1 °C.
Study design
The experimental protocol for the study is illustrated in Fig. 1. The participants visited the laboratory three times throughout the study. Physical examination, anthropometrical measurements, and a maximal cardiopulmonary exercise test (CPET) were performed during their first visit. The participants were asked to run for 60 min on a treadmill with a pace calculated from their CPET data on their second visit. Blood samples were collected before, immediately after, and 4 and 24 h after the 60-min running test, whereas HRV data were recorded before; immediately after; and 1, 4, and 24 h after the 60-min running task.
Physical examination and anthropometrical measurements
The participants had a general physical examination during their first visit to the laboratory. They had pulmonary function tests (slow vital capacity, forced vital capacity, and maximum voluntary ventilation) (b2 Cosmed, Italy), and their blood pressure was measured with a sphygmomanometer (ERKA, Germany). Resting 12-lead electrocardiography (ECG) recordings were also performed (Quark T12x, COSMED, Italy).
Body mass and height were determined with a scale (accurate to 20 g) (Kurdaklar Baskül, Turkey) and a stadiometer (accurate to 0.1 cm) (Sport Expert, Turkey), respectively. Anthropometric measurements were performed before the exercise by the same person. The participants removed their shoes and excess clothing prior to measurements.
Maximal CPET and individual running pace calculation
A maximal CPET was performed with an indirect calorimeter (PFT Cosmed, Italy) on a treadmill (HP Cosmos, Nussdorf–Traunstein, Germany). Volume and gas calibration of the system was performed using a 3-l calibration syringe and calibration gases (room air and a gas mixture composed of 16% O2 and 5% CO2), respectively. The heart rate was recorded continuously by telemetry using a heart rate monitor (Cosmed, Italy). The participants started the test at 6 km/h, and the speed increased by 1 km/h every minute until exhaustion. The treadmill’s incline was kept constant at 1% throughout the test. After the test, the data were collected, and individual gas exchange thresholds were calculated using the V-slope method [17]. The test was terminated if one or more of the following criteria were fulfilled: reaching up to 90% of the maximum heart rate according to the 220-age formula, formation of an oxygen uptake plateau (change less than 2 ml/kg body mass), or persistence of a non-protein respiratory quotient (npRQ) value of 1.15 or greater [18].
Sixty-minute running test
The participants were asked to run on a treadmill (LifeFitness CLST, USA) with a pace determined from their maximal CPET in the 60-min running test. The participants’ running pace was determined as the running speed corresponding to the oxygen uptake value at the midpoint between the maximal oxygen uptake and the gas exchange threshold point. There was a strong correlation (r = 0.84, p < 0.000) between the participants’ last half-marathon average pace (12.8 ± 1.7 km/h) and the calculated test pace (12.6 ± 1.4 km/h). Before the test, the athletes started walking at 6 km/h and 1% incline on the treadmill, and they voluntarily increased the speed up to their running speed for warm-up. At the 6th minute, the treadmill stopped, and all athletes stretched for 2 min.
After the warm-up period, the participants started to run at the previously determined pace with a 1% incline. The exclusion criteria were termination of the test before the end of 60 min and lowering the pace below the gas exchange threshold; none of the participants were excluded from the study.
Experimental procedures
A professional nurse performed all the blood sampling. The samples were withdrawn from the antecubital vein (10 ml) directly into anticoagulant-containing (EDTA) and anticoagulant-free vacutainer tubes (BD, US) for hemograms and blood biochemistry analysis, respectively. The tubes were inverted gently to mix the blood with the anticoagulant. The samples were kept cold in an insulated box throughout the blood collection period. When the blood sampling was complete, the samples were transported to the Çukurova University Faculty of Medicine Balcalı Hospital, Central Laboratory, certified by the JCI (Joint Commission International). The analytical kits for CK, CK-MB, and cTnI were provided by Beckmann Coulter (Beckmann Coulter, USA). Total CK was measured in serum using a kinetic enzymatic method (Beckmann Coulter AU5800, USA). CK-MB and cTnI were measured using the chemiluminescence method (Beckmann Coulter DXI600, US and Beckmann Coulter DXI600, USA, respectively). CK-MB is divided by total CK to calculate CK-Index, which estimates the relative contribution of cardiomyocyte damage [6].
Heart rate variability measurement
Inter beat interval (IBI) measurements were performed with Actiheart monitors (CamNtech, UK) using two Ag/AgCl pre-gelled electrodes (3 M Red Dot Electrodes, IL, USA) attached to the participants’ chest [19]. HRV measurements were performed before; immediately after; and 1, 4, and 24 h after the 60-min running test. The participants were instructed to sit comfortably and remain awake and calm with minimal body movement throughout the measurements. The participants were allowed to breathe spontaneously without talking, since the normal respiratory rate may not result in significantly different heart rate-derived indices compared with controlled breathing [20]. The participants were asked to refrain from exercise, alcohol, caffeine, or other stimulants at least 12 h before the testing days HRV was measured in the last 5 min of the 10-min period to ensure a stable data recording. At the end of the recording period, beat-to-beat HR was screened by visual inspection, converted to IBI, and exported in ASCII format for further analysis. IBI data were analyzed by Kubios HRV analysis software, version 3.2.0 (Biosignal Analysis and Medical Imaging Group—University of Kuopio, Finland). Ectopic beats and artifacts (< 3%) were automatically identified and corrected according to the manufacturer’s recommendations to generate normal-to-normal (NN) interval time series [21].
HRV analysis was performed by using time-domain, frequency-domain, and nonlinear measurements [22]. The time-domain indices included the standard deviation of all normal R-R intervals (SDNN), the root mean square difference of successive normal R-R intervals (RMSSD), mean heart rate (HR), and the percentage of successive R-R intervals that differed by more than 50 ms (pNN50).
Fast Fourier transform analysis based on Welch’s periodogram method was used for the frequency-domain calculations (window width: 300 s; window overlap: 50%; points in the frequency domain: 300 points/Hz). R-R intervals were examined as frequency-domain measures within the low-frequency (LF: 0.04–0.15 Hz) and high-frequency bands (HF: 0.15–0.40 Hz). The frequency-domain indices were expressed as absolute (ms2), normalized to total power (nu), and logarithm-transformed absolute (log ms2). The sympathovagal balance was quantified by the LF to HF (LF/HF) ratio.
The Poincaré plot was examined, and SD1 (the standard deviation of the Poincaré plot perpendicular to the identity line), SD2 (the standard deviation of the Poincaré plot along the line-of-identity), and SD1/SD2 ratio were calculated [22]. Additionally, the stress score (SS, a value directly proportional to the sympathetic modulation over the sinus node, calculated as the inverse of the SD2 multiplied by 1000; SS = 1000/SD2) and sympathetic-parasympathetic ratio (S/PS ratio, expressed as the ratio between SS and SD1) were calculated [23].
Time-domain measures (SDNN, RMSSD, and pNN50) are closely correlated with PNS activity and are used to evaluate vagal activity. In frequency domain measures, HF represents vagal modulation, whereas sympathetic and parasympathetic nervous system activities produce LF. LF/HF is accepted to reflect the sympathovagal balance [24]. In addition, Poincaré plot analysis is a nonlinear method that is the least common HRV analysis technique and reflects sympathetic–parasympathetic fluctuations. In the Poincaré plot, each IBI is plotted against its corresponding preceding interval, showing a qualitative picture of the variations between IBIs. The points are scattered around the identity line, and all the points converge into an ellipse shape. SD1, which is the transversal axis (standard deviation of points perpendicular to the line of identity), is correlated with parasympathetic activity, whereas SD2, which is the longitudinal axis (the standard deviation of points parallel to the identity line), is considered representative of long-term variability in the normal beat intervals [23, 25]. SD1/SD2 is correlated with the LF/HF ratio, and SS and SS/PS are novel indices used to improve the physiological meaning of HRV by the Poincaré plot analysis method [23].
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS for Windows Version 22.0, USA). The normality of values was assessed with the Shapiro–Wilk test. Repeated-measures ANOVA was performed to evaluate the differences in the mean values of the measured physiological parameters and HRV measures. The equality of variances between the data points was assessed with Mauchly’s test of sphericity. The results are presented as the mean ± SD. If the assumption of sphericity was violated, the Greenhouse–Geisser correction was applied. If there were any significant differences in the test of within-subject effects, post hoc comparisons using Bonferroni's method were performed to determine pairwise differences. The Wilcoxon signed-rank test was conducted for the nonparametric data. Instead of absolute values, the change in variables before and after the test was calculated as a percentage. In addition, the area under the curve for cTnI was calculated by integrating the cTnI plot from pre-running to 24 h post-running. To quantify the relationship between CK-Index, cTnI, and HRV measures, Spearman’s correlation analysis was performed between percent change values rather than absolute values. Statistical significance was set at p < 0.05.
Results
All of the participants completed the 60-min running test. The participants started the test at their individually calculated paces (12.6 ± 1.4 km/h) as described in the methodology section. Seven of the 18 participants did not change their running speed during the test; ten participants decreased their running speed, whereas only one increased his pace. The average running speed of the participants was 12.1 ± 1.4 km/h, and the distance covered was 12.0 ± 1.4 km.
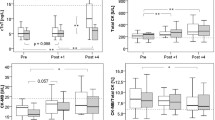
The changes in the CK, CK-MB, CK-Index, and cTnI are shown in Table 1. The CK levels increased significantly following the 60 min of running at a half marathon pace. Although 22% of the participants (four of the 18 participants) had CK levels above the upper limit before the test, which is a clinical indicator of the occurrence of muscle microinjuries, the ratio increased to 61% immediately after and 4 h after running (11 of the 18 participants). The ratio continued to increase and at the 24th hour following the test reached 78% (14 of the 18 participants).
The mean CK-MB value before the test was 4.4 ± 3.1 ng/ml and increased significantly following the end of the test (6.0 ± 4.2 ng/ml) (p < 0.001). While the number of people with a CK-MB value above the limit before the test was six (33%), the number of people above the limit immediately after, 4 h after and 24 h after the test increased to eight (44%), ten (56%) and 13 (72%), respectively (Table 1). In addition, the calculated CK-Index values 4 h after 60 min of running were significantly higher than the pre- and post-running values (p < 0.05). The 4-h post-running cTnI levels were significantly higher than the pre-running (p < 0.01) and immediate post-running (p < 0.001) values. The cTnI value was still significantly higher than the pre-running value 24 h after the 60-min running test (Table 1).
Table 2 presents heart rate variability indices before; immediately after; and 1, 4, and 24 h after 60 min of running at a half-marathon pace. Since one person could not be recorded at the end of the run (post-running), 17 HRV values were used for statistical analysis.
In the time domain, the mean heart rate increased significantly following the 60 min of running (p < 0.001) and stayed significantly higher than the pre-running values at 1 h (p < 0.001) and 4 h post-running (p < 0.01). The SDNN, RMSSD, and pNN50 values measured after running were significantly lower than the pre-running values (p < 0.05 for SDNN and p < 0.01 for RMSSD and pNN50). One hour and 4 h after running, these variables were still significantly lower than the pre-running values (p < 0.05 for SDNN and p < 0.01 for RMSSD and pNN50). At the 24th hour after running, the time domain measures were returned to pre-running values.
In the frequency domain, post-running high frequency (HF), relative high frequency, and log-transformed high frequency (log-HF) decreased significantly (p < 0.05). In contrast, low frequency (LF), relative low frequency, and log-transformed low frequency (log-LF) and the LF/HF ratio increased (p < 0.01) significantly compared to pre-running. One-hour post-running (p < 0.001) and 4-h post-running (p < 0.05) values were also significantly different from pre-running values. Similar to the time-domain measures, the frequency-domain measures took 24 h to return to the pre-running values.
In the nonlinear domain, after 60 min of running, the SD1 and SD2 indices decreased (p < 0.05), whereas the SD1/SD2 ratio increased (p < 0.01) significantly compared to pre-running. A statistically significant difference for the nonlinear-domain measures was also found at the 1st (p < 0.05 for SD2; and p < 0.01 for SD1 and SD1/SD2) and 4th hours after (p < 0.05 for SD1 and SD1/SD2; and p < 0.01 for SD1) the 60 min of running, and these measures took 24 h to return to their pre-running values.
The changes in the stress score and the sympathetic/parasympathetic ratio are given in Fig. 2A, B, respectively. The parameters showed a similar pattern following the 60 min of running, with a significant increase immediately after the run and a gradual decrease after that point. The stress scores calculated immediately post-running (39.2 ± 18.7), 1-h post-running (31.6 ± 11.1), and 4-h post-running (30.0 ± 12.0) were significantly higher than the pre-running (22.7 ± 9.0) (p < 0.05) and 24-h post-running (23.2 ± 11.2) scores (p < 0.05).
A Changes in stress score ratios before, immediately after, 1 h after, 4 h after, and 24 h after 60 min of running at a half-marathon pace. Values are the mean ± SD. * represents a significant difference from pre-running, and # represents a significant difference from 24 h post-running. B Changes in sympathetic/parasympathetic ratios before, immediately after, 1 h after, 4 h after, and 24 h after 60 min of running at a half-marathon pace. Values are the mean ± SD. * represents a significant difference from pre-running. & represents a significant difference from 1 h post-running, and # represents a significant difference from 24 h post-running
The sympathetic/parasympathetic ratio calculated after running (7.1 ± 7.3) was significantly higher than the pre-running (1.4 ± 1.3) (p < 0.01) and 24-h post-running (1.4 ± 1.3) (p < 0.01) values. The calculated ratios at 1 h post-running (3.6 ± 2.8) and 4 h post-running (3.2 ± 2.9) were also significantly higher than the pre-running (p < 0.01) and 24-h post-running values (p < 0.01).
The correlation between relative changes in HRV measures and changes in the CK-Index after the running task is given in Table 3. The CK-Index value was used because it had more correlations than CK, CK-MB, or cTnI.
The relationship between the changes in the stress score (post- and pre-running) and CK-Index (4 h post-running and pre-running) is given in Fig. 3A. There was a significant and moderate positive correlation between the changes in the stress score and the CK-Index. The relationship between the changes in the sympathetic/parasympathetic ratio (post- and pre-running) and CK-Index (4 h post-running and pre-running) is given in Fig. 3B. There was a significant, moderately strong positive correlation between the sympathetic/parasympathetic ratio changes and the CK-Index.
A Relationship between the changes in the stress score (SS) ratio (post- and pre-running) and the CK-Index (4 h post-running and pre-running). The solid line indicates the estimated association, and the dotted lines represent the pointwise 95% confidence interval. B Relationship between the changes in the sympathetic/parasympathetic (SS/PS) ratio (post- and pre-running) and the changes in the CK-Index (4 h post-running and pre-running). The solid line indicates the estimated association, and the dotted lines represent the pointwise 95% confidence interval
The correlation coefficients between relative changes in HRV measures (1-h post-running and pre-running) and the area under the cTnI curve, calculated by integrating the cTnI plot ranging from pre-running to 24 h post-running, are given in Table 4.
Discussion
This study evaluated the relationship between cardiac biochemical markers and HRV measures following 60 min of running at a half-marathon pace in trained middle-aged athletes. The main findings of the study are as follows: (a) HRV measures showed a significant decrease in cardiac parasympathetic activity following running compared to pre-running, and this reduction gradually increased to pre-running values in 24 h after the 60-min running task; (b) cardiac markers showed a significant elevation after 60 min of running and remained elevated up to 24 h; and (c) significant correlations were found between changes in HRV and cardiac biomarkers after 60 min of running, and there was a significant and moderate correlation between the changes in the HRV measures and the changes in the CK-Index. In addition, changes in the HRV measures are were correlated with total cTnI accumulation. The strong association of cardiac autonomic modulation changes with the CK-Index and the area under the cTnI curve is a novel observation.
Changes in heart rate variability measures
In our study, there was an apparent increase in sympathetic modulation and vagal withdrawal effect just after termination of 60 min of running. It took approximately 24 h to return to the resting levels; this value is consistent with previously published data [26,27,28]. The time-domain measures (RMSSD, SDNN, and pNN50) reflect an apparent parasympathetic withdrawal following running [22]. In addition, sympathovagal balance-related frequency-domain measures (LF/HF) and indices of nonlinear HRV measures (SD1, SD2, SS index, and S:PS ratio) showed that 60 min of running elevated sympathetic modulation [23, 29]. The changes in cardiac autonomic regulation are explained by the changes in systemic baroreflex and skeletal muscle metaboreflex responses together with the exercise-induced release of epinephrine into the systemic circulation, which may be prolonged up to 24–72 h [13]. Thus, a 60-min bout of running at a half-marathon pace was sufficient to induce vagal withdrawal.
Changes in cardiac markers
Plasma CK-MB elevation is one of the indicators of myocardial injury, and various investigators detected significant CK and CK-MB elevations after completing half-marathons [30], marathons [30,31,32], and ultramarathons [33, 34]. In our study, CK and CK-MB levels increased following 60 min of running, and even after 24 h, the values were still above the upper reference limit (URL), indicating possible cardiomyocyte damage[35, 36].
It was interesting to find significant correlations between changes in HRV and the CK-Index. This relationship suggests that a post-running reduction in parasympathetic activity may indicate a cardiovascular strain that might manifest with a significant increase in the CK-Index at the 4th hour after running. Since Weippert et al. mentioned that an elevation of this biomarker reflects a physiological rather than a pathological phenomenon in healthy exercising subjects, our findings may suggest that the possibility of cardiovascular strain should not be ignored [35,36,37]. It is also important to remember that elevation of cardiac biomarkers may not reflect an adverse cardiovascular event, and reduced cardiac parasympathetic modulation may require further evaluation for diagnosis.
Cardiac troponins are the preferred biomarkers for evaluating myocardial injury, defined as being present when blood levels of cTn increase above the 99th percentile URL [38]. It has been shown previously that serum cTnI and cTnT increase after prolonged and strenuous exercise [30, 39,40,41]. In our study, approximately 40% of the participants’ cTnI levels were above the URL (seven of the 18 participants) at the 4th hour post-running. As suggested by Engel et al. adding the CK-MB relative index (CK-Index) to troponin T increased the diagnostic sensitivity for detecting myocardial damage [42]. With this in mind, if our data are evaluated by the same criterion, 33% of the participants might have a transient myocardial injury, which may be a serious finding.
HRV and cardiac biomarker correlation
Although autonomic recovery after exercise is a hot topic [13, 29, 43], there have been few studies aiming to evaluate the relationship between changes in HRV and cardiac biomarkers following endurance events [3, 14, 15]. Evaluation of the cardiovascular consequences following a 160-km ultramarathon was investigated by Scott et al. In contrast to our data, cTnT levels did not change significantly, which may be related to the exercise intensity. In addition, the authors found little evidence of an association between changes in cTnT and HRV [14]. However, a 118-km mountain race increased high-sensitivity cTnT and decreased sample entropy, which measures the regularity and complexity of a time series. The magnitude of cardiac damage biomarkers increases, and cardiac autonomic modulation disturbance appears to be interrelated [15]. On the other hand, cardiac troponins reach their peak level approximately 3–6 h after the endurance event. Therefore, a single cTnT measurement immediately after the race may not reflect peak concentrations and may not be enough to predict a possible relationship between cardiac-specific biomarkers and HRV measures.
In our study, the significant time-dependent correlations between changes in HRV and CK-Index following 60 min of running were novel findings (Table 3). On the other hand, the significant correlations between changes in HRV and area under the cTnI curve (accumulation of cTnI) rather than relative changes in the cTnI concentrations is another important finding that may indicate possible myocardial injury [44]. The robust relationship between the area under the cTnI curve and changes in post-running HRV (time and nonlinear domains) indicates that individuals with greater autonomic alterations show higher cardiovascular strain following endurance running.
In the literature, various investigators have shown that the intensity/duration of exercise is correlated with an increase in cardiac markers and a change in cardiac autonomic modulation [13, 27, 30]. The exercise-induced elevation in cardiac troponins may be explained by the increase in membrane permeability of cardiac myocytes, which might be due to increased mechanical stress, overload with free radicals, increased body temperature, or prolonged acidosis [4]. In addition, Stanley et al. reported in a review that a reduction in postexercise parasympathetic activity correlates significantly with postexercise plasma epinephrine levels, blood lactate concentrations, blood acidosis, and arterial oxygenation [13]. Therefore, postexercise cardiac parasympathetic activity reduction may prolong the duration of cardiovascular strain, manifesting with the correlation between changes in HRV indices and cardiac biomarkers such as CK-Index and cTnI (Tables 3, 4).
Regular moderate-intensity exercise, without exposure to the level of cardiovascular strain, is suggested and prescribed for wellness and cardiovascular health in the general population. On the other hand, endurance athletes' training intensity and duration are much higher than recommended for beneficial effects; thus, exercise may be a double-edged sword for these individuals. Even if there are no typical cardiovascular symptoms, prolonged reductions in parasympathetic indices may indicate transient cardiac injury in endurance athletes. It is important to remember that repetitive cardiac microtraumas during endurance activities, as indicated by troponin release, might cause long-term cardiac maladaptation. We recommend that long-distance runners be cautious following intense training and races. The athletes should reduce the intensity of their training program and consult a professional for further examination in case of a reduction in parasympathetic measures in HRV. The results of our study indicate that evaluating post-race HRV changes might be a valuable noninvasive tool for detecting cardiovascular strain individually, serving as an alternative to blood sampling, which may be impractical, especially for marathons and other events with large numbers of participants. Therefore, noninvasive, time-efficient, and cost-effective HRV analysis may be a viable alternative method to inform athletes about the magnitude of cardiac stress to which they have been exposed.
Limitations
This study showed a significant relationship between changes in HRV and cardiac biomarkers following 60 min of running at a half-marathon pace. Even though there is a positive relationship between cardiac autonomic indices and cardiac biomarkers, increasing the number of participants may strengthen this correlation. There are reports of gender differences in HRV responses. In this study, we investigated the changes in HRV and cardiac biomarkers following a race-pace exercise in male participants. There were only two regularly trained women who volunteered to participate in this study. Thus, we selected only male participants due to the impracticality of achieving a balanced gender distribution. However, it may be important to investigate female athletes' HRV and cardiac biomarker responses to race-pace exercises. In addition, we chose an indoor activity that would mimic an endurance event; however, the participants were not affected by the environmental stress conditions they would typically encounter outdoors. It is well known that environmental stress may affect the physiological responses of athletes during exercise. Further evaluation of the changes in cardiac biomarkers and HRV during outdoor events may provide more information about the athletes’ exposure to cardiovascular strain.
Conclusions
Sixty minutes of running at a half-marathon pace is sufficient to increase cardiac parasympathetic modulation and increase cardiac stress biomarkers. After running-induced parasympathetic withdrawal, parasympathetic modulation took approximately 24 h to return to baseline levels; however, cardiac biomarkers were still above the pre-running values at this time. Moreover, the changes in HRV measures following 60 min of running correlate with the changes in the CK-Index and total cTnI accumulation. However, frequent blood sampling to detect possible cardiovascular strain is not practical, especially after a race. Therefore, noninvasive, time-efficient, and cost-effective HRV analysis may be an alternative approach to inform athletes about the magnitude of cardiac stress to which they have been exposed.
References
Parry-Williams G, Sharma S (2020) The effects of endurance exercise on the heart: panacea or poison? Nat Rev Cardiol 17:402–412. https://doi.org/10.1038/s41569-020-0354-3
Parry-Williams G, Gati S, Sharma S (2021) The heart of the ageing endurance athlete: The role of chronic coronary stress. Eur Heart J 42:2737–2744. https://doi.org/10.1093/eurheartj/ehab095
Aagaard P, Sahlén A, Bergfeldt L, Braunschweig F (2014) Heart rate and its variability in response to running—associations with troponin. Med Sci Sports Exerc 46:1624–1630. https://doi.org/10.1249/MSS.0000000000000270
Sedaghat-Hamedani F, Kayvanpour E, Frankenstein L et al (2015) Biomarker changes after strenuous exercise can mimic pulmonary embolism and cardiac injury—a metaanalysis of 45 studies. Clin Chem 61:1246–1255. https://doi.org/10.1373/clinchem.2015.240796
Donnellan E, Phelan D (2018) Biomarkers of cardiac stress and injury in athletes: what do they mean? Curr Heart Fail Rep 15:116–122. https://doi.org/10.1007/s11897-018-0385-9
Xue Y, Daniels LB, Maisel AS, Iqbal N (2014) Cardiac biomarkers. Reference module in biomedical sciences. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-801238-3.00022-2
La Gerche A, Burns AT, Mooney DJ et al (2012) Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 33:998–1006. https://doi.org/10.1093/eurheartj/ehr397
Mousavi N, Czarnecki A, Kumar K et al (2009) Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol 103:1467–1472. https://doi.org/10.1016/j.amjcard.2009.01.294
Yeo TJ, Ling LH, Lam CSP et al (2020) Cardiac and renal biomarkers in recreational runners following a 21 km treadmill run. Clin Cardiol 43:1443–1449. https://doi.org/10.1002/clc.23459
Franklin BA, Thompson CPD, Al-Zaiti SS et al (2020) Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective-an update: a scientific statement from the American Heart Association. Circulation. https://doi.org/10.1161/CIR.0000000000000749
Lüscher TF (2016) Sport, exercise, and daily activity: a double-edged sword revisited. Eur Heart J 37:2505–2507. https://doi.org/10.1093/eurheartj/ehw340
Cataldo A, Zangla D, Cerasola D et al (2016) Influence of baseline heart rate variability on repeated sprint performance in young soccer players. J Sports Med Phys Fitness 56:491–496
Stanley J, Peake JM, Buchheit M (2013) Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sport Med 43:1259–1277. https://doi.org/10.1007/s40279-013-0083-4
Scott JM, Esch BTA, Shave R et al (2009) Cardiovascular consequences of completing a 160-km ultramarathon. Med Sci Sports Exerc 41:25–33. https://doi.org/10.1249/MSS.0b013e31818313ff
Martínez-Navarro I, Sánchez-Gómez JM, Collado-Boira EJ et al (2019) Cardiac damage biomarkers and heart rate variability following a 118-km mountain race: relationship with performance and recovery. J Sport Sci Med 18:615–622
Huang C, Kong Z, Nie J et al (2021) Impact of high-intensity interval and moderate-intensity continuous exercise on heart rate variability and cardiac troponin. J Sports Med Phys Fit 61:1301–1308. https://doi.org/10.23736/S0022-4707.20.11657-8
Beaver WL, Wasserman K, Whipp BJ (2016) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 121:2020–2027
Balady GJ, Arena R, Sietsema K et al (2010) Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122:191–225
Brage S, Brage N, Franks PW et al (2005) Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr 59:561–570. https://doi.org/10.1038/sj.ejcn.1602118
Bloomfield DM, Magnano A, Bigger JT et al (2001) Comparison of spontaneous vs. metronome-guided breathing on assessment of vagal modulation using RR variability. Am J Physiol - Hear Circ Physiol 280:1145–1150. https://doi.org/10.1152/ajpheart.2001.280.3.h1145
Tarvainen MP, Niskanen JP, Lipponen JA et al (2014) Kubios HRV—heart rate variability analysis software. Comput Methods Programs Biomed 113:210–220. https://doi.org/10.1016/j.cmpb.2013.07.024
Shaffer F, Ginsberg JP (2017) An overview of heart rate variability metrics and norms. Front Public Heal 5:1–17. https://doi.org/10.3389/fpubh.2017.00258
Orellana JN, De La Cruz TB, Cachadiña ES et al (2015) Two new indexes for the assessment of autonomic balance in elite soccer players. Int J Sports Physiol Perform 10:452–457. https://doi.org/10.1123/ijspp.2014-0235
Malik M, Thomas Bigger J, John Camm A, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ (1996) Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17(3):354–381. https://doi.org/10.1093/oxfordjournals.eurheartj.a014868
Pham T, Lau ZJ, Chen SHA, Makowski D (2021) Heart rate variability in psychology: a review of HRV indices and an analysis tutorial. Sensors 21:1–20. https://doi.org/10.3390/s21123998
Bernardi L, Passino C, Robergs R, Appenzeller O (1997) Acute and persistent effects of a 46-kilometre wilderness trail run at altitude: cardiovascular autonomic modulation and baroreflexes. Cardiovasc Res 34:273–280. https://doi.org/10.1016/S0008-6363(97)00025-4
Fazackerley LA, Fell JW, Kitic CM (2019) The effect of an ultra-endurance running race on heart rate variability. Eur J Appl Physiol 119:2001–2009. https://doi.org/10.1007/s00421-019-04187-6
Mertová M, Botek M, Krejčí J, McKune AJ (2017) Heart rate variability recovery after a skyrunning marathon and correlates of performance. Acta Gymnica 47:161–170. https://doi.org/10.5507/ag.2017.021
Michael S, Graham KS, Oam GMD (2017) Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals-a review. Front Physiol 8:1–19. https://doi.org/10.3389/fphys.2017.00301
Lara B, Salinero JJ, Gallo-Salazar C et al (2019) Elevation of cardiac troponins after endurance running competitions. Circulation 139:709–711. https://doi.org/10.1161/CIRCULATIONAHA.118.034655
Kim YJ, Ahn JK, Shin KA et al (2015) Correlation of cardiac markers and biomarkers with blood pressure of middle-aged marathon runners. J Clin Hypertens 17:868–873. https://doi.org/10.1111/jch.12591
Siegel AJ, Silverman LM, Holman BL (1981) Elevated creatine kinase MB isoenzyme levels in marathon runners normal myocardial scintigrams suggest noncardiac source. Jama 246:2049–2051
Hohl R, Nazário de Rezende F, Millet GY et al (2019) Blood cardiac biomarkers responses are associated with 24 h ultramarathon performance. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e01913
Son HJ, Lee YH, Chae JH, Kim CK (2015) Creatine kinase isoenzyme activity during and after an ultra-distance (200 km) run. Biol Sport 32:267–272. https://doi.org/10.5604/20831862.1163384
Kurapati R, Soos MP (2022) CPK-MB. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 32491523.. https://pubmed.ncbi.nlm.nih.gov/32491523/
Williams T, Walz E, Lane AR et al (2015) The effect of estrogen on muscle damage biomarkers following prolonged aerobic exercise in eumenorrheic women. Biol Sport 32:193–198. https://doi.org/10.5604/20831862.1150300
Weippert M, Behrens M, Mau-Moeller A et al (2018) Relationship between morning heart rate variability and creatine kinase response during intensified training in recreational endurance athletes. Front Physiol 9:1–7. https://doi.org/10.3389/fphys.2018.01267
Thygesen K, Alpert JS, Jaffe AS et al (2018) Fourth universal definition of myocardial infarction. Circulation. https://doi.org/10.1161/CIR.0000000000000617
Sedaghat-hamedani F, Katus HA (2016) Cardiac biomarker changes after endurance sports. 1–7. https://www.acc.org/latest-in-cardiology/articles/2016/04/14/09/58/cardiac-biomarker-changes-after-endurance-sports
Le Goff C, Lennartz L, Vranken L et al (2019) Comparison of cardiac biomarker dynamics in marathon, semi-marathon and untrained runners: what is the impact on results interpretation? J Lab Precis Med 4:6–6. https://doi.org/10.21037/jlpm.2019.01.04
Li F, Hopkins WG, Wang X et al (2021) Kinetics, moderators and reference limits of exercise-induced elevation of cardiac troponin T in athletes: a systematic review and meta-analysis. Front Physiol. https://doi.org/10.3389/fphys.2021.651851
Engel G, Rockson SG (2007) Rapid diagnosis of myocardial injury with troponin T and CK-MB relative index. Mol Diagnosis Ther 11:109–116. https://doi.org/10.1007/BF03256230
Seiler S, Haugen O, Kuffel E (2007) Autonomic recovery after exercise in trained athletes: intensity and duration effects. Med Sci Sports Exerc 39:1366–1373. https://doi.org/10.1249/mss.0b013e318060f17d
Pegg TJ, Maunsell Z, Karamitsos TD et al (2011) Utility of cardiac biomarkers for the diagnosis of type V myocardial infarction after coronary artery bypass grafting: Insights from serial cardiac MRI. Heart 97:810–816. https://doi.org/10.1136/hrt.2010.213462
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Özgünen, K., Günaştı, Ö., Özdemir, Ç. et al. The relationship between cardiac damage biomarkers and heart rate variability following 60 min of running. Clin Auton Res 32, 249–260 (2022). https://doi.org/10.1007/s10286-022-00878-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-022-00878-x