Abstract
Purpose
The aim of this study was to determine the effects of high-intensity aerobic interval training (AIT) on exercise hemodynamics in metabolic syndrome (MetS) volunteers.
Methods
Thirty-eight, MetS participants were randomly assigned to a training (TRAIN) or to a non-training control (CONT) group. TRAIN consisted of stationary interval cycling alternating bouts at 70–90% of maximal heart rate during 45 min day−1 for 6 months.
Results
CONT maintained baseline physical activity and no changes in cardiovascular function or MetS factors were detected. In contrast, TRAIN increased cardiorespiratory fitness (14% in VO2PEAK; 95% CI 9–18%) and improved metabolic syndrome (−42% in Z score; 95% CI 83–1%). After TRAIN, the workload that elicited a VO2 of 1500 ml min−1 increased 15% (95% CI 5–25%; P < 0.001). After TRAIN when subjects pedaled at an identical submaximal rate of oxygen consumption, cardiac output increased by 8% (95% CI 4–11%; P < 0.01) and stroke volume by 10% (95% CI, 6–14%; P < 0.005) being above the CONT group values at that time point. TRAIN reduced submaximal exercise heart rate (109 ± 15–106 ± 13 beats min−1; P < 0.05), diastolic blood pressure (83 ± 8–75 ± 8 mmHg; P < 0.001) and systemic vascular resistances (P < 0.01) below CONT values. Double product was reduced only after TRAIN (18.2 ± 3.2–17.4 ± 2.4 bt min−1 mmHg 10−3; P < 0.05).
Conclusions
The data suggest that intense aerobic interval training improves hemodynamics during submaximal exercise in MetS patients. Specifically, it reduces diastolic blood pressure, systemic vascular resistances, and the double product. The reduction in double product, suggests decreased myocardial oxygen demands which could prevent the occurrence of adverse cardiovascular events during exercise in this population.
Clinicaltrials.gov identifier
NCT03019796.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is a cluster of conditions compiling elevated waist circumference, blood lipids and glucose and blood pressure that increases the risk of developing cardiovascular disease (i.e., CVD; Ingelsson et al. 2007). In intervention trials, it has been established that aerobic interval training improves cardiorespiratory fitness (i.e., CRF measured as VO2max; Mora-Rodriguez et al. 2014; Tjonna et al. 2008) in MetS subjects. Endurance aerobic training increases CRF mainly by increasing maximal cardiac output and active limb blood flow (Roca et al. 1992). Endurance training not only increases cardiovascular system functionally when exercising at maximal workloads but importantly readjust its hemodynamics during submaximal exercise to reduce cardiovascular work. This is, for a given absolute exercise intensity, there is a reduced heart rate and increased stroke volume while cardiac output is either maintained (Saltin et al. 1968) or slightly decreased (Andrew et al. 1966; Ekblom et al. 1968; Wilmore et al. 2001).
The other hemodynamics readjustment that reduces cardiovascular stress after aerobic training is the decrease in exercise blood pressure for a given workload. Early experiments training only one leg while maintaining the other untrained as a control revealed that the training induced reduction in vascular resistances is specific to the vasculature of the muscle trained (Klausen et al. 1982; Saltin et al. 1976). MetS patients are prone to develop arterial stiffness and endothelial dysfunction (Edgell et al. 2012) that results in increased vascular resistances. However, it is unknown if exercise training in MetS patients results in reduction in vascular resistances to blood flow during exercise and if that reflects on parallel reductions in blood pressure.
Myocardial oxygen consumption during exercise has been estimated using the non-invasive rate-pressure product (i.e., double product; DP) which is the result of multiplying heart rate (HR) and systolic blood pressure (SBP). This easily measured hemodynamic variable is a good predictor of myocardial VO2 during exercise in healthy individuals (Kitamura et al. 1972), in patients with ischemic heart disease (Gobel et al. 1978) either when exercising with the upper or lower body musculature (Balady et al. 1986). DP has been used in cardiac rehabilitation to identify workloads at which signs and symptoms of ischemia occur, and to assess improvements in functional capacity in people with coronary artery diseases (Moore et al. 2016). In people without heart diseases, but with a high risk to develop cardiovascular diseases (e.g., metabolic syndrome patients) a lowering of DP for a given exercise workload evidences a reduced metabolic demand to the myocardium and may reduce the chances of suffering an adverse cardiovascular event triggered by exercise.
Cardiovascular training adaptations are classically measured when exercising at the same absolute workload before and after training. However, aerobic training lowers oxygen consumption for an absolute workload due to improved efficiency (Amati et al. 2008). One of the most important functions of the cardiovascular system during exercise is to deliver oxygen to the contracting muscles. Thus, the reduced oxygen demand during submaximal exercise after training (i.e., improved efficiency) could account for a portion of the reduction in heart rate and cardiac output. Thus, to isolate the effects of training, it seems logical to compare the hemodynamic response before and after training at the same submaximal oxygen consumption, even though workload ought to be increased to compensate for the effects of improved efficiency. However, to our knowledge, no study provides information of the hemodynamic response to such a comparison.
Although the information on the cardiovascular adaptations to aerobic training in healthy-young people is abundant, studies examining whether circulatory function is improved in MetS after endurance training are scarce. Therefore, the primary aim of the present study was to test if aerobic training results in significant reductions in vascular resistances and double product during submaximal exercise. This primary outcome will be assessed by monitoring the blood pressure, heart rate and stroke volume responses to training when subjects pedaled at an identical submaximal rate of oxygen consumption. The hypothesis is that training will reduce blood pressure and heart rate while increasing stroke volume despite increases in workload to achieve similar submaximal oxygen consumption.
Materials and methods
Participants
Thirty-four obese (BMI 32 ± 3 kg m−2) adult (age 54 ± 9 year old) women (17%) and men completed this study. All participants were physically inactive reporting less than 100 min per week of moderate–vigorous activity. Participants were enrolled based on fulfilling ≥3 MetS criteria as per harmonized definition (Alberti et al. 2009) using Europid waist circumference cut points (80 cm for women and 94 cm for men). Exclusion criteria included cardiovascular, respiratory, renal or any disease associated with exercise intolerance. Screening included physical examination to measure BMI, resting blood pressure, waist circumference, 12-lead ECG at rest and during a graded exercise stress test (GXT), and blood biochemistry. All subjects provided written, witnessed, informed consent and the study was approved by the local Hospital’s Ethics Committee in accordance with the Declaration of Helsinki.
Study design
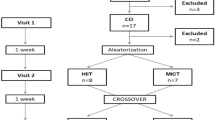
After screening, subjects were randomly assigned to either a training group (TRAIN; N = 20) or non-exercise control (CONT; N = 20) group. Two participants in the TRAIN group and four in the CONT group dropped out after the initial weeks owing to conflicts with working schedule or injuries unrelated to training (Fig. 1). Outcome variables were analyzed on both groups before and after the intervention period (6 months). Subjects in CONT were advised to continue with their moderate level of physical activity and fill out a physical activity questionnaire every month (IPAQ; Craig et al. 2003). All subjects were advised to maintain their normal dietary habits and continue with their current medication prescriptions during the whole study. They were requested to fill out a 3-day nutritional diary that was analyzed using a software (CESNICD, Barcelona, Spain) and feedback provided monthly. In the TRAIN group, post-training tests were scheduled at least 48 h after the last exercise session to examine the chronic effects of exercise training rather than the acute most recent exercise session. All subjects underwent a familiarization session 1 week prior the onset of testing to reduce the variability in the results of the rebreathing technique to assess cardiac output.
Resting measurements
Before and after the intervention, subjects arrived to the lab after an overnight fast. Body mass was measured on an electronic scale (Toledo Hawk, Metler, USA). After 20 min of supine rest, blood pressure was measured (Tango; Sun Tech Medical, NC, USA) in triplicate and then a 6-ml blood sample was drawn into a blood collection tube containing EDTA (Vacutainer; Becton–Dickinson, USA) and analyzed for hemoglobin and hematocrit (BC 5800 Mindray, Bio-Medical Electronics Ltd, China). Resting and changes in blood and plasma volume were estimated from body weight (Sawka et al. 1992) and changes in hemoglobin and hematocrit (Dill and Costill 1974). The remaining portion of the blood was analyzed for MetS parameters (HDL-c, triglycerides, glucose; Mindray BS 400 Chemistry Analyzer, Mindray Medical Instrumentation, USA). Percent body fat was determined by dual energy X-ray absorptiometry scan (DXA Hologic Serie Discovery Wi QDR, Bedford, USA).
Submaximal exercise
After the resting measurements, participants cycled (Ergoselect 200, Ergoline, Germany) for 20 min at a submaximal intensity. After 5 min of warm-up, workload was increased to elicit a target VO2 of ~1500 ml min−1. From min 8 to 12, VO2 (Quark b2, Cosmed, Italy), heart rate (RS400, Polar, Finland) and blood pressure (BP; Tango; Sun Tech Medical, NC, USA) were monitored. From min 12 to 20 cardiac output (Q) was measured in duplicate using CO2 rebreathing technique (Wilmore et al. 1982). In brief, after recording end-tidal CO2 pressure (P ETCO2) during normal breathing, subjects were switched to rebreathe from a rubber bag containing a mixture of CO2 and O2 until expired CO2 readings reached an equilibrium within 20 s of rebreathing (i.e., P EQCO2). Partial pressure and contents were corrected for blood hemoglobin concentration and capillary oxygen saturation (pulse oximeter, Quark b2, Cosmed, Italy) and Q calculated as follows:
In turn, stroke volume (SV) was derived by dividing the estimated Q by the measured HR at the time of the Q determination (i.e., SV = Q/HR). Systemic vascular resistance (SVR) was calculated as follows:
where 80 is a factor to convert values to dyn s cm−5. Gross efficiency was calculated as work accomplished divided by energy expenditure calculated from VO2 and VCO2 measurements (Brouwer 1957). Double product (DP) was calculated as follows:
Maximal exercise
Subjects were then provided with 333 ml of juice to rehydrate and rested seated during 20 min. Next, VO2PEAK was measured using a continuous graded exercise test (GXT) on the same electronic cycle ergometer starting at 50 W for men and 30 W for women with increases of 20 and 15 W every minute, respectively. VO2 and VCO2 were recorded in a breath-by-breath basis, whereas HR was monitored every 5 s. Participants met or exceeded the criteria that indicated maximal effort with RPEMAX ≥18, RERMAX ≥1.1 and HRMAX ≥85% of the age predicted maximum HR.
Training program
The TRAIN group underwent 45 min of pedaling 3 days per week for a total of 75 sessions in 6 months. In every exercise session participants wore a heart rate monitor and workloads were self-adjusted to reach four bouts of 90% HRMAX during 4 min alternated with 3 min of active recovery periods at 70% HRMAX as described previously (Morales-Palomo et al. 2017). This AIT protocol was previously shown to both improve CRF and be tolerable in this population (Mora-Rodriguez et al. 2014, 2016; Stensvold et al. 2010). Maximal heart rate was re-evaluated monthly during a maximal cycling bout to exhaustion and workloads adjusted accordingly to maintain training stimulus. Participants in CONT were instructed to maintain their baseline physical activity and dietary pattern during the intervention period.
Statistical analysis
The Shapiro–Wilk test evaluated normality. Differences between groups at baseline were analyzed using a t test for independent samples. To evaluate the effects of the interventions a two-way (Group × Time) mixed-model ANOVA was used using sex and age as covariant. When an interaction existed, Bonferroni post hoc test were performed to identify the time point where treatments significantly differed. Cohen’s formula for effect size (ES; Cohen 1988) was used, and the results were based on the following criteria; >0.70 large effect; 0.30–0.69 moderate effect; ≤0.30 small effect. Gender-specific Z score equations were used to account for variations from the MetS harmonized definition (Alberti et al. 2009). The sum of the Z scores for each MetS components was divided by 5 to compile the MetS risk score with units of SD (Brage et al. 2004). All analyses were performed with SPSS version 21 (Chicago, IL). Data presented as mean ± 95% CI. Statistical significance level was set at P ≤ 0.05.
Results
Body composition and MetS components
No differences existed between groups at pre-intervention baseline for age, body weight, percent fat, BMI or any of the MetS components (i.e., MetS Z score; Table 1). The major outcome measures did not differ between sexes or age and thus responses in the whole group are reported. TRAIN attendance to the exercise sessions averaged 95%. TRAIN resulted in small but significant reductions in body weight (92.6 ± 13.1 vs. 90.7 ± 11.2 kg, P = 0.021; ES 0.201), BMI (33.2 ± 3.1 vs. 32.9 ± 2.9 kg m−2, P = 0.032; ES 0.167) percent body fat (37.2 ± 7.1 vs. 35.6 ± 6.7%, P = 0.0105; ES 0.152). With TRAIN waist circumference decreased from baseline (107 ± 7.3 vs. 105 ± 6.7 cm; P = 0.001; ES 0.280) being lower than CONT at that time point (Table 1). TRAIN reduced 8% mean blood pressure (101.7 ± 11.7 vs. 93.9 ± 7.6 mmHg; P < 0.001; ES 0.594) resulting in differences form CONT at the end of treatment (Table 1). TRAIN did not affect blood glucose, triglycerides, and HDL-c that remain at the levels of CONT at the end of treatment.
Changes in blood and plasma volume
Estimated PV at rest was not significantly different between groups pre-intervention. However, TRAIN reduced hemoglobin concentration by 2.5% (95% CI 1.2–3.8%; P = 0.002; ES 0.349) while hematocrit increased (43.5 ± 3.1–44.3 ± 2.8%; P = 0.038; ES 0.365). Calculated plasma volume remained at pre-training levels (3606 ± 353 vs. 3657 ± 474 ml; P = 0.28; ES 0.124) but blood volume increased by 2.6% (95% CI 1.1–4.0%; P = 0.001; ES 0.232). Blood or plasma volume did not change in CONT.
Submaximal exercise
As designed, oxygen consumption during submaximal exercise was similar before and after treatment in both groups (~1500 ml O2 min−1; Table 2). The power output to elicit that rate of oxygen consumption was not significantly different between TRAIN and CONT at baseline (76 ± 37 vs. 79 ± 38 W; P = 0.293; Table 2). However, TRAIN increased by 15% (95% CI 5–25%) the power output to achieve the target VO2 raising it from 76 ± 37 to 88 ± 42 W (P < 0.001; ES 0.287; Table 2). At the end of the intervention, power output was higher in the TRAIN than in the CONT group (88 ± 42 vs. 80 ± 35 W; P < 0.05; Table 2). Gross efficiency also increased from 14 ± 4 to 16 ± 4% with TRAIN (P < 0.001; ES 0.571) being higher than CONT at the end of the treatment. Fat oxidation also increased with TRAIN but values were not different from CONT at the end of the intervention (Table 2).
In the TRAIN group, along with the increase in workload, Q increased from 10.9 ± 2.5 to 12.0 ± 4 l min−1 after training (P < 0.001; ES 0.571; Fig. 2a) being higher than the values of CONT at that time point (Fig. 2a). In contrast, HR decreased from 109 ± 15 to 106 ± 13 beats min−1 after TRAIN (P < 0.05; ES 0.215; Fig. 2b) although values were not different from CONT at the end of the intervention. Calculated stroke volume increased from 101 ± 24 ml beat−1 before training to 113 ± 23 ml beat−1 after training (P = 0.004; ES 0.513; Fig. 2c) being different from CONT at the end of the intervention (Fig. 2c).
a Cardiac output, b heart rate and c calculated stroke volume, during submaximal exercise before and after the 6-month intervention (TRAIN or CONT groups). Data are mean ± SD. Asterisk significantly different from baseline within that group (P < 0.05). Dagger significantly different from CONT group at that time point
TRAIN did not alter systolic blood pressure during submaximal exercise (166 ± 14 vs. 164 ± 15, P = 0.59; Fig. 3a) but there was a significant reduction in diastolic blood pressure (83 ± 8 vs. 75 ± 8 mmHg, P = 0.001; ES 0.990; Fig. 3b). Systemic vascular resistances were lowered in TRAIN when compared to baseline values (933 ± 203 vs. 844 ± 234 dyn s cm−5, P = 0.009; ES 0.404) and to CONT values at the end of the intervention (i.e., Fig. 3c). The calculated double product decrease from baseline values in TRAIN (18.2 ± 3.2–17.4 ± 2.4 bt min−1 mmHg 10−3; P = 0.043; ES 0.395) while it remain unchanged in CONT.
a, b Systolic and diastolic pressure and c calculated vascular resistances, during submaximal exercise before and after the 6-month intervention (TRAIN or CONT groups). Data are mean ± SD. Asterisk significantly different from baseline within that group (P < 0.05). Dagger significantly different from CONT group at that time point
Maximal exercise
VO2PEAK was not different between TRAIN and CONT at baseline. TRAIN increased subjects VO2PEAK by 14% (95% CI 9–18%) from 23.0 ± 6.4 vs. 26.1 ± 6.8 ml O2 kg min−1 (P = 0.001; ES 0.479) along with increased maximal work rate (W MAX; 176 ± 65 vs. 210 ± 75 W, P = 0.001; ES 0.483). None of these variables changed in the CONT group.
Discussion
We measured the effects of aerobic interval training (AIT) on the cardiovascular response to exercise in a sample of participants with metabolic syndrome (MetS). As previously reported (Mora-Rodriguez et al. 2014; Stensvold et al. 2010; Tjonna et al. 2008), AIT efficiently increased maximal oxygen consumption in MetS. What this study adds to the current body of literature is that TRAIN reduced systemic vascular resistances allowing increased cardiac output (Q) during submaximal exercise at the same oxygen consumption rate than before training (Fig. 1). The elevated Q was produced by increasing stroke volume while heart rate (HR) was slightly reduced (i.e., 3 beats min−1). There is a direct relationship between the product of systolic blood pressure by heart rate (double product) and myocardial oxygen requirements during exercise (Balady et al. 1986; Gobel et al. 1978; Kitamura et al. 1972). Of note, the lowering of double product in the TRAIN group, took place despite a 15% increase in workload to achieve a similar VO2. Thus, in MetS patients, 6 months of AIT improves the cardiovascular response for a given submaximal VO2 lowering HR, blood pressure and likely myocardial energy requirements.
The cardiovascular response to exercise training has been studied for decades (Ekblom et al. 1968; Klausen et al. 1982; Saltin et al. 1976; Wilmore et al. 2001). In most studies, the cardiovascular submaximal exercise responses are compared after training at the same absolute workload and thus at lower VO2 because of improved efficiency. Thus, some of the reported reductions in HR and blood pressure during submaximal exercise after training may be due to the lower metabolic demands. Since the prime function of the cardiovascular system during exercise is to deliver oxygen to contracting muscles, we though that it was important to test the cardiovascular response to exercise at the same oxygen consumption rate before and after training. To achieve similar oxygen consumption rate after training we had to increase the workload 15% due to the improved efficiency post-training (Amati et al. 2008; Wilmore et al. 2001). Despite the increased workload to achieve the same VO2, we observe the classical reduction in HR (3%) and increased stroke volume (10%) after aerobic training (Fig. 2).
Increases in exercise stroke volume (SV) with endurance training are largely attributed to increase end-diastolic volume as a consequence of left ventricular increase in chamber size (Gledhill et al. 1994), increased heart compliance (Arbab-Zadeh et al. 2004), plasma volume expansion (Hagberg et al. 1998) and faster diastolic filling rates. Cross-sectional data suggest that individuals with MetS have impaired left ventricular relaxation as measured by the ratio between early and late diastolic peak filling velocities measured at the mitral valve (Edgell et al. 2012). Neither, ten days of treadmill walking at 70–75% of VO2PEAK (Baynard et al. 2008) nor one year of combined endurance and resistance training reduces that ratio in MetS subjects (Lalande et al. 2013). However, isovolumetric relaxation time (IVRT) is reduced after training in MetS patients reflecting an enhanced ability of the heart to start the diastolic filling phase (Baynard et al. 2008). Baynard and co-workers found a tight correlation (r = 0.79; P = 0.017) between the change in IVRT and diastolic blood pressure with short-term aerobic training explaining 20% of the reductions in IVRT. Although we did not measure heart hemodynamics, we observed a reduction in diastolic blood pressure during exercise that could be indicative of an increased heart’s ability to fill during early diastole. This enhanced heart pre-load could be contributing to the increased SV observed in TRAIN group (Fig. 2c).
In contrast with studies comparing the same absolute intensity before and after training, we found increased Q when exercising at the same VO2, however, higher workload after training. An increased Q in conjunction with reduced diastolic blood pressure (Fig. 2b) suggest a reduction in heart after-load permitting blood flow augmentation. In fact, calculated systemic vascular resistance (SVR) significantly decreased in the TRAIN group (Fig. 3c) which could be due to augmented vessel compliance in the active muscles. Classic (Klausen et al. 1982) and recent (Mortensen et al. 2013) studies using one-leg exercise training protocol indicate that one of the main adaptations to aerobic training in the active skeletal muscle is the reduction in resistance to blood flow.
The increased blood flow to the contracting skeletal muscle could stem from reduced afferent feedback (lower K+, lactate and pH) and lower activation of the sympathetic outflow to active muscles (Mortensen et al. 2013). Alternatively, the extra Q after training (Fig. 2a) could be diverted to other vascular beds that were overlying constricted when detrained. Rowel (1986) showed that splanchnic and renal flow are inversely related to the percent of maximal oxygen uptake which in our data decreased from 71 to 64% VO2PEAK from baseline to follow-up in TRAIN. Alternatively, structural factor (i.e., angiogenesis inducing increased capillarity; Egginton 2011), functional sympatholysis (Strandell and Shepherd 1967) increased vasodilating hormones (MacDonald 2002) and local muscle autoregulation factors (i.e., temperature, ATP; Gonzalez-Alonso et al. 2015) are possibly implicated in the lowering of SVR with training.
Investigators have found that the blood volume (BV) expansion induced by training could account for up to half of the increase in VO2MAX observed after 12 weeks of interval training (Warburton et al. 2004). However, these investigators used 25 normally active and relatively young (30 years old) males and their BV expansion after 12 weeks of training amounted to 12%. In contrast, our middle-aged MetS subjects elevated BV by 2.5% after 24 weeks of AIT. Similarly, in a 4-week long training study in obese women, sprint interval training expanded plasma volume by only 4% and contributed little to the increase in SV after exercise (Trilk et al. 2011). Likewise, the small increase in blood volume in our subjects unlikely accounted for all the reported increases in SV and Q during submaximal exercise in the TRAIN group.
Our study has several limitations that are worth to mention. Blood volume was estimated and not directly measured and changes in hydration status of the subjects could have confounded the effects due to training. We do not provide any direct measure of myocardial hemodynamics and thus the cause of the increased SV after training (i.e., reduction in heart pre-load, post-load, or increased contractility) is speculative. The increase in muscle efficiency with training could be biased by a “learning effect” of the cycling technique by subjects with repeated testing. However, we provided a familiarization session before pre-training and it is likely that the reported improvements represent true increase in metabolic efficiency. Finally, subjects continued taking their prescribed medication during the duration of the study, and thus, a possible interaction between medication and exercise training could have existed in our data. To study the pure effect of exercise training, ideally, all patients should have suppressed medication during the study. However, our ethical committee deemed inappropriate to withhold medication since this could increase cardiovascular event risk. Nevertheless, since baseline and follow-up tests were conducted holding the same dose and type of medication in each subject, it is unlikely that medication could be responsible for the differences in hemodynamic reported in this study.
In summary, 6 months of aerobic interval training (TRAIN group) resulted in previously described increases in cardiorespiratory (15%; VO2MAX) and muscular (19%; W MAX) fitness in MetS patients. TRAIN reduced systemic vascular resistances and blood pressure permitting increased blood flow when exercising at a given submaximal VO2. On the other hand, the increased Q was delivered by increasing stroke volume while heart rate was slightly reduced (i.e., 3 beats min−1). The reduced heart rate in combination with the lowering of blood pressure, decrease myocardial energy demands (i.e., double product) and may lower the occurrence of a cardiovascular adverse event during exercise.
Abbreviations
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- CRF:
-
Cardiorespiratory fitness
- DBP:
-
Diastolic blood pressure
- DP:
-
Double product
- GXT:
-
Graded exercise testing
- MetS:
-
Metabolic syndrome
- SBP:
-
Systolic blood pressure
- W MAX :
-
Maximal power output
- VO2PEAK :
-
Peak oxygen consumption rate
References
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SCJ (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645
Amati F, Dube JJ, Shay C, Goodpaster BH (2008) Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J Appl Physiol 105(3):825–831. doi:10.1152/japplphysiol.90384.2008
Andrew GM, Guzman CA, Becklake MR (1966) Effect of athletic training on exercise cardiac output. J Appl Physiol 21(2):603–608
Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD (2004) Effect of aging and physical activity on left ventricular compliance. Circulation 110(13):1799–1805. doi:10.1161/01.CIR.0000142863.71285.74
Balady GJ, Schick EC Jr, Weiner DA, Ryan TJ (1986) Comparison of determinants of myocardial oxygen consumption during arm and leg exercise in normal persons. Am J Cardiol 57(15):1385–1387
Baynard T, Carhart RL Jr, Ploutz-Snyder LL, Weinstock RS, Kanaley JA (2008) Short-term training effects on diastolic function in obese persons with the metabolic syndrome. Obesity (Silver Spring) 16(6):1277–1283
Brage S, Wedderkopp N, Ekelund U, Franks PW, Wareham NJ, Andersen LB, Froberg K, European Youth Heart S (2004) Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS). Diabetes Care 27(9):2141–2148
Brouwer E (1957) On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (Oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl 6:795–802
Cohen J (1988) Statistical power analysis for the behavioural sciences, 2nd edn. Lawrence Erlbaum Associates, New Jersey, p 569
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35(8):1381–1395. doi:10.1249/01.MSS.0000078924.61453.FB
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37(2):247–248
Edgell H, Petrella RJ, Hodges GJ, Shoemaker JK (2012) Central versus peripheral cardiovascular risk in metabolic syndrome. Front Physiol 3:38. doi:10.3389/fphys.2012.00038
Egginton S (2011) Physiological factors influencing capillary growth. Acta Physiol (Oxf) 202(3):225–239. doi:10.1111/j.1748-1716.2010.02194.x
Ekblom B, Astrand PO, Saltin B, Stenberg J, Wallstrom B (1968) Effect of training on circulatory response to exercise. J Appl Physiol 24(4):518–528
Gledhill N, Cox D, Jamnik R (1994) Endurance athletes’ stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc 26(9):1116–1121
Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y (1978) The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 57(3):549–556
Gonzalez-Alonso J, Calbet JA, Boushel R, Helge JW, Sondergaard H, Munch-Andersen T, van Hall G, Mortensen SP, Secher NH (2015) Blood temperature and perfusion to exercising and non-exercising human limbs. Exp Physiol 100(10):1118–1131. doi:10.1113/EP085383
Hagberg JM, Goldberg AP, Lakatta L, O’Connor FC, Becker LC, Lakatta EG, Fleg JL (1998) Expanded blood volumes contribute to the increased cardiovascular performance of endurance-trained older men. J Appl Physiol 85(2):484–489
Ingelsson E, Sullivan LM, Murabito JM, Fox CS, Benjamin EJ, Polak JF, Meigs JB, Keyes MJ, O’Donnell CJ, Wang TJ, D’Agostino RB Sr, Wolf PA, Vasan RS (2007) Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes 56(6):1718–1726. doi:10.2337/db07-0078
Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y (1972) Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol 32(4):516–522
Klausen K, Secher NH, Clausen JP, Hartling O, Trap-Jensen J (1982) Central and regional circulatory adaptations to one-leg training. J Appl Physiol 52(4):976–983
Lalande S, Petrella RJ, Shoemaker JK (2013) Effect of exercise training on diastolic function in metabolic syndrome. Appl Physiol Nutr Metab 38(5):545–550. doi:10.1139/apnm-2012-0383
MacDonald JR (2002) Potential causes, mechanisms, and implications of post exercise hypotension. J Hum Hypertens 16(4):225–236. doi:10.1038/sj.jhh.1001377
Moore G, Durstine JL, Painter P (2016) ACSM’s exercise management for persons with chronic diseases and disabilities, 4th edn. Human Kinetics, Champaign
Morales-Palomo F, Ramirez-Jimenez M, Ortega JF, Pallares JG, Mora-Rodriguez R (2017) Cardiovascular drift during training for fitness in patients with metabolic syndrome. Med Sci Sports Exerc 49(3):518–526. doi:10.1249/MSS.0000000000001139
Mora-Rodriguez R, Ortega JF, Hamouti N, Fernandez-Elias VE, Canete Garcia-Prieto J, Guadalupe-Grau A, Saborido A, Martin-Garcia M, Guio de Prada V, Ara I, Martinez-Vizcaino V (2014) Time-course effects of aerobic interval training and detraining in patients with metabolic syndrome. Nutr Metab Cardiovasc Dis 24(7):792–798. doi:10.1016/j.numecd.2014.01.011
Mora-Rodriguez R, Ortega JF, Guio de Prada V, Fernandez-Elias VE, Hamouti N, Morales-Palomo F, Martinez-Vizcaino V, Nelson RK (2016) Effects of simultaneous or sequential weight loss diet and aerobic interval training on metabolic syndrome. Int J Sports Med 37(4):274–281. doi:10.1055/s-0035-1564259
Mortensen SP, Svendsen JH, Ersboll M, Hellsten Y, Secher NH, Saltin B (2013) Skeletal muscle signaling and the heart rate and blood pressure response to exercise: insight from heart rate pacing during exercise with a trained and a deconditioned muscle group. Hypertension 61(5):1126–1133. doi:10.1161/HYPERTENSIONAHA.111.00328
Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD (1992) Effects of training on muscle O2 transport at VO2max. J Appl Physiol 73(3):1067–1076
Rowell LB (1986) Circulatory adjustments to dynamic exercise. Human circulation; regulation during physical stress. Oxford University Press, New York, pp 213–256
Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Wildenthal K, Chapman CB (1968) Response to exercise after bed rest and after training. Circulation 38(5):VII1–VII78
Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B, Gollnick D (1976) The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand 96(3):289–305. doi:10.1111/j.1748-1716.1976.tb10200.x
Sawka MN, Young AJ, Pandolf KB, Dennis RC, Valeri CR (1992) Erythrocyte, plasma, and blood volume of healthy young men. Med Sci Sports Exerc 24(4):447–453
Stensvold D, Tjonna AE, Skaug EA, Aspenes S, Stolen T, Wisloff U, Slordahl SA (2010) Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol 108(4):804–810. doi:10.1152/japplphysiol.00996.2009
Strandell T, Shepherd JT (1967) The effect in humans of increased sympathetic activity on the blood flow to active muscles. Acta Med Scand Suppl 472:146–167
Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slordahl SA, Kemi OJ, Najjar SM, Wisloff U (2008) Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation 118(4):346–354. doi:10.1161/CIRCULATIONAHA.108.772822
Trilk JL, Singhal A, Bigelman KA, Cureton KJ (2011) Effect of sprint interval training on circulatory function during exercise in sedentary, overweight/obese women. Eur J Appl Physiol 111(8):1591–1597. doi:10.1007/s00421-010-1777-z
Warburton DE, Haykowsky MJ, Quinney HA, Blackmore D, Teo KK, Taylor DA, McGavock J, Humen DP (2004) Blood volume expansion and cardiorespiratory function: effects of training modality. Med Sci Sports Exerc 36(6):991–1000
Wilmore JH, Farrell PA, Norton AC, Cote RW 3rd, Coyle EF, Ewy GA, Temkin LP, Billing JE (1982) An automated, indirect assessment of cardiac output during rest and exercise. J Appl Physiol 52(6):1493–1497
Wilmore JH, Stanforth PR, Gagnon J, Rice T, Mandel S, Leon AS, Rao DC, Skinner JS, Bouchard C (2001) Cardiac output and stroke volume changes with endurance training: the HERITAGE Family Study. Med Sci Sports Exerc 33(1):99–106
Acknowledgements
This study was partially funded by a grant from the Spanish Ministry of Economy and Competivity (DEP-2014-52930-R).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Carsten Lundby.
Rights and permissions
About this article
Cite this article
Mora-Rodriguez, R., Fernandez-Elias, V.E., Morales-Palomo, F. et al. Aerobic interval training reduces vascular resistances during submaximal exercise in obese metabolic syndrome individuals. Eur J Appl Physiol 117, 2065–2073 (2017). https://doi.org/10.1007/s00421-017-3697-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3697-7