Abstract
This study verifies if caffeine supplementation delays the onset of fatigue, enhancing performance, and whether this effect is due to modulation of the caffeine in the rating of perceived exertion, motor drive activation, autonomic nervous system, and energy metabolism. Nine recreational athletes were submitted to two constant load tests after supplementation of caffeine and placebo. In both conditions, the fatigue was not identified by the motor drive activity, autonomic nervous system, and energy metabolism. CAF group improved performance (total time until exhaustion) in 27 % compared with PLA (1220 ± 245 vs 963 ± 243 s, P < 0.05). When compared with the absolute time, the RPE presents a reduction on the final term of the caffeine condition on the same time when compared with the placebo condition (17 ± 2 vs 19 ± 1, P < 0.05). This ergogenic effect has not changed cardiac autonomic activity and energy metabolism. We showed that caffeine supplementation increases the time associated with a reduction in the ratings of perceived exertion concluding that fatigue is controlled by central mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The governor central model (GCM) of fatigue states that physical performance is regulated by the central nervous system (CNS) [1, 2]. According to the GCM, exercise is regulated by the brain to prevent catastrophic failure of biological systems. The ergogenic resources that act in the CNS can modulate this regulation postponing the onset of fatigue, enhancing physical performance [1, 3, 4]. Investigating the effects triggered by these feature is a way to understand the physiological mechanisms involved in fatigue.
Caffeine is a nutritional ergogenic aid commonly used during physical exercise, aiming to delay fatigue, enhancing physical performance [5]. The caffeine supplementation has low risk, is inexpensive, easily accessible, and socially acceptable [5]. After being removed from the list of prohibited substances of the World Anti-Doping Agency in 2004 [6], prevalence rates show that three out of four athletes use caffeine during competitions [7]. The delay in the onset of fatigue and improvement in performance due to supplementation of caffeine is attributed generally to its mechanism of action in the CNS [8]. Due to its lipophilic nature, caffeine crosses the blood brain barrier and cellular membranes from other tissues [9, 10]. Thus, it is also attributed to the ergogenic effects of caffeine on fatigue mechanisms of action in peripheral systems as well [8, 10, 11].
Assuming that fatigue is regulated by the CNS and that the caffeine has central and peripheral action, this study aimed to verify if caffeine supplementation delays the onset of fatigue, enhancing performance, and whether this effect is due to modulation of the caffeine in the perception of effort, activation of the motor drive, autonomic nervous system, and energy metabolism.
Methods
Subjects
This study included nine trained men, experienced in cycling. The subjects were allowed to participate of the study after prior medical evaluation, which found the absence of any impediment of cardiological, endocrine-metabolical, cardiorespiratory, or muscular joint order. The sample characteristics are described in Table 1. The institutional research ethics committee (Federal University of São Paulo) approved the study, and the subjects provided informed consent before participation.
Experimental design
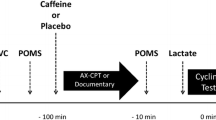
This study was a double blinded, randomized, placebo-controlled, crossover study with a repeated measures design. Subjects performed three visits to the laboratory with an average interval of 72 h between each visit. In the first visit anamnesis, anthropometric evaluation and maximal incremental cycle ergometer test were made to determine the second ventilatory threshold (VT2) and maximal oxygen uptake (VO2max). In the second and third visit, subjects were submitted to a constant load test corresponding to VT2 intensity after ingestion of caffeine or placebo. The subjects were instructed to refrain from the practice of intense exercise and alcohol consumption for at least 24 h before the experimental sessions, avoid the consumption of foods and beverages containing caffeine and/or stimulants for at least 48 h before the experimental sessions and to repeat the same feeding before the tests. All tests were performed on a cycle ergometer with mechanical braking (MONARK ®, model 828E). The pedaling cadence was maintained at 80 rpm [12], and the laboratory temperature was at 21 °C. Tests were always performed in the same period and time of day.
Maximal incremental test
Subjects were submitted a maximal incremental cycle ergometer test after 3 min of warm-up load of 0.5 Kp with increments of 0.5 Kp every 3 min, the test was conducted up to exhaustion. To ensure that the maximum stress was reached, the subjects were verbally encouraged (BARON et al. [15]). The test was terminated when indicators of fatigue (the subject report or inability to maintain pedaling cadence at 80 rpm) were observed. Heart rate (HR) was measured continuously during the test by means of a heart rate monitor (RS800CX, Polar®, Finland).
Measurements of VO2, carbon dioxide production (VCO2), ventilation (VE), respiratory exchange ratio (RER), and other ventilatory parameters were carried out during the test using a metabolic gas analyzer (K4b2, Cosmed, Rome, Italy). The exhaled breath gases were measured breathing (breath by breath), and the results were analyzed by means every 15 s. Before each test, the system for analysis of oxygen (O2) was calibrated with ambient air and with a known concentration of O2 and carbon dioxide (CO2) according to the manufacturer’s instructions (K4b2 instruction manual). The measurement of the flow of the K4b2’s turbine was calibrated using a 3 L syringe.
VT2 was determined by three ventilatory parameters: (1) second loss in linearity of ventilation; (2) increasing the curve representing the ventilatory equivalent for CO2 (VE/VCO2); and (3) decreasing in end tidal CO2 (% FeCO2) [13], and VO2max was determined by the highest value obtained every 15 s [14].
Constant load tests
After 3 min of warm-up load of 0.5 Kp, the subjects underwent in two cycle ergometer tests on separate days, corresponding to VT2 intensity until exhaustion. To ensure that the maximum stress was reached, the subjects were verbally encouraged [15]. The tests were terminated when indicators of fatigue (voluntary reporting or inability to maintain pedaling cadence) were observed.
Ventilation and HR parameters were measured continuously during the constant load tests in accordance with the procedures used in the maximal incremental test. The parameters listed are expressed in three points t 0 % (beginning of test), t 50 % (half of the test) and t 100 % (point of fatigue) or by the mean time obtained in absolute caffeine and placebo conditions.
Heart rate varialility
The heart rate variability (HRV) was recorded during constant load tests using a heart rate monitor (RS800CX, Polar®, Finland) and subsequently analyzed by specific software (v.2.0 Kubios HRV, Biosignal and Medical Image Analysis Group, Department of Physics, University of Kuopio, Finland). RR intervals (RRi) below 300 ms and above 2.000 ms were considered artifacts and edited manually. The resulting signal was filtered by software eliminating ectopic beats and artifacts. Each RRi edited was replaced by the mean value maintaining the trend of the time series of RRi. Segments whose edition of premature beats or noise exceeded 15 % of the signal were excluded from analysis. The filtering techniques have been described in previous studies [16, 17].
HRV was analyzed by frequency domain. Low frequency (LF, 0.04–0.15 Hz), which is associated with both the intensity of the parasympathetic and sympathetic modulation of the ANS on the heart; from the power spectrum density of the following components were obtained; high frequency (HF, 0.15–0.4 Hz), expressing the parasympathetic modulation acting on the sinoatrial node; rate LF/HF, indicating the index of sympathovagal balance [18]. The power spectra were calculated in normalized units (nu) [18].
Caffeine and placebo administration
Subjects ingested pure caffeine (6-mg kg−1 body mass) [19] or placebo prepared and packaged in capsules with similar physical characteristics, 15 min before the constant load test [10].
Evaluation of the rate of perceived exertion
All subjects self-rated overall perceived exertion using the Borg scale (6–20 points) [20] every 5 min of the time-to-exhaustion test and at the volitional fatigue moment.
Collection and processing of the electromyographyc signal
The acquisition of the surface electromyographic signal (sEMG) happened in the presence of caffeine and placebo during the tests. For the acquisition was used bipolar electrodes coated with silver and silver chloride (Ag/AgCl) positioned on the belly of the rectus femoris (RF), vastus lateralis (VL), and vastus medialis (VM), respecting the distance between the electrodes of 20 mm as well as the standards set by surface electromyography for the non-invasive assesment of muscle (SENIAM) and Hermens et al. [21]. To reduce possible interferences in the passage of electrical stimulation were performed, before the placement of the electrodes, shaving and cleaning of the skin with alcohol, at the level of the studied muscle. The reference electrode was placed on the styloid process of the ulna. To obtain the data of the surface electromyographic signals, was used a eight channels module of biological signals acquisition (Miotool wireless/USB ®) which were calibrated with a gain of 1000 times; with a high-pass filter of 10 Hz and a low-pass filter of 500 Hz with a sampling frequency of 2000 Hz; and a common rejection module of 120 dB. The signals were stored in a specific software (Miotec®), in which the test parameters consisted of continuous collection and were exported for processing digital signals. The EMG signal processing was performed in the frequency domain by applying the fast Fourier transform (FFT) (Hamming window processing, 512 points), followed by obtaining the mean frequency (MDF) of the spectrum. The signal processing was performed in the Matlab 7.0 (MathWorks ®) software.
Statistical analysis
For the presentation of the data, a descriptive statistics (mean ± SD) was used. As the index of HRV, LF, HF, and LF/HF, exhibit great variability and inter intrasubjects, and they were transformed into natural logarithms (LFln, HFln, and LF/HFln) allowing the use of parametric statistical tests. The Shapiro–Wilk test was performed to verify the normality of the data and then applied the paired Students’ t test to compare the time to exhaustion, the effort subjective perception and electromyographic activity, index of HRV, and cardiorespiratory parameters between caffeine and placebo groups in constant load tests. Significance level of P ≤ 0.05 was accepted.
Results
The values obtained in the maximum incremental test are shown in Table 2.
Figure 1 shows that there was a 27 % increase in time to exhaustion in the group that exercised under effect of caffeine supplementation (pla 963 ± 243 s vs caf 1.220 ± 245 s, P = 0.035).
The CAF intervention had longer time to exhaustion than PLA (over 4 min). When PLA group finished the task (volitional fatigue), the CAF group kept exercising, and at this moment (~16 min), the RPE was higher to PLA than CAF. However, when we compare the time to exhaustion of PLA (~16 min) with CAF (~20 min) the difference did not remain (Fig. 2).
Figure 3 shows that there was no difference in RPE between caffeine and placebo conditions when expressed as percentage of total time-to-exhaustion at point t 0 % (P = 0.76), t 50 % (P = 0.14) and t 100 % (P = 0.34).
Figure 4 shows that, in both experimental conditions, there was a constant behavior of neuromuscular activity throughout the test. Comparing the caffeine and placebo conditions, the muscles of VL showed higher activity in the neuromuscular in the caffeine condition at point t 50 % (P = 0.017).
There was no significant difference between groups in the natural logarithms of the components of HRV low frequency (LFln) (P = 0.46), high frequency (HFln) (P = 0.23) rate, and sympathovagal balance (LF/HFln) (P = 0.32) (Fig. 5).
The RER showed no significant difference between groups after caffeine and placebo administration at point t 10 % (P = 0.73), t 50 % (P = 0.17) and t 100 % (P = 0.28) (Fig. 6).
Discussion
Caffeine supplementation showed positive effects postponing the onset of fatigue (Fig. 1). This effect can be attributed to central factors. When the central regions related to the construction of the RPE are stimulated, this result in better physical performance associated with lower RPE [22]. It has been shown, on average, an increase of 11.2 % in the exhaustion time and a decrease of 5.6 % in the RPE in studies with caffeine supplementation [23]. Our data showed an increase in time to exhaustion by 27 and 7 % reduction in RPE on the caffeine group.
The caffeine condition had lower RPE than placebo treatment when analized by absolute time (Fig. 2). Lower values of RPE are associated with better performance [24]. Therefore, CAF supplementation positively modulates the physical performance via less RPE, but differences between the RPE disappear when the data are expressed as a function in expressed in percentage of total time-to-exhaustion. (Figure 3) [22, 25]. The lack of difference is consistent with the teleoanticipation mechanism, according to the GCM [26].
This study is probably the first to investigate the effect of caffeine supplementation on the ANS activity reflected by HRV during exercise performed until exhaustion in VT2. The modulation of sympathetic and parasympathetic activity was not altered by caffeine supplementation. Previous studies found that the ANS activity is modulated in response to exercise and not by of caffeine supplementation [27]. During physical exercise, reduction of vagal activity and increased sympathetic activity on the HR through its action on automaticity in the sinus node occurs [27]. Thus, it is evident that regardless of the administered substance, caffeine or placebo, changes in cardiac autonomic activity are responses to physical exercise and not the supplemental caffeine.
It is suggested that caffeine increases the production of catecholamines [8]. This increase in plasma catecholamines promotes greater free fatty acids mobilization from adipose tissue and/or intramuscular, increasing lipid oxidation and decreasing carbohydrate oxidation, sparing glycogen [28, 29]. However, in this study, no differences in RER between groups were observed. Thus, it denies that the improved physical performance is related to the action of caffeine on energy metabolism, as proposed by classical and contemporary studies [28, 30]. These data indicate that modulation of fatigue through caffeine action is due to central and not peripheral mechanisms.
The antagonistic action of caffeine on the CNS promotes increased release of excitatory neurotransmitters and low thresholds for neural activation [31]. In experimental models, caffeine administration increases time to exhaustion in treadmill running [32]. In humans, the excitation of brain structures involved in the construction of perceived exertion, as insular cortex, thalamus, and anterior cingulate cortex, is closely linked to positive changes in performance [22]. Due to the complexity of the interactions among these brain structures, the literature does not accurately identify how caffeine modulates the RPE in each structure. However, Nybo and Nielsen [33] found an association between the RPE and brain patterns analyzed by an increase in blood flow in the middle cerebral artery, which distributes blood to the lateral sulcus (including the insular cortex) structure. Considering the high density of adenosine A1 type found in these regions [34, 35] plus the greater availability of caffeine through the middle cerebral artery, we point a hypothesis reported in the literature by Graham [36] in which there is a greater antagonizing receptors in this region. Such evidence shows the potential modulator of caffeine as an adenosine antagonist and promotes changes in performance per central actions related to RPE.
During the experimental conditions, there was a pattern of constant activation of the drive motor (Fig. 4), unlike the literature data which could identify neuromuscular fatigue by a decrease in the electromyographic signal linearity [37]. Our data show that central mechanisms together with the RPE have a primary action in deciding the ending point of the exercise. These data corroborate the GCM in which CNS is the main factor limiting performance [38].
Caffeine induced an increase in neuromuscular activation of the VL muscle at time t 50 %. Despite these changes do not remain constant, they will meet the literature associated with the presence of neurotransmitters (serotonin and norepinephrine) resulting from antagonizing caffeine. These responses have an ergogenic action due to the increase in spinal excitability [39] and increased self-sustaining firing of motoneurons [40].
Conclusions
The caffeine supplementation increases time to exhaustion by a reduction in the rating of perceived exertion and increasing the drive motor activity, allowing the athlete to access for a longer time their physiological reserves to maintain the activity. This effect is neither due to action mechanisms of caffeine in modulating SNA activity nor the action mechanisms of the respiratory system and energy metabolism. Fatigue was regulated by the caffeine action in the CNS.
References
Swart J, Lamberts RP, Lambert MI, Gibson ASC, Lambert EV, Skowno J, Noakes TD (2009) Exercising with reserve: evidence that the central nervous system regulates prolonged exercise performance. Br J Sports Med 43(10):782–788
Baron B, Moullan F, Deruelle F, Noakes TD (2011) The role of emotions on pacing strategies and performance in middle and long duration sport events. Br J Sports Med 45(6):511–517
Noakes TD, St Clair Gibson A, Lambert EV (2005) From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans: summary and conclusions. Br J Sports Med 39(2):120–124. doi:10.1136/bjsm.2003.010330
Roelands B, Meeusen R (2010) Alterations in central fatigue by pharmacological manipulations of neurotransmitters in normal and high ambient temperature. Sports Med 40(3):229–246
Sökmen B, Armstrong LE, Kraemer WJ, Casa DJ, Dias JC, Judelson DA, Maresh CM (2008) Caffeine use in sports: considerations for the athlete. J Strength CondRes 22(3):978–986
WADA (2010) World Anti Doping Agency. The 2010 prohibited list international standard
Del Coso J, Muñoz G, Muñoz-Guerra J (2011) Prevalence of caffeine use in elite athletes following its removal from the World Anti-Doping Agency list of banned substances. Appl Physiol Nutr Metab 36(4):555–561
Graham T (2001) Caffeine and exercise: metabolism, endurance and performance. Sports Med 31(11):785–807
Magkos F, Kavouras SA (2005) Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit Rev Food Sci Nutr 45(7–8):535–562
Goldstein ER, Ziegenfuss T, Kalman D, Kreider R, Campbell B, Wilborn C, Taylor L, Willoughby D, Stout J, Graves BS (2010) International society of sports nutrition position stand: caffeine and performance. J Int Soc Sports Nutr 7(1):5
Ganio MS, Klau JF, Casa DJ, Armstrong LE, Maresh CM (2009) Effect of caffeine on sport-specific endurance performance: a systematic review. J Strength Cond Res 23(1):315
Santalla A, Earnest CP, Marroyo J, Lucia A (2012) The Tour de France: an updated physiological review. Int J Sports Physiol Perform 7(3):200–209
Bhambhani Y, Singh M (1985) Ventilatory thresholds during a graded exercise test. Respiration 47(2):120–128
Rossiter H, Kowalchuk J, Whipp B (2006) A test to establish maximum O2 uptake despite no plateau in the O2 uptake response to ramp incremental exercise. J Appl Physiol 100(3):764–770
Baron B, Noakes TD, Dekerle J, Moullan F, Robin S, Matran R, Pelayo P (2008) Why does exercise terminate at the maximal lactate steady state intensity? Br J Sports Med 42(10):828–833
Jurca R, Church TS, Morss GM, Jordan AN, Earnest CP (2004) Eight weeks of moderate-intensity exercise training increases heart rate variability in sedentary postmenopausal women. Am Heart J 147(5):e8–e15
Karavirta L, Tulppo MP, Laaksonen DE, Nyman K, Laukkanen RT, Kinnunen H, Häkkinen A, Häkkinen K (2009) Heart rate dynamics after combined endurance and strength training in older men. Med Sci Sports Exerc 41(7):1436
Montenegro RA, Farinatti PdTV, Fontes EB, Soares PPdS, Cunha FAd, Gurgel JL, Porto F, Cyrino ES, Okano AH (2011) Transcranial direct current stimulation influences the cardiac autonomic nervous control. Neurosci Lett 497(1):32–36
Desbrow B, Leveritt M (2006) Awareness and use of caffeine by athletes competing at the 2005 Ironman Triathlon World Championships. Int J Sport Nutr Exerc Metab 16(5):545
Borg G (1998) Borg’s perceived exertion and pain scales. Human Kinetics Publishers, Champaign
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10(5):361–374
Okano AH, Fontes EB, Montenegro RA, Farinatti PTV, Cyrino ES, Li LM, Bikson M, Noakes TD (2013) Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br J Sports Med 49(18):1213–1218
Doherty M, Smith P (2005) Effects of caffeine ingestion on rating of perceived exertion during and after exercise: a meta-analysis. Scand J Med Sci Sports 15(2):69–78
Doherty M, Smith PM (2005) Effects of caffeine ingestion on rating of perceived exertion during and after exercise: a meta-analysis. Scand J Med Sci Sports 15(2):69–78
Eston R, Faulkner J, Gibson ASC, Noakes TD, Parfitt G (2007) The effect of antecedent fatiguing activity on the relationship between perceived exertion and physiological activity during a constant load exercise task. Psychophysiology 44(5):779–786
Noakes TD (2012) Fatigue is a brain-derived emotion that regulates the exercise behavior to ensure the protection of whole body homeostasis. Front Physiol 3:82. doi:10.3389/fphys.2012.00082
Karapetian G, Engels H, Gretebeck K, Gretebeck R (2012) Effect of caffeine on LT, VT and HRVT. Int J Sports Med 33(07):507–513
Costill D, Dalsky GP, Fink W (1978) Effects of caffeine ingestion on metabolism and exercise performance. Med Sci Sports 10(3):155
Spriet L, MacLean D, Dyck D, Hultman E, Cederblad G, Graham T (1992) Caffeine ingestion and muscle metabolism during prolonged exercise in humans. Am J Physiol Endocrinol Metab 262(6):E891–E898
Olcina G, Munoz D, Kemp J, Timon R, Maynar J, Caballero MJ, Maynar M (2012) Total plasma fatty acid responses to maximal incremental exercise after caffeine ingestion. J Exerc Sci Fit 10(1):33–37
Kalmar JM, Caferelli E (2004) Caffeine: a valuable tool to study central fatigue in humans? Exerc Sport Sci Rev 32(4):143–147
Davis JM, Alderson NL, Welsh RS (2000) Serotonin and central nervous system fatigue: nutritional considerations. Am J Clin Nutr 72(2 Suppl):573S–578S
Nybo L, Nielsen B (2001) Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol 91(5):2017–2023
Svenningsson P, Hall H, Sedvall G, Fredholm BB (1997) Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse 27(4):322–335
Bauer A, Holschbach MH, Meyer PT, Boy C, Herzog H, Olsson RA, Coenen HH, Zilles K (2003) In vivo imaging of adenosine A1 receptors in the human brain with [18F] CPFPX and positron emission tomography. Neuroimage 19(4):1760–1769
Graham TE (2001) Caffeine and exercise. Sports med 31(11):785–807
Dingwell JB, Joubert JE, Diefenthaeler F, Trinity JD (2008) Changes in muscle activity and kinematics of highly trained cyclists during fatigue. IEEE Trans Biomed Eng 55(11):2666–2674
Noakes TD, Tucker R (2008) Do we really need a central governor to explain brain regulation of exercise performance? A response to the letter of Dr. Marcora. Eur J Appl Physiol 104(5):933–935
Walton C, Kalmar JM, Cafarelli E (2003) Caffeine increases spinal excitability in humans. Muscle Nerve 28(3):359–364
Walton C, Kalmar JM, Cafarelli E (2002) Effect of caffeine on self-sustained firing in human motor units. J Physiol 545(2):671–679
Acknowledgments
We thank all participants for their kind participation in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Human Ethics Committee of the Federal University of São Paulo and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Pereira, P.E., Motoyama, Y., Esteves, G.J. et al. Caffeine supplementation delays the fatigue through central nervous system modulation. Sport Sci Health 12, 239–245 (2016). https://doi.org/10.1007/s11332-016-0281-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-016-0281-1