Abstract
Purpose
We tested the hypothesis that low-volume high-intensity swimming has a larger impact on insulin sensitivity and glucose control than high-volume low-intensity swimming in inactive premenopausal women with mild hypertension.
Methods
Sixty-two untrained premenopausal women were randomised to an inactive control (n = 20; CON), a high-intensity low-volume (n = 21; HIT) or a low-intensity high-volume (n = 21; LIT) training group. During the 15-week intervention period, HIT performed 3 weekly 6–10 × 30-s all-out swimming intervals (average heart rate (HR) = 86 ± 3 % HRmax) interspersed by 2-min recovery periods and LIT swam continuously for 1 h at low intensity (average HR = 73 ± 3 % HRmax). Fasting blood samples were taken and an oral glucose tolerance test (OGTT) was conducted pre- and post-intervention.
Results
After HIT, resting plasma [insulin] was lowered (17 ± 34 %; P < 0.05) but remained similar after LIT and CON. Following HIT, 60-min OGTT plasma [insulin] and [glucose] was lowered (24 ± 30 % and 10 ± 16 %; P < 0.05) but remained similar after LIT and CON. Total area under the curve for plasma [glucose] was lower (P < 0.05) after HIT than LIT (660 ± 141 vs. 860 ± 325 mmol min L−1). Insulin sensitivity (HOMA-IR) had increased (P < 0.05) by 22 ± 34 % after HIT, with no significant change after LIT or CON, respectively. Plasma soluble intracellular cell adhesion molecule 1 was lowered (P < 0.05) by 4 ± 8 and 3 ± 9 % after HIT and CON, respectively, while plasma soluble vascular cell adhesion molecule 1 had decreased (P < 0.05) by 8 ± 23 % after HIT only.
Conclusions
These findings suggest that low-volume high-intensity intermittent swimming is an effective and time-efficient training strategy for improving insulin sensitivity, glucose control and biomarkers of vascular function in inactive, middle-aged mildly hypertensive women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lack of physical activity is considered a global pandemic and one of the most serious public health problems of the 21st century (Kohl et al. 2012). Physical inactivity is an independent risk factor for major chronic diseases and the annual global mortality attributable to physical inactivity is estimated at approximately 3.2 million (World Health Organization 2016). Type 2 diabetes mellitus (T2DM), an example of a pathological condition associated with an inactive lifestyle, shows an increasing growth rate, imposing a significant health and economic burden on the health care system. In the US alone, the incidence of T2DM increased 117 % from 1980 to 2011 (Al Tunaiji et al. 2014). The population attributable fraction of T2DM related to physical inactivity is reported to be higher in women than in men (Al Tunaiji et al. 2014).
Women experience a decline in health status in the years following the menopause (Appelman et al. 2015), including a higher prevalence of insulin resistance, which plays a major role in the pathophysiology of T2DM (Rizzoli et al. 2014; Shufelt et al. 2015). In addition, there is evidence that a relationship exists between insulin resistance and markers of endothelial dysfunction which also contributes to cardiovascular disease (Chen et al. 2000; Kim et al. 2006). Two markers of endothelial dysfunction that have been shown to predict cardiovascular disease include soluble intracellular cell adhesion molecule 1 (sICAM-1] and soluble vascular cell adhesion molecule 1 [sVCAM-1] (Weyer et al. 2002), which are mediators of platelet rolling and cell attachment (Marsh and Coombes 2005). It is well known that exercise training has a preventive effect on the development of lifestyle-related deficiencies (Chedraui and Perez-Lopez 2013); thus, exercise-interventions targeting an improvement in glucose control, insulin sensitivity and endothelial function are clearly warranted for inactive middle-aged women approaching the menopause period.
High-intensity interval training protocols have garnered attention in recent years as a time-efficient exercise option for improving metabolic health, and they may be particularly effective for improving blood glucose homeostasis in individuals with, or at risk of, T2DM (Little and Francois 2014). In a recent study by Little and Francois (2014), high-intensity interval cycling induced greater and longer-lasting effects on postprandial blood glucose control than continuous moderate-intensity exercise in obese middle-aged adults. Moreover, 10 × 1 min of high-intensity intermittent cycling caused a reduction in insulin resistance measured using the homeostatic model assessment of insulin resistance (HOMA-IR) in inactive women (Durrer et al. 2015), which is supported by studies on patients with T2DM (Shaban et al. 2014) and non-diabetic subjects (Adams 2013). These studies, and the vast majority of studies on the health effects of high-intensity training regimes have deployed running or cycling exercise, while a limited number of studies have deployed exercise modalities such as swimming, which involves a significant taxation of the upper body.
Swimming is a popular physical leisure activity among the female population and may be considered a good choice of exercise training for obese, middle-aged and elderly individuals due to the minimal weight-bearing stress involved. In addition, swimming highly engages the upper-body muscles, where the adaptive capacity of the skeletal muscle has shown to be higher than the lower limbs due to the bipendular nature of humans (Nordsborg et al. 2015). For example, low-intensity swimming has been shown to be more efficient for improving insulin resistance than walking at a matched intensity in 50- to 70-year-old sedentary women (Cox et al. 2010). In addition, in obese mice (Motta et al. 2015) and rats (Song et al. 2014) swimming has been demonstrated to facilitate glucose control and insulin sensitivity. Moreover, 1 year of participation in low-intensity swimming has been demonstrated to increase insulin sensitivity in young hypertensive patients (Chen et al. 2010). However, limited information exists on the impact of high-intensity intermittent swimming, and, as far as we know, no studies have compared the effects of different swimming protocols on insulin resistance and blood glucose control in middle-aged, inactive women.
Thus, the objective of the present study was to investigate the effects of low-volume high-intensity swimming compared to high-volume low-intensity swimming, on insulin sensitivity and blood glucose control in middle-aged, inactive women with mild hypertension. It was hypothesised that short-term high-intensity interval swimming is a more efficient training method than prolonged, continuous low-intensity swimming for improving metabolic fitness in untrained middle-aged mildly hypertensive women.
Materials and methods
Participants
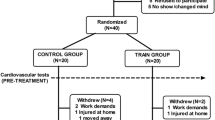
Sixty-two inactive premenopausal women with mild to moderate arterial hypertension were recruited for the study. The subjects were selected from 262 volunteers based on training history, medication, blood pressure and body mass index. A total of 83 participants were recruited in the original sample. Sixty-two took part in the present study, for which the cardiovascular data are presented in Mohr et al. (2014b) and 21 were randomly assigned to a football group as part of another study (Mohr et al. 2014a). Data for the adaptive capacity of skeletal muscle for the above-mentioned participants were also presented in Nordsborg et al. (2015). In addition, the control group (20 participants) in the present study was also the controls in the above-mentioned studies (Mohr et al. 2014a, b; Nordsborg et al. 2015) The study was approved by the ethical committee of the Faroe Islands as well as the Sport and Health Sciences Research Ethics Committee at the University of Exeter, Exeter, United Kingdom, and conducted in accordance with the Declaration of Helsinki (1964). After being informed verbally and in writing of the experimental procedures and associated risks, all participants gave their written consent to take part in the study.
Experiment design
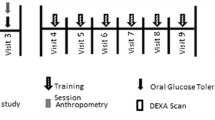
The study was designed as a randomised controlled trial. After initial screening of the 262 volunteers, 62 participants were enrolled in the present study based on the selection criteria: an inactive lifestyle for the last 2 years; mild to moderate hypertension (mean arterial pressure 96–110 mmHg); and body mass index >25. Participants undergoing treatment with adrenergic beta-antagonists were excluded. Participants using diuretics and ACE inhibitors (n = 4) were not excluded from the study, but none of the four subjects changed their medication during the intervention period. The participants were randomised into a high-intensity intermittent swim-training group (HIT: age; 44 ± 5 (±SD) (range 36–49) years, height; 164 ± 6 cm, weight; 76.5 ± 8.8 kg; n = 21), a low-intensity continuous swimming group (LIT: age; 46 ± 4 (38–48) years, height; 165 ± 5 cm, weight; 83.8 ± 18.8 kg; n = 21) and a control group (CON: age; 45 ± 4 (35–48) years, height; 166 ± 6 cm, weight; 77.3 ± 10.4 kg; n = 20). The randomization process first separated the participants in groups and thereafter determined the type of intervention. Both steps were carried out in random and blinded conditions. The training groups took part in two types of swim training involving three training sessions per week for 15 weeks, while CON had no training or lifestyle changes in the same period. There were no drop-outs from the study, but one subject in the LIT group suffered aquatic phobia and was therefore moved to CON after the randomization process. The participants also performed an oral glucose tolerance test (OGTT) and had a resting blood sample taken. Dietary intake was not controlled during the training period and the testing periods were not timed in relation to the menstrual cycle.
Training intervention
The HIT participants completed a total of 44 ± 1 (39–50) training sessions over the 15-week intervention period, corresponding to 2.9 ± 0.1 (2.6–3.3) sessions per week. Each session lasted ~15–25 min (3–5 min of effective swimming) and consisted of 6–10 30-s all-out free-style swimming (front crawl) intervals interspersed by 2 min of passive recovery according to training principles previously described (Iaia et al. 2009; Mohr et al. 2007). In the first 6 weeks of training each comprised 6 intervals, the following 6 weeks comprised 8 intervals and the final 3 weeks comprised 10 intervals. The LIT group completed a total of 43 ± 1 (37–49) training sessions over 15 weeks, corresponding to 2.9 ± 0.1 (2.5–3.3) training sessions per week. All LIT training sessions lasted 1 h and consisted of continuous front-crawl swimming, with the participants encouraged to swim as far as possible in every session. Five trained swimming coaches were present during all training sessions to give technical advice, control the intensity and duration of the training, and secure a safe training environment. There were no injuries from the swim-training during the 15 wks. Heart rate was measured during one training session in week 1 and one session in week 15 of the training intervention, and the swimming distance was noted in every session. Average mean and peak HR during HIT training in the first and last weeks of the intervention was 158 ± 5 and 176 ± 2 bpm, respectively, corresponding to 86 ± 3 and 96 ± 1 % HRmax, respectively, which was higher (P < 0.05) than average values in LIT (132 ± 4 and 144 ± 3 bpm equivalent to 73 ± 3 and 79 ± 1 % HRmax).
Resting blood sampling and OGTT testing
On the day of testing, the participants reported to the laboratory after transport by car or bus. No training was performed 48-72 h prior to the testing and participants were instructed to avoid physical activity the day prior to the OGTT tests. A resting blood sample was collected under standardised conditions from an antecubital vein between 7 and 8 a.m. after an overnight fast using venipuncture technique. The blood was rapidly centrifuged for 30 s and the plasma was collected and analysed by an automatic analyzer (Cobas Fara, Roche, France) using enzymatic kits (Roche Diagnostics, Germany) to determine the relevant variables. Furthermore, 100 μl of plasma was used to determine concentrations of sICAM-1 and sVCAM-1 by immunoassays (Elisa, R&D Systems, Minneapolis, MN) using an Emax precision microplate reader (Molecular Devices, Sunnyvale, CA, USA). The intra-assay coefficient of variations for sICAM-1 and sVCAM-1 are 4.9 ± 3.5 and 5.1 ± 5.9 %, respectively.
Also, an OGTT test was performed as previously described (Durrer et al. 2015), with blood samples taken after 0, 30, 60 and 120 min and analysed for plasma [glucose] using enzymatic kits (Roche Diagnostics, Germany) and [insulin]. Insulin sensitivity was evaluated for all subjects using the homeostatic model assessment method (HOMA-IR); (Yokoyama et al. 2003). Changes in plasma [glucose] and [insulin] during the OGTT were quantified using total area under the curve (tAUC) analyses employing the trapezium rule (GraphPad Prism, San Diego, CA, USA). The intra-assay coefficient of variations for glucose and insulin are 3.0 ± 3.5 and 4.8 ± 3.6 %, respectively.
Statistical analysis
Data are presented as mean ± SD. Statistical analyses were performed using SPSS v.22. A two-factor mixed ANOVA design with the between factor ‘group’ (HIT versus LIT versus CON) and repeated factor ‘condition’ (pre-training versus post-training) was used to analyse all data apart from the 2-h OGTT. Responses to the OGTT were analysed using a three-factor mixed ANOVA design, with the factors ‘group’ (HIT versus LIT versus CON), ‘condition’ (pre-training versus post-training) and ‘time’ (0, 30, 60 and 120 min). When significant interactions or main effects were detected, data were subsequently analysed using Bonferroni post hoc t tests. The significance level was P < 0.05.
Results
Plasma [glucose] and [insulin]
Pre-intervention fasting plasma [glucose] was 4.9 ± 0.3, 5.8 ± 2.3, and 4.9 ± 0.5 mmol L−1 in HIT, LIT and CON, respectively, with a tendency for higher (P = 0.07) values in LIT than HIT. Fasting plasma [glucose] was unaltered after the intervention period (Fig. 1). Plasma [glucose] increased (main effect for time, P < 0.05) in all three groups after 30 and 60 min during the OGTT, but was normalised after 120 min pre- and post-training. In HIT, plasma [glucose] was 10 ± 16 % lower after 60 min post- compared to pre-intervention, with the concentration being lower (condition x time x group interaction, P < 0.05) than the corresponding concentration in LIT and tending (P = 0.054) to be lower than in CON (Fig. 1). For plasma [glucose] tAUC, there were no significant differences between groups prior to the training intervention (HIT: 700 ± 155, LIT: 833 ± 324 and CON: 764 ± 186 mmol min L−1), respectively. However, following the training intervention HIT tAUC (660 ± 141 mmol min L−1) was significantly lower than LIT tAUC (860 ± 325 mmol min L−1, group–condition interaction, P < 0.05).
Pre intervention fasting plasma [insulin] was 9.6 ± 4.2, 10.2 ± 7.3 and 9.9 ± 4.6 μIU·ml−1 in HIT, LIT and CON, respectively, with no significant differences between groups (Fig. 2). A training-induced decrease of 17 ± 34 % in baseline plasma [insulin] occurred following HIT (group–condition interaction, P < 0.05), while levels remained constant after LIT and CON. Plasma [insulin] increased in all three groups during the OGTT, being higher than at baseline at 30-, 60- and 120-min pre- and post-intervention (main effect for time, P < 0.05, Fig. 2). However, plasma [insulin] was only lower (24 ± 30 %) at 60 min into the OGTT following HIT which was lower than the corresponding value for LIT (condition x time x group interaction, P < 0.05). There was also a tendency (P = 0.09) for a lower concentration following HIT at the 120 min time point (Fig. 2). There were no differences between pre- and post-plasma [insulin] during the OGTT in LIT and CON (Fig. 2). For plasma [insulin] tAUC, there were no significant differences between groups prior to (HIT: 9121 ± 7606, LIT: 6879 ± 3621, and CON: 6947 ± 3116 μIU ml min−1, respectively) and following (HIT: 6634 ± 3449, LIT: 6809 ± 2973, and CON: 6712 ± 2999 μIU ml min−1, respectively) the training intervention. However, there was a tendency (P = 0.09) for plasma [insulin] tAUC to be lower after HIT.
Insulin sensitivity: HOMA-IR
Insulin sensitivity data are shown in Fig. 3. There were no significant differences in insulin sensitivity between HIT, LIT and CON pre intervention (HIT: 1.3 ± 0.5, LIT: 1.4 ± 1.0, and CON: 1.2 ± 0.5). Following the training-intervention, there was a 22 ± 34 % improvement in insulin sensitivity after HIT (group–condition interaction, P < 0.05), with no change in LIT and CON.
Plasma [sICAM-1] and [sVCAM-1]
There were no significant differences in plasma [sICAM-1] between HIT, LIT and CON groups pre intervention (146 ± 68, 142 ± 56 and 134 ± 67 ng ml−1), but it was lowered (group–condition interaction, P < 0.05) by 4 ± 8 and 3 ± 9 % post-intervention after HIT and CON only, respectively (Fig. 4a). There were no significant differences in plasma [sVCAM-1] between groups pre intervention (624 ± 154, 667 ± 106 and 553 ± 198 ng ml−1 in HIT, LIT and CON, respectively; Fig. 4b). However, plasma [sVCAM-1] was lower (group–condition interaction, P < 0.05) by 8 ± 23 % after HIT (Fig. 4b), with no change after LIT and CON.
Discussion
For the first time, the effects of low-volume high-intensity versus high-volume low-intensity swim training on insulin sensitivity and blood glucose control have been studied in middle-aged, inactive women. The major findings were that short-term high-intensity intermittent swim training lowered the fasting plasma [insulin], as well as plasma [glucose] and [insulin] during an oral glucose tolerance test, demonstrating an improvement in insulin sensitivity. In contrast, no changes occurred in those parameters after prolonged low-intensity swimming despite a ~12-fold longer efficient swimming time and ~fivefold longer swimming distance covered during training.
Although previous literature on high-intensity swim training is lacking, Chen et al. (2010), Nualnim et al. (2012), and Cox et al. (2010) have implemented 3-12 months of moderate-intensity swim training for a combination of hypertensive and normotensive young (21 years), and older adults (>50 years) in which there were no significant improvements in insulin sensitivity, fasting [glucose], [insulin] or [glucose] AUC. In contrast the present study displayed a 22 % improvement in insulin sensitivity after HIT. However, it should be noted that fasting values for Nualnim et al. (2012) and the present study were already within the normal range. Interestingly, HIT in the present study decreased [glucose] values by 10 % at 60 min during the OGTT. Thus, HIT swimming may represent an attractive alternative to moderate-intensity swimming.
In the present study, 17 % decrease in fasting [insulin] was also reported for HIT along with a 24 % improvement at 60 min of the OGTT. The present findings are comparable with effects on metabolic health from other forms of high-intensity exercise interventions. For example, Little et al. (2011) demonstrated that 10 × 60 s of high intensity cycling over only 2 weaks reduced hyperglycemia and improved muscle mitochondrial capacity in patients with type 2 diabetes. Ciolac et al. (2010) found that 16 wks of high-intensity aerobic interval running 3 times per week (1 min running interspersed with 2 min walking for 40 min) for young females (20–30 years) at risk of hypertension resulted in a 35 % improvement in fasting [insulin]. In addition, Trapp et al. (2008) conducted 15 wks of high-intensity intermittent cycling 3 times per week (8-s all-out interspersed with 12 s of slow cycling for 60 repetitions) for inactive women (18–30 years) which resulted in a 31 % and 33 % improvement in fasting [insulin] and HOMA-IR score whereas the steady-state exercise group only resulted in a 9 % and 11 % improvement. The slightly lower improvement in fasting [insulin] in the present study compared to Ciolac et al. (2010) and Trapp et al. (2008) may be due to the exercise modality given that swimming differs in medium, position, breathing pattern and muscle groups used, providing a different training stimulus to cardiovascular parameters due to differences in ventricular volumes (Lazar et al. 2013; Sundstedt et al. 2003). It may also be speculated that the less pronounced systemic adaptation may be due to the smaller muscle mass activated during swimming compared to other forms of exercise (Nordsborg et al. 2015). Although the positive effect of high-intensity training on insulin sensitivity has been confirmed in numerous studies, the additional knowledge provided by this study is that high-intensity swimming provides a useful alterative for inactive middle-aged women compared to other modes of exercise.
On the other hand, not all high-intensity training interventions have reported insulin improvements. For example, Arad et al. (2015), Gillen et al. (2013) and Keating et al. (2015) found no change in fasting [insulin] and [glucose] and insulin sensitivity following 8–16 weeks of cycling interval training in overweight females (27–40 years). One reason for the discrepancy between these studies and the present study may be due to this study applying front-crawl training primarily taxing the upper-body muscles, which have a lower baseline training status than the legs and, hence, a higher improvement potential (Nordsborg et al. 2015).
Studies of swim training in rats have reported increased upregulation of molecules related to insulin signalling transduction in the skeletal muscle. For example, Fujimoto et al. (2010) previously reported that 5 days of non-exhaustive high-intensity intermittent swimming in male Sprague–Dawley rats provided an excellent stimulus for improving GLUT4 expression in skeletal muscle. In addition, in a study by Terada et al. (2001), male Sprague–Dawley rats performed short-term bouts of extremely high, relatively high and low intensity swimming over an 8-day period. GLUT4 content in the three training groups was increased following the exercise period, with no differences between groups. In support of these changes, Burgomaster et al. (2007) found increased muscle GLUT4 content after 1 week of sprint interval training, which remained higher during detraining of young (22 years) active men, which is supported by findings by Little et al. (2011). GLUT4 has been shown to be responsible for insulin-stimulated and contraction-induced glucose uptake (Daugaard and Richter 2001). As shown by Nordsborg et al. (2015), muscle glycogen in the deltoideus muscle of the study’s participants increased by 63 % following HIT, which could strengthen the notion that muscular uptake of glucose increased via GLUT4 transporters.
While little research is available on [sICAM-1] and [sVCAM-1] following high-intensity swimming in middle-aged women, the improvement in vascular function as indicated by the 4 and 8 % reductions in [sICAM-1] and [sVCAM-1] following HIT is of interest as endothelial dysfunction is known to initiate not only the process of atherosclerosis, but also the development of insulin resistance (Glowinska et al. 2005). The present findings are not as notable as the improvements presented by Nyberg et al. (2014) following a 12-week floorball training intervention for premenopausal women ([sVCAM-1] pre: 674 and post: 535 ng ml−1; [sICAM-1] pre: 166 and post: 122 ng ml−1, however, this may be due to the exercise modality. On the other hand, the swimming data of the present study appears to be more promising than those of cycling based training, for example, Sabatier et al. (2008) failed to show a significant improvement in [sICAM-1] following a 14 week cycling exercise intervention in healthy premenopausal women with a baseline [sICAM-1] of 169 ng ml−1. In addition, although not significantly correlated (data not shown), the reductions in [sICAM-1] and [sVCAM-1] after HIT were also accompanied by improvements in glucose handling which was not the case following LIT, which is in agreement with previous studies incorporating continuous aerobic training (Padilla et al. 2008). As shear stress on endothelial cells lining conduit blood vessels increases in direct proportion to intensity (Padilla et al. 2008), it may be speculated that HIT has greater potential for improving endothelial function compared to LIT (Wisloff et al. 2009). The improvement in glucose handling following HIT may have been enhanced by vascular actions of insulin in endothelium leading to an increased stimulation of the production of nitric oxide (NO). It has previously been reported that the NO-dependent increase in vasodilatation and skeletal muscle blood flow accounts for 25–40 % of the increase in glucose uptake in response to insulin stimulation (Kim et al. 2006). It may therefore be postulated that to induce changes in [sVCAM-1] and [sICAM-1] improvements are likely to occur quicker when exercising at high intensity and quite possibly without the need to change the individual’s diet.
Despite HIT only exercising for one-third of the time of LIT, similar changes in whole-body fat and lean body mass occurred, although leg lean mass increased by 6 % after HIT with no changes after LIT (data not shown). As the speed of swimming increases, drag forces increase exponentially, reducing the mechanical efficiency of front crawl by 5–9.5 % (Lazar et al. 2013). This may have increased the energy requirements of the lower limbs in HIT and, in turn, increased leg lean mass. It is also possible that the number of muscles recruited during HIT and LIT swimming differ, possibly causing a faster depletion of glycogen during HIT, and, together with increased circulating catecholamines, this may contribute to an increase in glucose utilisation and an improved insulin response (Vollestad and Blom 1985). The favourable change in body composition may also have been brought about by an increased capacity for fat oxidation. Previous research has shown that endurance exercise (Holloszy and Booth 1976) and high-intensity exercise (Talanian et al. 2007) enhance the capacity for fat oxidation and mitochondrial enzyme activity. This has been shown to improve the potential for muscle to utilise lipids as a substrate for energy and is also associated with improved insulin sensitivity (Goodpaster et al. 2003). It is interesting to note that, in this study, HIT participants of this study increased mitochondrial enzyme activity more than LIT (data published in Nordsborg et al. (2015)). If the training had continued for a longer period (6 months to 1 year), it is possible that the decrease in [insulin] would have led to higher fat oxidation and fat loss in the long term (Ferrannini et al. 1997). However, it cannot be ruled out that changes in body composition could have been due to unreported changes in diet, as diet was not directly controlled. As stated by Chen et al. (2010), interventions that enhance insulin sensitivity can alleviate hypertension, and the findings of the present study, together with those of Nordsborg et al. (2015), may help explain the reduction in blood pressure in the present participants shown by Mohr et al. (2014b).
Conclusions
In conclusion, 15 weeks of low-volume high-intensity intermittent, but not prolonged low intensity high-volume, swim training improves insulin sensitivity and reduces biomarkers of endothelial activation in inactive premenopausal women with mild hypertension. Consequently, high-intensity intermittent swim training appears to be an appealing non-weight bearing training regime with a very low risk of injury.
Abbreviations
- ANOVA:
-
Analysis of variance
- CON:
-
Control
- HIT:
-
High-intensity training
- HOMA-IR:
-
Homeostatic model assessment of insulin resistance
- HR:
-
Heart rate
- OGTT:
-
Oral glucose tolerance test
- LIT:
-
Low-intensity training
- SEM:
-
Standard error of the mean
- sICAM-1:
-
Soluble intracellular cell adhesion molecule 1
- sVCAM-1:
-
Soluble vascular cell adhesion molecule 1
- T2DM:
-
Type 2 diabetes mellitus
- tAUC:
-
Total area under the curve
References
Adams OP (2013) The impact of brief high-intensity exercise on blood glucose levels. Diabetes Metab Syndr Obes 6:113–122. doi:10.2147/DMSO.S29222
Al Tunaiji H, Davis JC, Mackey DC, Khan KM (2014) Population attributable fraction of type 2 diabetes due to physical inactivity in adults: a systematic review. BMC Public Health 14:469. doi:10.1186/1471-2458-14-469
Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA (2015) Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 241:211–218. doi:10.1016/j.atherosclerosis.2015.01.027
Arad AD, DiMenna FJ, Thomas N, Tamis-Holland J, Weil R, Geliebter A, Albu JB (2015) High-intensity interval training without weight loss improves exercise but not basal or insulin-induced metabolism in overweight/obese African American women. J Appl Physiol 119:352–362. doi:10.1152/japplphysiol.00306.2015
Burgomaster KA, Cermak NM, Phillips SM, Benton CR, Bonen A, Gibala MJ (2007) Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol 292:R1970–R1976. doi:10.1152/ajpregu.00503.2006
Chedraui P, Perez-Lopez FR (2013) Nutrition and health during mid-life: searching for solutions and meeting challenges for the aging population. Climacteric 16(Suppl 1):85–95. doi:10.3109/13697137.2013.802884
Chen NG, Azhar S, Abbasi F, Carantoni M, Reaven GM (2000) The relationship between plasma glucose and insulin responses to oral glucose, LDL oxidation, and soluble intercellular adhesion molecule-1 in healthy volunteers. Atherosclerosis 152:203–208. doi:10.1016/S0021-9150(99)00460-8
Chen HH, Chen YL, Huang CY, Lee SD, Chen SC, Kuo CH (2010) Effects of one-year swimming training on blood pressure and insulin sensitivity in mild hypertensive young patients. Chin J Physiol 53:185–189. doi:10.4077/cjp.2010.amk042
Ciolac EG, Bocchi EA, Bortolotto LA, Carvalho VO, Greve JM, Guimaraes GV (2010) Effects of high-intensity aerobic interval training vs. moderate exercise on hemodynamic, metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypertens Res 33:836–843. doi:10.1038/hr.2010.72
Cox KL, Burke V, Beilin LJ, Puddey IB (2010) A comparison of the effects of swimming and walking on body weight, fat distribution, lipids, glucose, and insulin in older women-the Sedentary Women Exercise Adherence Trial 2. Metabolism 59:1562–1573. doi:10.1016/j.metabol.2010.02.001
Daugaard JR, Richter EA (2001) Relationship between muscle fibre composition, glucose transporter protein 4 and exercise training: possible consequences in non-insulin-dependent diabetes mellitus. Acta Physiol Scand 171:267–276. doi:10.1046/j.1365-201x.2001.00829.x
Durrer C et al (2015) Differential impact of acute high-intensity exercise on circulating endothelial microparticles and insulin resistance between overweight/obese males and females. PLoS ONE 10:e0115860. doi:10.1371/journal.pone.0115860
Ferrannini E, Camastra S, Coppack SW, Fliser D, Golay A, Mitrakou A (1997) Insulin action and non-esterified fatty acids. The European Group for the Study of Insulin Resistance (EGIR). Proc Nutr Soc 56:753–761
Fujimoto E, Machida S, Higuchi M, Tabata I (2010) Effects of nonexhaustive bouts of high-intensity intermittent swimming training on GLUT-4 expression in rat skeletal muscle. J Physiol Sci 60:95–101. doi:10.1007/s12576-009-0072-4
Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA, Gibala MJ (2013) Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity 21:2249–2255. doi:10.1002/oby.20379
Glowinska B, Urban M, Peczynska J, Florys B (2005) Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension, and diabetes. Metabolism 54:1020–1026. doi:10.1016/j.metabol.2005.03.004
Goodpaster BH, Katsiaras A, Kelley DE (2003) Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52:2191–2197. doi:10.2337/diabetes.52.9.2191
Holloszy JO, Booth FW (1976) Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 38:273–291. doi:10.1146/annurev.ph.38.030176.001421
Iaia FM, Hellsten Y, Nielsen JJ, Fernstrom M, Sahlin K, Bangsbo J (2009) Four weeks of speed endurance training reduces energy expenditure during exercise and maintains muscle oxidative capacity despite a reduction in training volume. J Appl Physiol 106:73–80. doi:10.1152/japplphysiol.90676.2008
Keating SE et al (2015) Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol 63:174–182. doi:10.1016/j.jhep.2015.02.022
Kim JA, Montagnani M, Koh KK, Quon MJ (2006) Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113:1888–1904. doi:10.1161/CIRCULATIONAHA.105.563213
Kohl HW, Craig CL, Lambert EV, Inoue S, Alkandari JR, Leetongin G, Kahlmeier S (2012) The pandemic of physical inactivity: global action for public health. Lancet 380:294–305. doi:10.1016/s0140-6736(12)60898-8
Lazar JM, Khanna N, Chesler R, Salciccioli L (2013) Swimming and the heart. Int J Cardiol 168:19–26. doi:10.1016/j.ijcard.2013.03.063
Little JP, Francois ME (2014) High-intensity interval training for improving postprandial hyperglycemia. Res Q Exerc Sport 85:451–456. doi:10.1080/02701367.2014.963474
Little JP et al (2011) Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 111:1554–1560. doi:10.1152/japplphysiol.00921.2011
Marsh SA, Coombes JS (2005) Exercise and the endothelial cell. Int J Cardiol 99:165–169. doi:10.1016/j.ijcard.2004.02.005
Mohr M, Krustrup P, Nielsen JJ, Nybo L, Rasmussen MK, Juel C, Bangsbo J (2007) Effect of two different intense training regimens on skeletal muscle ion transport proteins and fatigue development. Am J Physiol Regul Integr Comp Physiol 292:R1594–R1602. doi:10.1152/ajpregu.00251.2006
Mohr M, Lindenskov A, Holm PM, Nielsen HP, Mortensen J, Weihe P, Krustrup P (2014a) Football training improves cardiovascular health profile in sedentary, premenopausal hypertensive women. Scand J Med Sci Sports 24(Suppl 1):36–42. doi:10.1111/sms.12278
Mohr M et al (2014b) High-intensity intermittent swimming improves cardiovascular health status for women with mild hypertension. BioMed research international 2014:728289. doi:10.1155/2014/728289
Motta VF, Aguila MB, Mandarim-De-Lacerda CA (2015) High-intensity interval training (swimming) significantly improves the adverse metabolism and comorbidities in diet-induced obese mice. The Journal of Sports Medicine and Physical Fitness 54:203–209
Nordsborg NB, Connolly L, Weihe P, Iuliano E, Krustrup P, Saltin B, Mohr M (2015) Oxidative capacity and glycogen content increase more in arm than leg muscle in sedentary women after intense training. J Appl Physiol 119:116–123. doi:10.1152/japplphysiol.00101.2015
Nualnim N, Parkhurst K, Dhindsa M, Tarumi T, Vavrek J, Tanaka H (2012) Effects of swimming training on blood pressure and vascular function in adults >50 years of age. Am J Cardiol 109:1005–1010. doi:10.1016/j.amjcard.2011.11.029
Nyberg M, Seidelin K, Andersen TR, Overby NN, Hellsten Y, Bangsbo J (2014) Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: effect of exercise training. Am J Physiol Regul Integr Comp Physiol 306:R510–R517. doi:10.1152/ajpregu.00539.2013
Padilla J, Harris RA, Rink LD, Wallace JP (2008) Characterization of the brachial artery shear stress following walking exercise. Vasc Med 13:105–111. doi:10.1177/1358863x07086671
Rizzoli R et al (2014) The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 79:122–132. doi:10.1016/j.maturitas.2014.07.005
Sabatier MJ, Schwark EH, Lewis R, Sloan G, Cannon J, McCully K (2008) Femoral artery remodeling after aerobic exercise training without weight loss in women. Dyn Med. doi:10.1186/1476-5918-7-13
Shaban N, Kenno KA, Milne KJ (2014) The effects of a 2 week modified high intensity interval training program on the homeostatic model of insulin resistance (HOMA-IR) in adults with type 2 diabetes. J Sports Med Phys Fitness 54:203–209
Shufelt C, Braunstein GD, Pepine CJ, Bairey Merz CN (2015) Recognizing Sex Similarities in Cardiovascular Disease Research. J Am Coll Cardiol 65:2152–2153. doi:10.1016/j.jacc.2015.02.066
Song A, Wang C, Ren L, Zhao J (2014) Swimming improves high-fat induced insulin resistance by regulating lipid and energy metabolism and the insulin pathway in rats. Int J Mol Med 33:1671–1679. doi:10.3892/ijmm.2014.1738
Sundstedt M, Jonason T, Ahren T, Damm S, Wesslen L, Henriksen E (2003) Left ventricular volume changes during supine exercise in young endurance athletes. Acta Physiol Scand 177:467–472. doi:10.1046/j.1365-201X.2003.01098.x
Talanian JL, Galloway SD, Heigenhauser GJ, Bonen A, Spriet LL (2007) Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol 102:1439–1447. doi:10.1152/japplphysiol.01098.2006
Terada S, Yokozeki T, Kawanaka K, Ogawa K, Higuchi M, Ezaki O, Tabata I (2001) Effects of high-intensity swimming training on GLUT-4 and glucose transport activity in rat skeletal muscle. J Appl Physiol 90:2019–2024
Trapp EG, Chisholm DJ, Freund J, Boutcher SH (2008) The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes (Lond) 32:684–691. doi:10.1038/sj.ijo.0803781
Vollestad NK, Blom PC (1985) Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand 125:395–405. doi:10.1111/j.1748-1716.1985.tb07735.x
Weyer C, Yudkin JS, Stehouwer CD, Schalkwijk CG, Pratley RE, Tataranni PA (2002) Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima Indians. Atherosclerosis 161:233–242
Wisloff U, Ellingsen O, Kemi OJ (2009) High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev 37:139–146. doi:10.1097/JES.0b013e3181aa65fc
World Health Organization (2016) Physical Inactivity: A Global Public Health Problem. http://www.who.int/dietphysicalactivity/factsheet_inactivity/en/. Accessed 11/03/16 2016
Yokoyama H et al (2003) Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care 26:2426–2432
Acknowledgments
The authors would like to express their appreciation for the outstanding efforts and positive attitude of the participants. In addition, they are extremely grateful for the professional assistance given by swimming coaches Remi Lamhauge, Brynhild Klein, Pauli Øssursson Mohr, Heini Rasmussen, Oluffa á Høvdanum, and the late Jákup Mohr, and for the technical support provided by Ivy Hansen, Gunnrið Jóannesarson, Guðrið Andórsdóttir, Hergerð Joensen, Ann Østerø, Ebba Andreassen, Maud av Fløtum, Liljan av Fløtum Petersen, Marjun Thomsen, Annika Lindenskov, Jann Mortensen, David Childs, Sarah R. Jackman and Jens Jung Nielsen. The study was supported by a Grant from the Faroese Research Council, as well as by The Faroese Confederation of Sports and Olympic Committee (Ítróttarsamband Føroya), and the Danish Sports Confederation (Danmarks Idrætsforbund). In addition, financial support was obtained from Eik Bank.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Michael Lindinger.
Rights and permissions
About this article
Cite this article
Connolly, L.J., Nordsborg, N.B., Nyberg, M. et al. Low-volume high-intensity swim training is superior to high-volume low-intensity training in relation to insulin sensitivity and glucose control in inactive middle-aged women. Eur J Appl Physiol 116, 1889–1897 (2016). https://doi.org/10.1007/s00421-016-3441-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3441-8