Abstract
Introduction
Isometric resistance training has repeatedly shown to be an effective exercise modality in lowering resting blood pressure (BP), yet associated mechanisms and sex differences in the response to training remain unclear. Exploration into potential sex differences in the response to isometric resistance training is necessary, as it may allow for more optimal and sex-based exercise prescription, thereby maximizing the efficacy of the training intervention.
Purpose
Therefore, we investigated, in normotensives, whether sex differences exist in the response to isometric handgrip (IHG) training.
Methods
Resting BP and endothelium-dependent vasodilation (brachial artery flow-mediated dilation; FMD) were assessed in 11 women (23 ± 4 years) and 9 men (21 ± 2 years) prior to and following 8 weeks of IHG training (four, 2-min unilateral contractions at 30 % of maximal voluntary contraction; 3 days per week).
Results
Main effects of time were observed (all P < 0.05), whereby IHG training reduced systolic BP (Δ 8 ± 6 mmHg), diastolic BP (Δ 2 ± 3 mmHg), mean arterial pressure (Δ 4 ± 3 mmHg), and pulse pressure (Δ 5 ± 7 mmHg), accompanied by increases in absolute (Δ 0.09 ± 0.15 mm) and relative (Δ 2.4 ± 4.1 %) brachial artery FMD; however, no significant sex differences were observed in the magnitude of post-training change in any variable assessed (all P > 0.05).
Conclusion
IHG training effectively lowers resting BP and improves endothelium-dependent vasodilation in men and women, without significant sex differences in the magnitude of response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise training represents a widely recommended component in the treatment and prevention of high blood pressure (BP) (Pescatello et al. 2004). Indeed, aerobic exercise training has consistently proven to effectively reduce resting and ambulatory BP (Pescatello et al. 2004). More recently, a large and emerging body of evidence supports isometric resistance training as an effective exercise modality to lower resting BP in both normotensive and hypertensive populations (Wiley et al. 1992; Ray and Carrasco 2000; Howden et al. 2002; Taylor et al. 2003; Peters et al. 2006; McGowan et al. 2007a, b; Millar et al. 2008; Devereux et al. 2010; Wiles et al. 2010; Baross et al. 2012; Millar et al. 2013; Badrov et al. 2013a, b; Gill et al. 2015). Two recent meta-analyses suggest that isometric resistance training may be capable of eliciting greater BP reductions than traditional aerobic and resistance exercise training (Cornelissen and Smart 2013; Carlson et al. 2014). Based on this accumulating evidence, the American Heart Association suggests that isometric resistance training, and in particular, isometric handgrip (IHG) training, may be used as a potential alternative strategy to lower resting BP (Class IIB, Level of Evidence C; Brook et al. 2013).
An important and overlooked consideration is whether sex differences exist in the magnitude of BP attenuation that occurs following isometric resistance training. While differences between men and women in several aspects of BP control are well established (Hart et al. 2012), limited data exists pertaining to the effect of sex on the hypotensive response to exercise training. In the aerobic and resistance training literature, some evidence suggests that BP decreases similarly in men and women (Ishikawa et al. 1999; Kelley et al. 2001; Cornelissen and Smart 2013), while other data shows that women may respond with greater reductions (Hagberg et al. 2000; Collier et al. 2011; Morita and Okita 2013). In the isometric resistance training literature, early exploratory evidence suggests that older normotensive women may be more responsive to the IHG stimulus than older men (Millar et al. 2008), while a recent systematic review and meta-analysis observed a non-significant trend towards larger reductions in men (Inder et al. 2016). Furthermore, the associated mechanisms responsible for the hypotensive response remain elusive and are likely to differ across populations, including sex (Millar et al. 2014).
To date, the influence of sex on the response to IHG training has yet to be directly nor prospectively investigated. Examination of potential sex differences in the response to IHG training is required as it may allow for more optimized and sex-based exercise prescription, thereby maximizing the efficacy of the training intervention. Therefore, we explored potential sex differences in the BP lowering effects of, and peripheral vascular adaptations (endothelium-dependent vasodilation) to, IHG training in young normotensives. We hypothesized that women would experience greater post-IHG training BP reductions in comparison to men (Millar et al. 2008), concomitant with greater improvements in endothelium-dependent vasodilation.
Methods
Participants
Twenty normotensive (<140/<90 mmHg) women (n = 11) and men (n = 9) participated in the current investigation. Women were aged 23 ± 4 years and were 165 ± 5 cm in height and 58 ± 6 kg in weight (Body mass index = 21 ± 2 kg m−2). Men were aged 21 ± 2 years and were 179 ± 8 cm in height and 77 ± 12 kg in weight (Body mass index = 24 ± 2 kg m−2). All participants were recreationally active (≥two exercise sessions per week), non-smoking, and free of any overt disease (established via a standardized health questionnaire). Women were tested during the early follicular phase of their menstrual cycle (if not on oral contraceptives) or low hormone phase (if on oral contraception) at all time points. No changes in diet, exercise, or medication use occurred during the study period.
The investigation was cleared by the University of Windsor Research Ethics Board and all procedures were performed in accordance with the Declaration of Helsinki. All participants provided informed written consent and were familiarized to all testing protocols prior to study participation.
Experimental design
This study utilized a prospective cohort design. Participants completed a laboratory testing session to assess resting BP and heart rate (HR), and endothelium-dependent vasodilation. This laboratory testing session was repeated following 8 weeks of IHG training.
Isometric handgrip training
Participants trained 3 days per week for 8 weeks. IHG exercise was performed using a programmed handgrip dynamometer (IBX H-101, MD Systems, Inc., Westerville, Ohio, USA). Each exercise session consisted of four, 2-min unilateral contractions at 30 % of maximal voluntary contraction (MVC; determined at the start of each exercise session via electronic linear load cells contained within each handgrip device). All exercise was performed using the non-dominant hand and each contraction was separated by a 4-min rest period.
Experimental measures
All testing was conducted in a quiet, darkened, and temperature-controlled laboratory (20–24 °C) following at least a 4-h fast, a 12-h abstinence from caffeine and other stimulants, and a 24-h abstinence from exercise and alcohol. Participants voided their bladder prior to testing commencement. All repeat testing (i.e., following 8 weeks of IHG training) was conducted within 2 h of the initial baseline testing time of day.
Resting blood pressure
Resting BP was measured in the seated position in the brachial artery of the dominant arm using automated brachial oscillometry (Dinamap Carescape v100, Critikon, Tampa, FL, USA). Four BP and HR measurements, each separated by 2 min, were obtained following 10 min of seated rest. The latter three measures were averaged and used in the final analysis.
Endothelium-dependent vasodilation
Endothelium-dependent vasodilation was assessed using brachial artery flow-mediated dilation (FMD; Celermajer et al. 1992), in accordance with published guidelines (Harris et al. 2010; Thijssen et al. 2011). Participants rested in the seated position with their non-dominant arm extended at heart level and fitted with a pneumatic cuff (Hokanson SC12D, D.E. Hokanson, Inc., Bellevue, Washington, USA) 2–4 cm distal to the antecubital fossa. A 13-MHz linear array probe attached to a high-resolution Doppler ultrasound machine (Vivid i, GE Healthcare, Pittsburgh, PA, USA) was used to image the brachial artery approximately 3–5 cm proximal to the antecubital fossa. Simultaneous B-mode images and continuous Doppler were obtained for the determination of brachial artery diameter and blood velocity, respectively. All recordings were obtained using an insonation angle of 60° and a sample volume spanning the entire width of the brachial artery, but kept clear of the vessel walls. HR was continuously obtained from two sets of three electrodes on the chest to generate ECG-gated ultrasound images and blood velocity samples. Following 20 min of seated rest, baseline resting measures assessing brachial artery diameter and blood velocity were acquired for 1-min. Upon completion of baseline resting measures, the pneumatic cuff was inflated via a rapid cuff inflator (Hokanson E20 Cuff Inflator, D.E. Hokanson, Inc., Bellevue, Washington, USA) to a pressure above systolic BP (~200 mmHg) for 5 min, which provided the ischemic stimulus responsible for reactive hyperemia and subsequent FMD. Measures of brachial artery diameter and blood velocity were acquired 30 s prior to cuff deflation and continued for 5 min post-deflation.
Resting and reactive hyperemic brachial artery images were saved at end-diastole in Digital Imaging and Communications in Medicine (DICOM) format for off-line analysis. Individual end-diastolic frames were stacked and saved in a new digital DICOM file using DICOM editing software (Sante DICOM Editor, Version 3.0.12; Santesoft LTD, Athens, Greece). Brachial artery diameters were analyzed by the same trained investigator using semi-automated edge detection software (Artery Measurement System (AMS) v2, Image and Data Analysis, Gothenburg, Sweden). Briefly, a region of interest (ROI) was chosen to include both the near and far wall of the brachial artery. Brachial artery diameter was determined from leading edge to leading edge throughout the subsequent frames of the DICOM file. The AMS software performs approximately 100 diameter measures within the selected ROI to determine a mean brachial artery diameter for each frame of the DICOM file. If necessary, the investigator could manually correct the chosen borders. Mean blood velocity was analyzed from the continuous Doppler mode spectra. All brachial artery diameter and mean blood velocity measures were analyzed using 3-s average time-bins.
From the synchronized brachial artery diameter and blood velocity data, blood flow (product of blood vessel cross-sectional area and mean blood velocity) and shear rate (eight times mean blood velocity divided by blood vessel diameter) were calculated. Brachial artery FMD is reported as the absolute (mm) and relative (%) diameter change from baseline.
Statistical analysis
All data were analyzed using two-way analyses of variance with repeated measures to test for the effects of sex (men vs. women) and time (pre- vs. post-). Shapiro-Wilk tests were used to assess for normality of all variables, with non-normally distributed data undergoing logarithmic transformation. When required, Tukey’s post hoc procedures were used to evaluate specific differences between means. Estimates of effect size are provided as Cohen’s d values (d = 0.2, small effect; d = 0.5, medium effect; d = 0.8, large effect; Cohen 1992). All statistical analyses were performed using SigmaPlot 12.0 (Systat Software, San Jose, CA, USA) and the significance level was set at P < 0.05. Data are presented as mean ± SD.
Results
All participants adhered to the exercise prescription, completing all 24 exercise sessions over the 8-week training period. As expected, at baseline, women had significantly lower height, weight, body mass index, systolic BP, and pulse pressure in comparison to men (all P < 0.05). Furthermore, at baseline, women had lower resting and peak brachial blood flow and a smaller brachial artery diameter (all P < 0.05). There were no significant differences between men and women in any other baseline characteristic. IHG training had no effect on MVC strength in either women (22.1 ± 4.3 to 23.3 ± 6.3 kg; P > 0.05, d = 0.22) or men (37.5 ± 8.0 to 40.9 ± 10.8 kg; P > 0.05, d = 0.36).
Effects of IHG training on resting blood pressure
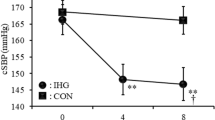
Analysis of resting BP revealed no sex differences in the magnitude of change following IHG training (all sex × time interactions, P > 0.05). However, significant main effects of time were observed, whereby IHG training significantly lowered resting systolic BP (P < 0.001; Women: ∆ 6 ± 4 mmHg, d = 1.08; Men: ∆ 9 ± 9 mmHg, d = 1.11), diastolic BP (P < 0.01; Women: ∆ 3 ± 4 mmHg, d = 0.52; Men: ∆ 2 ± 2 mmHg, d = 0.32), mean arterial pressure (P < 0.001; Women: ∆ 4 ± 4 mmHg, d = 0.90; Men: ∆ 4 ± 4 mmHg, d = 0.79), and pulse pressure (P < 0.01; Women: ∆ 3 ± 4 mmHg, d = 0.41; Men: ∆ 8 ± 9 mmHg, d = 1.07) (Fig. 1).
Effects of IHG training on endothelium-dependent vasodilation
Analysis of brachial artery FMD revealed no significant sex × time interactions for absolute or relative brachial artery FMD (all P > 0.05). However, significant main effects of time were observed, whereby IHG training significantly improved absolute (P < 0.05; Women: ∆ 0.09 ± 0.11 mm, d = 0.72; Men: ∆ 0.08 ± 0.20 mm, d = 0.57) and relative (P < 0.05; Women: ∆ 2.8 ± 3.7 %, d = 0.68; Men: ∆ 1.9 ± 4.7 %, d = 0.59) brachial artery FMD (Fig. 2). Resting vascular and vascular reactivity characteristics are presented in Table 1. No sex differences or changes were observed in resting brachial artery diameter, resting blood flow, or resting and peak shear rate following IHG training (all P > 0.05). However, a main effect of time was observed for peak reactive hyperemic blood flow, which increased following IHG training (P < 0.05; Women: ∆ 63.1 ± 66.5 mL min−1, d = 1.37; Men: ∆ 64.0 ± 185.2 mL min−1, d = 0.38); however, no difference between sexes in the magnitude of improvement existed (P > 0.05).
Discussion
To date, the majority of IHG training research has sampled combined populations of men and women without direct investigation into potential sex differences. To our knowledge, this is the first study to directly and prospectively assess differences between men and women in the effects of IHG training on resting BP and endothelium-dependent vasodilation, a potential mechanism involved in the BP change. Our results suggest that IHG training effectively lowers resting BP in both men and women, without sex differences in the magnitude of attenuation. Furthermore, exploration into potential mechanisms responsible revealed improvements in endothelium-dependent vasodilation, without significant differences between men and women.
Effects of IHG training on resting blood pressure
We found no differences between men and women in the magnitude of resting BP reduction following IHG training in our young, normotensive population. However, significant effects of IHG training were observed, whereby all indices of resting BP were lowered significantly following the IHG intervention. Our results demonstrate the strength of the IHG training effect, as 85 % of participants in the present study experienced clinically relevant reductions in BP (i.e., ≥2 mmHg; Pescatello et al. 2004), despite already normal baseline BP. Our findings add to an impressive body of literature attesting to the substantial hypotensive benefits of IHG training in populations with and without high BP (Wiley et al. 1992; Ray and Carrasco 2000; Taylor et al. 2003; Peters et al. 2006; McGowan et al. 2007a, b; Millar et al. 2008, 2013; Badrov et al. 2013a, b). In contrast to previously reported trends in the literature (Millar et al. 2008; Inder et al. 2016), we show for the first time, using a prospective cohort design, that young men and women respond to IHG training with similar reductions in resting BP. Essential to the implementation of IHG training as a therapeutic intervention in the management of BP is specificity in exercise prescription to sex, age, and pathology. Moving forward, large-scale randomized controlled trials should be employed to further investigate sex differences in the BP response to IHG training in various age and patient groups to fully maximize the benefit of this treatment modality.
Effects of IHG training on endothelium-dependent vasodilation
While the BP lowering effects of IHG training are well established, the mechanistic pathway(s) mediating this response have yet to be fully elucidated. A reduction in total peripheral resistance through an improvement in endothelium-dependent vasodilation has been proposed as a potential contributor to the observed reductions in resting BP following IHG training (Millar et al. 2014). Whether the effect of IHG training on brachial artery FMD differs between men and women is unknown. In the current study, reductions in resting BP were concomitant with improvements in both absolute and relative brachial artery FMD; however, there were no differences between sexes in the magnitude of change.
Previously, we observed no change in brachial artery FMD following an identical 8-week IHG training intervention in a group of young normotensives (McGowan et al. 2007a). A potential explanation for the discrepancy in results may be the larger sample size (N = 20 vs. N = 13) studied in the current study, increasing power to detect a change in brachial artery FMD. Similarly, studies to date in healthy subjects on the effect of localized exercise training (i.e., handgrip training) on endothelial function have yielded somewhat inconsistent results. For example, rhythmic handgrip exercise in healthy men improved brachial artery FMD at weeks 2, 4, and 6 of training (Tinken et al. 2010), whereas 4 weeks of rhythmic handgrip training, also in men, had no effect (Hunt et al. 2012). Furthermore, several studies have shown enhanced endothelium-dependent vasodilation following handgrip training interventions in young individuals (Allen et al. 2003; Credeur et al. 2010), yet other studies have observed no such change (Green et al. 1994; Franke et al. 1998). Differences in handgrip training protocols and intervention lengths make direct comparison to the current study difficult, as these studies typically employed rhythmic handgrip training protocols for a period of 4 weeks, in comparison to the 8-week interventions used commonly with isometric resistance exercise. However, our current findings are consistent with previous reports in hypertensive subjects (McGowan et al. 2006, 2007b), in which conduit artery endothelial function was improved significantly following 8 weeks of IHG training. The improvement in brachial artery FMD following IHG training may be due to shear-stress mediated increases in nitric oxide bioavailability and/or improved oxidative stress (Green et al. 2004), the latter of which has been shown to occur following IHG training (Peters et al. 2006). Yet, it cannot be ruled out that improvements were due to altered vascular structure and/or changes in endothelium-independent vasodilation, both of which were not measured in the current study. However, the fact that IHG training has no effect on brachial artery compliance (Visocchi et al. 2004), and has previously improved brachial artery FMD without changes in nitroglycerin-mediated vasodilator capacity (McGowan et al. 2006), makes this conjecture unlikely.
Also noteworthy, and in accordance with previous work in normotensives (McGowan et al. 2007a), is our finding of increased peak reactive hyperemic blood flow following the IHG training intervention, which may be indicative of functional and/or structural changes in the resistance vessel vasculature (Naylor et al. 2005). This supports our recent work (Badrov et al. 2013a), in which resistance vessel endothelial function (assessed using venous strain-gauge plethysmography) was improved in young normotensives following 8 weeks of IHG training. However, in this study (Badrov et al. 2013a), BP was reduced after 4 weeks of training without accompanying change in resistance vessel function, suggesting that multiple pathways may interact to mediate the effect of IHG training. Taken together, our current results suggest that improvements in conduit and resistance vessel endothelial function may play a role in the observed BP reductions following IHG training in both men and women, although other mechanisms are likely involved (Millar et al. 2014). Nonetheless, brachial artery FMD has proven to be an independent predictor of future cardiovascular disease in healthy adults (Yeboah et al. 2009), and therefore, improvements with IHG training yield great benefit independent of the potential contribution to the BP lowering effects of the exercise.
In conclusion, we demonstrate that an 8-week IHG training intervention reduces resting BP in men and women with normal BP, without sex differences in the magnitude of reduction. Furthermore, reductions in resting BP occurred simultaneously to improvements in conduit and resistance vessel endothelium-dependent vasodilation in both men and women, suggesting that improvements in vascular endothelial function may contribute to the post-training BP response.
Abbreviations
- AMS:
-
Artery measurement system
- BP:
-
Blood pressure
- DICOM:
-
Digital imaging and communications in medicine
- FMD:
-
Flow mediated dilation
- HR:
-
Heart rate
- IHG:
-
Isometric handgrip
- MVC:
-
Maximal voluntary contraction
- ROI:
-
Region of interest
- SD:
-
Standard deviation
References
Allen JD, Geaghan JP, Greenway F, Welsch MA (2003) Time course of improved flow-mediated dilation after short-term exercise training. Med Sci Sports Exerc 35:847–853
Badrov MB, Bartol CL, DiBartolomeo MA, Millar PJ, McNevin NH, McGowan CL (2013a) Effects of isometric handgrip training dose on resting blood pressure and resistance vessel endothelial function in normotensive women. Eur J Appl Physiol 113:2091–2100
Badrov MB, Millar PJ, Horton S, McGowan CL (2013b) Cardiovascular stress reactivity tasks successfully predict the hypotensive response of isometric handgrip training in hypertensives. Pyschophysiology 50:407–414
Baross AW, Wiles JD, Swaine IL (2012) Effects of the intensity of leg isometric training on the vasculature of trained and untrained limbs and resting blood pressure in middle-aged men. Int J Vasc Med 2012:964697
Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano JD, Elliott WJ, Fuchs FD, Hughes JW, Lackland DT, Staffileno BA, Townsend RR, Rajagopalan S, on behalf of the American Heart Association Professional Education Committee of the Council on High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity, and Metabolism (2013) Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension 61:1360–1383
Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA (2014) Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clin Proc 89:327–334
Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE (1992) Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340:1111–1115
Cohen J (1992) A power primer. Psychol Bull 112:155–159
Collier SR, Frechette V, Sandberg K, Schafer P, Ji H, Smulyan H, Fernhall B (2011) Sex differences in resting hemodynamics and arterial stiffness following 4 weeks of resistance versus aerobic exercise training in individuals with pre-hypertension to stage 1 hypertension. Biol Sex Differ 2:1–7
Cornelissen VA, Smart NA (2013) Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2:e004473
Credeur DP, Hollis BC, Welsch MA (2010) Effects of handgrip training with venous restriction on brachial artery vasodilation. Med Sci Sports Exerc 42:1296–1302
Devereux GR, Wiles JD, Swaine IL (2010) Reductions in resting blood pressure after 4 weeks of isometric exercise training. Eur J Appl Physiol 109:601–606
Franke WD, Stephens GM, Schmid PG III (1998) Effects of intense exercise training on endothelium-dependent exercise-induced vasodilatation. Clin Physiol 18:521–528
Gill KF, Arthur ST, Swaine I, Devereux GR, Huet YM, Wikstrom E, Cordova ML, Howden R (2015) Intensity-dependent reductions in resting blood pressure following short-term isometric exercise training. J Sports Sci 33:616–621
Green DJ, Cable NT, Fox C, Rankin JM, Taylor RR (1994) Modification of forearm resistance vessels by exercise training in young men. J Appl Physiol 77:1829–1833
Green DJ, Maiorana A, O’Driscoll G, Taylor R (2004) Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561:1–25
Hagberg JM, Park J, Brown MD (2000) The role of exercise training in the treatment of hypertension: an update. Sports Med 30:193–206
Harris RA, Nishiyama SK, Wray DW, Richardson RS (2010) Ultrasound assessment of flow-mediated dilation. Hypertension 55:1075–1085
Hart EC, Joyner MJ, Wallin BG, Charkoudian N (2012) Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 590:2069–2079
Howden R, Lightfoot JT, Brown SJ, Swaine IL (2002) The effects of isometric exercise training on resting blood pressure and orthostatic tolerance in humans. Exp Physiol 87:507–515
Hunt JEA, Walton LA, Ferguson RA (2012) Brachial artery modifications to blood flow-restricted handgrip training and detraining. J Appl Physiol 112:956–961
Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA (2016) Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res 39:88–94
Ishikawa K, Ohta T, Zhang J, Hashimoto S, Tanaka H (1999) Influence of age and gender on exercise training-induced blood pressure reduction in systemic hypertension. Am J Cardiol 84:192–196
Kelley GA, Kelley KA, Tran ZV (2001) Aerobic exercise and resting blood pressure: a meta-analytic review of randomized, controlled trials. Prev Cardiol 4:73–80
McGowan CL, Levy AS, Millar PJ, Guzman JC, Morillo CA, McCartney N, MacDonald MJ (2006) Acute vascular responses to isometric handgrip exercise and effects of training in persons medicated for hypertension. Am J Physiol Heart Circ Physiol 291:H1797–H1802
McGowan CL, Levy AS, McCartney N, MacDonald MJ (2007a) Isometric handgrip training does not improve flow-mediated dilation in subjects with normal blood pressure. Clin Sci 112:403–409
McGowan CL, Visocchi A, Faulkner M, Verduyn R, Rakobowchuk M, Levy AS, McCartney N, MacDonald MJ (2007b) Isometric handgrip training improves local flow-mediated dilation in medicated hypertensives. Eur J Appl Physiol 99:227–234
Millar PJ, Bray SR, MacDonald MJ, McCartney N (2008) The hypotensive effects of isometric handgrip training using an inexpensive spring handgrip training device. J Cardiopulm Rehabil Prev 28:203–207
Millar PJ, Levy AS, McGowan CL, McCartney N, MacDonald MJ (2013) Isometric handgrip training lowers blood pressure and increases heart rate complexity in medicated hypertensive patients. Scand J Med Sci Sports 23:620–626
Millar PJ, McGowan CL, Cornelissen VA, Araujo CG, Swaine IL (2014) Evidence for the role of isometric exercise training in reducing blood pressure: potential mechanisms and future directions. Sports Med 44:345–356
Morita N, Okita K (2013) Is gender a factor in the reduction of cardiovascular risk with exercise training? Circ J 77:646–651
Naylor LH, Weisbrod CJ, O’Driscoll G, Green DJ (2005) Measuring peripheral resistance and conduit arterial structure in humans using Doppler ultrasound. J Appl Physiol 98:2311–2315
Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA (2004) Exercise and hypertension. Med Sci Sports Exerc 36:533–553
Peters PG, Alessio HM, Hagerman AE, Ashton T, Nagy S, Wiley RL (2006) Short-term isometric exercise reduces systolic blood pressure in hypertensive adults: possible role of reactive oxygen species. Int J Cardiol 110:199–205
Ray CA, Carrasco DI (2000) Isometric handgrip training reduces arterial pressure at rest without changes in sympathetic nerve activity. Am J Physiol Heart Circ Physiol 279:H245–H249
Taylor AC, McCartney N, Kamath MV, Wiley RL (2003) Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc 35:251–256
Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ (2011) Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300:H2–H12
Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ (2010) Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55:312–318
Visocchi A, McGowan CL, Faulkner M, Verdun R, McCartney N, MacDonald M (2004) The effect of isometric arm or leg exercise on resting blood pressure and arterial distensibility in persons medicated for hypertension (Abstract). Physiologist 47:285
Wiles JD, Coleman DA, Swaine IL (2010) The effects of performing isometric training at two exercise intensities in healthy young males. Eur J Appl Physiol 108:419–428
Wiley RL, Dunn CL, Cox RH, Hueppchen NA, Scott MS (1992) Isometric exercise training lowers resting blood pressure. Med Sci Sports Exerc 24:749–754
Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM (2009) Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120:502–509
Acknowledgments
The authors would like to thank Don A. Clarke for his technical assistance and Cassandra L. Bartol and Matthew A. DiBartolomeo for their help with data collection. The IHG dynamometers used in this study were donated by Zona Health (Boise, ID, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the University of Windsor (Grant #s 810043; 809264; 808316; CLM), Heart and Stroke/Richard Lewar Centre of Excellence Postdoctoral Fellowship (PJM), and an Ontario Graduate Scholarship (MBB).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Massimo Pagani.
Rights and permissions
About this article
Cite this article
Badrov, M.B., Freeman, S.R., Zokvic, M.A. et al. Isometric exercise training lowers resting blood pressure and improves local brachial artery flow-mediated dilation equally in men and women. Eur J Appl Physiol 116, 1289–1296 (2016). https://doi.org/10.1007/s00421-016-3366-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3366-2