Abstract
Purpose
The purpose of the present study was to develop and describe a simple method to evaluate the rate of ion reabsorption of eccrine sweat glands in human using the measurement of galvanic skin conductance (GSC) and local sweating rate (SR). This purpose was investigated by comparing the SR threshold for increasing GSC with following two criteria of sweat ion reabsorption in earlier studies such as (1) the SR threshold for increasing sweat ion was at approximately 0.2–0.5 mg/cm2/min and (2) exercise heat acclimation improved the sweat ion reabsorption ability and would increase the criteria 1.
Methods
Seven healthy non-heat-acclimated male subjects received passive heat treatment both before and after 7 days of cycling in hot conditions (50 % maximum oxygen uptake, 60 min/day, ambient temperature 32 °C, and 50 % relative humidity).
Results
Subjects became partially heat-acclimated, as evidenced by the decreased end-exercise heart rate (p < 0.01), rate of perceived exhaustion (p < 0.01), and oesophageal temperature (p = 0.07), without alterations in whole-body sweat loss, from the first to the last day of training. As hypothesized, we confirmed that the SR threshold for increasing GSC was near the predicted SR during passive heating before exercise heat acclimation, and increased significantly after training (0.19 ± 0.09–0.32 ± 0.10 mg/cm2/min, p < 0.05).
Conclusions
The reproducibility of sweat ion reabsorption by the eccrine glands in the present study suggests that the relationship between GSC and SR can serve as a new index for assessing the maximum rate of sweat ion reabsorption of eccrine sweat glands in humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The human eccrine sweat gland reabsorbs sodium from sweat that passes through the sweat duct (Sato et al. 1989). Two processes are relevant to the amount and concentration of various ions (Na+, Cl−) secretion of sweat from the sweat gland. Firstly, isosmotic precursor sweat is produced in the proximal secretory coil (Buono et al. 2008; Shamsuddin et al. 2005b; Sato et al. 1989). Secondly, sodium and chloride ions are reabsorbed from precursor sweat in the distal duct, rendering sweat appearing on the skin to be hypo-osmotic (Buono et al. 2008; Shamsuddin et al. 2005b; Sato et al. 1989). When the sodium secretion rate exceeds that of reabsorption, the sweated sodium concentration increases linearly with sweating rate (SR) (Shamsuddin et al. 2005b; Buono et al. 2008). Therefore, the sodium concentration in sweat should always be evaluated with respect to the actual SR (Sato et al. 1989; Allan and Wilson 1971; Buono et al. 2007, 2008; Shamsuddin et al. 2005b; Kuno 1956). Furthermore, the SR at which the sweated ion (e.g., sodium) concentration begins to rapidly increase is believed to reflect the point where the maximum rate of sweated ion reabsorption in sweat gland (SR threshold) is reached (Shamsuddin et al. 2005a, b). Given that sweat ion reabsorption in human eccrine sweat gland is a fundamental physiological function to prevent excess mineral loss through sweating during heat stress and exercise, it is relevant to evaluate the rate of sweated ion reabsorption in human eccrine sweat glands for widespread applications in sports and clinical situations.
Conventionally, filter paper, gauze pads, or commercial sweat collectors have been used to collect sweat for measurement of electrolyte concentrations (Buono et al. 2007; Inoue et al. 1998; Allan and Wilson 1971; Verde et al. 1982; Araki et al. 1981; Hamouti et al. 2011). However, these methods are unable to detect the SR threshold because they require sweating rates over 0.4 mg/cm2/min (Allan and Wilson 1971; Shamsuddin et al. 2005b), and a minimum sampling period of 5 min, to obtain sufficient amounts of sweat (Araki et al. 1981). On the other hand, we previously developed a method to continuously measure sweat conductivity (an index of sweat ion concentration) at lower levels of sweating, and determined the SR threshold at which the conductivity increases steeply with increases in SR (Shamsuddin et al. 2005a, b). However, based on our experience, measurement of sweat conductivity requires a special technique and equipment that perfuses purified water through a tube and a chamber attached to the skin (e.g., the purified water must be perfused through the chamber without any contamination by bubbles that would induce substantial disturbance of the electrical signal) (Shamsuddin et al. 2005a, b). This is technically rather difficult and research in this topic area has not advanced. Hence, for more widespread applications, an alternative index of sweat ion concentration is required. This must be capable of continuous measurement to detect the SR thresholds where the sweated ion concentration rises at lower levels of sweating.
Galvanic skin conductance (GSC), a measure of electrical conductivity between two electrodes attached to the skin, is an easily measurable index of sweat gland activity (Vetrugno et al. 2003; Wang 1957; Gutrecht 1994; Gerrett et al. 2013). For example, GSC correlates with the number of activated sweat glands (Thomas and Korr 1957) and also this was used as an index of precursor sweating (Machado-Moreira and Taylor 2012). The GSC is considered to be influenced by the presence and concentration of electrolytes in sweat in the duct and on the skin surface (Thomas and Korr 1957; Boucsein et al. 2012; Fowles 1986), which increases with increasing SR. Therefore, it is reasonable to assume that the relationship between GSC and the local SR could serve as an index for determining the point of reaching the maximum rate of sweat ion reabsorption of the gland. We previously reported that the maximum SR at which the eccrine sweat gland can completely reabsorb sweated ion ranges from 0.2 to 0.5 mg/cm2/min during passive heating and cycling (Shamsuddin et al. 2005a, b). In addition, the sodium in sweat is likely reabsorbed to the extent of approximately 90 % by the duct when the SR attains 0.2 mg/cm2/min (Buono et al. 2008). Furthermore, it has been reported that exercise heat acclimation improves the ability of sweat glands to reabsorb sweat sodium, as evidenced by a rightward shift of the regression line obtained when the sweated sodium concentration–SR relationship is graphed, with or without changes in the slope (Buono et al. 2007; Allan and Wilson 1971). In light of the aforementioned observations, this study was designed to investigate whether the GSC–SR relationship is able to reproduce the characteristics of sweated ion reabsorption by human eccrine sweat glands of non-glabrous skin during thermal stimulation. We hypothesized that: (1) the SR threshold for an increasing GSC would appear at a SR of approximately 0.2 to 0.5 mg/cm2/min, and, (2) this threshold would shift rightward, to higher local sweat rates, when the sweat gland capacity to reabsorb sweat ion was improved by exercise heat acclimation. To test this hypothesis, we continuously and simultaneously measured both GSC and SR using the ventilated capsule method, on the forearm, during passive heating, both before and after a typical exercise heat acclimation protocol run over 7 days.
Materials and methods
Ethics approval
Prior to the study, each subject was informed of the study purpose and the procedures involved. All subjects provided written informed consent. The study was approved by the Human Subjects Committee of the Graduate School of Human Development and Environment, Kobe University (Kobe, Japan), and conformed to the standards set forth in the latest revision of the Declaration of Helsinki.
Participants
Seven healthy young male students were recruited for the present study. The subjects had not performed any regular physical activity for at least the past 3 years. No subjects were taking any medication, and all were non-smokers. Average age, height, weight, and body surface area before exercise heat acclimation were 21.3 ± 0.6 years, 168.3 ± 1.4 cm, 60.7 ± 2.5 kg, and 1.70 ± 0.04 m2, respectively. The study was performed in May (one subject), and between October and December (the other six subjects) to avoid any effect of heat acclimatization during the summer in Japan.
General procedures
Although it is considered that the GSC is affected by the changes in sweat electrolytes concentration, to our knowledge, no one has investigated whether the changes in GSC would actually depend on the changes in sweat electrolytes concentration. Therefore, as a technical preliminary study, we have confirmed if the changes in GSC depend on the changes in sweat electrolyte concentrations. For the main experiment, we measured maximum oxygen uptake (\({{\dot{\text{V}}}\text{O}}_{{ 2 {\text{max}}}}\)), SR, and GSC of the forearm during passive heating on different days before and after exercise heat acclimation for 7 days. Subjects reported to the laboratory at similar times of the day (within 2 h), at least 2 h after their last meal, and having abstained from caffeine and alcohol for 24 h prior to testing.
Technical study
We measured sweat electrolytes concentration and GSC on the chest during a stepwise cycling exercise. We employed endogenous heat load (cycling exercise) rather than the exogenous because exercise is easier to produce a large amount of sweat. A healthy active male subject was recruited in the study. This subject conducted a 4-step cycling exercise at an ambient temperature of 30 °C and relative humidity of 50 % with target chest sweat rate of 0.2, 0.8, 1.4, and 2.0 mg/cm2/min measured by ventilated capsule method as described below. Once the sweat rate reached to the given value, the sweat on the skin was wiped and a Macroduct sweat collector (Wescor, UT, USA) was quickly and firmly placed on the chest to collect sweat samples. We used a rubber band to attach the sweat collector on the skin for preventing a leakage and contamination of sweat. Exercise intensity was manipulated during the period of sweat collection to maintain the target local sweat rate. The sweat collector was removed when the minimum amount of sweat was collected for analysis of sweat electrolytes concentration. Sweat electrolytes concentration was measured by a sweat conductivity analyzer (3120 Sweat chek; Wescor, UT, USA) which provide an unit of mmol/L (equivalent sodium chloride: NaCl) based on the calculation from the sweat conductivity. This equipment would satisfy an estimation of approximate actual Na+ and Cl− concentrations in sweat (Boisvert and Candas 1994). The changes in GSC were plotted against the equivalent NaCl concentrations of sweat during exercise.
\({{\dot{\text{V}}}\text{O}}_{{ 2 {\text{max}}}}\)
Subjects cycled on an ergometer-equipped stationary bicycle at a pedaling frequency of 60 rpm. After a 2 min warm-up at 20 W, the workload was increased by 15 W each minute until exhaustion. Respired gases obtained from samples drawn continuously from a face mask were analyzed using a gas analyzer (AE300S; Minato Medical Science, Osaka, Japan). The \({{\dot{\text{V}}}\text{O}}_{{ 2 {\text{max}}}}\) was calculated as the average oxygen consumption over the final 60 s of the test.
Exercise heat acclimation
All subjects performed a cycle ergometer exercise at 50 % \({{\dot{\text{V}}}\text{O}}_{{ 2 {\text{max}}}}\) for 60 min/day, consisting of two sets of 30 min of exercise, each followed by a 10 min rest, for 7 consecutive days, in a hot environment (32 °C with 50 % relative humidity). Heart rate (HR), oesophageal temperature (T es), rate of perceived exertion (RPE), and body weight reduction (BWR) during exercise were recorded on the first and seventh days. HR was continuously monitored via a telemetric transmitter strapped around the chest (RS400; Polar Electro Oy, Kempele, Finland). Each subject was asked to rate his RPE on a scale from 6 to 20 at the end of each exercise session (Borg 1982). Dry nude weight was measured on a balance scale (ID1 s; Mettler-Toledo, Greifensee, Switzerland) before and immediately after each exercise session. T es was measured as described in “Passive heating test”.
Passive heating test
Passive heating was measured by partial immersion in a hot water bath. Subjects wore only shorts and rested in a semi-supine position for 50 min before heating, while instruments were attached. After recording baseline data for 5 min, both lower legs were inserted into hot water (43 °C) for 50 min in an environmental chamber (SR-3000; Nagano Science, Osaka, Japan) maintained at an ambient temperature of 27 °C and a relative humidity of 50 %, with minimal air movement.
During passive heating, T es, local skin temperature at 10 sites (forehead, chest, right and left scapula, lateral lumbar, biceps, forearm, thigh, calf, and palm), SR and GSC of the left forearm, HR, and arterial blood pressure, were recorded. T es and local skin temperatures were measured using a copper-constantan thermocouple. For T es, the tip of the thermocouple was covered with silicone, and T es was measured at a distance of one-fourth of the standing height from the external nares. Mean skin temperature (\(\overline{T}_{\text{sk}}\)) was calculated using the following formula (Hardy and DuBois 1938):
SR of the forearm (the centre of the left ventral forearm) was measured continuously using the ventilated capsule method. Dry nitrogen gas was flushed through the apparatus for at least 1 h prior to each experiment to ensure stable readings. The humidity of the nitrogen flowing out of the capsule was measured using a capacitance hygrometer (HMP50 YAN1A1X; Vaisala, Helsinki, Finland). Tes, local skin temperature, and SR were recorded every second by a data logger (MX100; Yokogawa, Tokyo, Japan). GSC of the forearm was measured every second using Biopac systems (MP100 and GSR100C; Biopac, Goleta, CA, USA). Two Ag/AgCl electrodes (Vitrode J, Nihon Kohden, Tokyo, Japan) were transversely attached to the forearm after cleaning with alcohol, separated by 3 cm, and the sweat capsule was located between the electrodes. HR and arterial blood pressure were continuously measured on the left middle finger using a Finometer system, to check for physiological stress developing during passive heating (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands); mean arterial pressure (MAP) was subsequently calculated.
Oesophageal/mean body temperature thresholds and slopes for increasing SR and GSC
All variables were averaged over each minute during passive heating. SR and GSC were expressed as changes from baselines (ΔSR and ΔGSC, respectively). Oesophageal and mean body temperatures (T b) were used to calculate the thresholds and slopes of ΔSR and ΔGSC during passive heating. T b was calculated as 0.8 × T es + 0.2 × \(\overline{T}_{\text{sk}}\) (Stolwijk and Hardy 1966). The T es/T b thresholds for the onset of sweating and the slope of the response were defined using the segmented linear regression analysis method of Cheuvront et al. (2009). This method was also used to determine ΔGSC. When the ΔGSC increased slightly before a rapid increase in the response, we excluded the values obtained before the slight increase.
SR threshold and slope of increasing GSC
Figure 1 shows data obtained from one subject in a pilot study, and indicates that the relationship between ΔGSC and ΔSR during passive heating can be separated into three phases: (a) increased ΔGSC without a change in ΔSR, representing isosmotic precursor sweat production in the proximal secretory coil (Machado-Moreira and Taylor 2012; Thomas and Korr 1957; Darrow 1964); (b) increased ΔSR without an increase in ΔGSC, which represents reabsorption of sweated ions in the duct (Shamsuddin et al. 2005b; Bulmer and Forwell 1956); and, (c) a proportional increase in ΔGSC with increasing ΔSR after the rate of sweat ion secretion exceeds its reabsorption limit in the duct (Bulmer and Forwell 1956; Buono et al. 2008; Shamsuddin et al. 2005b). The data of “b” and “c” were used to calculate the ΔSR threshold and slope of the increasing ΔGSC during passive heating using the segmented linear regression analysis method (Cheuvront et al. 2009). When phase “b” was not observed, which could be attributable to low-level ion reabsorption by the sweat gland, the intercept of phases “a” and “c” was used to identify the SR threshold for the increasing ΔGSC.
Exercise heat acclimation
End-exercise physiological values on the first and seventh days of training were calculated as the averages over the final 1 min of exercise.
Statistics
Paired t tests were used to compare differences in physiological parameters pre- and post-heat acclimation, and between the first and seventh days of training. Two-way repeated measured analysis of variance (ANOVA) was performed to evaluate differences during passive heating (exercise training × passive heating time). All data exhibited homogeneity of variance. When the normality test (Shapiro–Wilk test) was violated, Wilcoxon’s signed-rank test (comparing pre- and post-exercise training data) was performed. The statistical significance was set at p < 0.05.
Results
Technical study
As the results of manipulation of exercise intensity, average sweat rate on the chest throughout the sweat collection during stepwise exercise test were 0.24, 0.84, 1.45, and 2.10 mg/cm2/min, respectively. Figure 2 shows a relationship between GSC and equivalent NaCl concentration of sweat during the exercise. In general, the changes in GSC is dependent on the changes in equivalent NaCl concentration of sweat evidenced by a significant high correlation between these parameters (p < 0.05, R = 0.95, Fig. 2).
Exercise heat acclimation and \({{\dot{\text{V}}}\text{O}}_{{ 2 {\text{max}}}}\)
Exercise heat acclimation induced a significant reduction in end-exercise HR (172 ± 3 and 155 ± 4 beats/min on the first and seventh days of training, respectively; p < 0.01); RPE (15.6 ± 0.6 and 12.9 ± 0.3, respectively; p < 0.01); and T es (38.09 ± 0.09 and 37.74 ± 0.14 °C, respectively; p = 0.07). On the other hand, BWR during exercise was unchanged between the first and seventh days of training (0.52 ± 0.03 and 0.50 ± 0.04 kg/m2, respectively; p = 0.19), while \({{\dot{\text{V}}}\text{O}}_{{ 2 {\text{max}}}}\) increased 12 % following heat acclimation (40.2 ± 1.3 and 45.1 ± 1.5 ml/kg/min, respectively; p < 0.01).
Passive heating
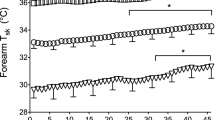
Changes in HR, T es, and T b during passive heating were significantly reduced following heat acclimation, while ΔSR and ΔGSC were uninfluenced by acclimation (Table 1). In contrast, the effect of heat acclimation on ΔSR and ΔGSC during passive heating, as function of Tes and Tb, differed. As shown in Fig. 3, the relationship between ΔGSC and ΔTb was significantly shifted to the right following heat acclimation, while the relationship with SR was virtually unaffected (p < 0.05). Although the T es and T b thresholds showed similar trends, the statistical inferences were inconsistent, likely due to an influence of T sk during passive heating both before and after heat acclimation (Table 2). Furthermore, the slopes of both parameters were unaffected by heat acclimation (Table 2; Fig. 3).
When ΔGSC was plotted against ΔSR during passive heating, a significant increase in ΔSR threshold for an increasing ΔGSC was evident following heat acclimation (Fig. 4; Table 3), indicating an increased rate of sweat ion reabsorption to that of secretion. While the relationship appeared linear before heat acclimation, this was not the case after acclimation. Nevertheless, the calculated ΔSR threshold for increasing ΔGSC was 0.19 ± 0.09 mg/cm2/min before heat acclimation, which increased significantly to 0.32 ± 0.10 mg/cm2/min after acclimation (p < 0.05, Fig. 4; Table 3). However, the slope of the relationship between ΔGSC and ΔSR was uninfluenced by heat acclimation (p > 0.05, Fig. 4; Table 3).
Discussion
The present study was designed to investigate whether a continuously measured GSC–SR relationship reflected the previously reported characteristics of sweated ion reabsorption by the human eccrine sweat glands of non-glabrous skin. As hypothesized, we confirmed the capability of the new method to determine the ΔSR threshold for increasing ΔGSC during passive heating. In addition, the threshold was determined to develop at the rate of 0.19 mg/cm2/min SR before exercise heat acclimation, similar to the predicted maximum rate of sweated ion reabsorption of eccrine sweat glands (Buono et al. 2008; Shamsuddin et al. 2005a, b). Furthermore, the ΔSR threshold was significantly shifted to the right following heat acclimation, but this did not affect the slope of the GSC–SR relationship above the threshold. This is in agreement with previous studies that reported the effect of exercise heat acclimation on sweated sodium ion versus SR (Allan and Wilson 1971; Buono et al. 2007). Based on these findings, we believe that the ΔSR threshold for increasing ΔGSC as evident in the GSC–SR relationship can serve as a new index of the maximum rate of sweated ion reabsorption of eccrine sweat glands. Perhaps the most remarkable finding of the present study is the utility of the method; GSC measurement is simple and can be easily performed in basic laboratories.
GSC has been used in psychophysiological studies as a sensitive index of bodily arousal related to emotion and attention, and is usually measured on glabrous skin where the sweat gland density is high (Vetrugno et al. 2003; Gutrecht 1994; Wang 1957). More recently, GSC has also been used as an index of precursor sweating (Machado-Moreira and Taylor 2012) and skin wetness in thermal comfort studies (Gerrett et al. 2013). Regardless of such a widespread application of GSC, it was unclear whether the conductance actually reflects to the changes in sweat electrolytes concentration. Our follow-up study showed that the overall changes in GSC depend on the sweat electrolytes concentration (Fig. 2), suggesting that the GSC seems appropriate as an index of sweat electrolytes concentration against the changes in local sweat rate in the present study.
We directly compared GSC and thermoregulatory sweating of non-glabrous skin based on continuous measurement of local SR, as well as the relevance of body temperature changes. We previously evaluated the capacity of sweat glands to reabsorb sweated ion using the relationship between sweat conductance and local SR (Shamsuddin et al. 2005a, b). Although we strongly believe that GSC can serve as an index of sweat ion concentration, replacing sweat conductance, it is important to note that sweat conductance and skin conductance may be influenced by different features. More specifically, sweat conductance is mainly influenced by the electrolyte concentrations of sweat that actually appears on the skin, while GSC is influenced by both the concentrations of sweat in the duct and stratum corneum (Thomas and Korr 1957; Boucsein et al. 2012), which may increase during passive heating upon leakage of sweat from the sweat gland duct and subsequent appearance on the skin surface. Irrespective of these differences between sweat conductance and GSC, we found that the ΔSR thresholds associated with ΔGSC were similar to those associated with sweat conductance, as determined in previous studies (Shamsuddin et al. 2005a, b). In addition, an effect of heat acclimation on the ΔSR threshold was evident. These results suggest that the GSC–SR relationship is valid for assessing a threshold in SR reflecting the maximum rate of sweat ion reabsorption of the eccrine sweat glands, irrespective of differences between sweat and skin conductance.
Buono et al. (2007) measured sweat sodium concentrations and SR relationships during exercise, as well as pre- and post-exercise heat acclimation. A macroduct collector was used to collect sweat for 30 min during three exercise bouts, data from which were then plotted to evaluate the influence of exercise heat acclimation on sweat sodium concentration. It was shown that the slope of the sweat sodium–SR relationship shifted significantly to the right following heat acclimation, as evidenced by a significant reduction in the y-intercept of the relationship without any change in slope (Buono et al. 2007). The results of our present study are consistent with these findings on the effect of heat acclimation on the capacity of sweat glands to reabsorb sweated sodium, and further demonstrate that the shift in the slope is caused by a significant increase in the SR threshold at low levels of sweating (0.13 mg/cm2/min).
Our results provide important information on the use of GSC as an index of sweat gland activity. For example, the relationship between ΔSR and ΔTb was independent of heat acclimation, whereas the relationship between ΔGSC and ΔTb shifted significantly to the right without affecting the slope (Fig. 3). Furthermore, a ΔSR threshold at an increasing ΔGSC was indeed evident, and was shifted to the right following heat acclimation, without affecting the slope (Fig. 4). These results suggest that the changes in GSC are not a simple function of the amount of sweat appearing on the skin, rather, would be affected by the rate of ion reabsorption in sweat glands as well as the amount of sweat on the skin surface. Therefore, caution should be exercised when using GSC as a direct index of SR at low levels of sweating, especially in individuals who may have higher sweat ion reabsorption capacities, including those who are both heat-acclimated and highly aerobic (Buono et al. 2007; Allan and Wilson 1971).
It is well known that exercise heat acclimation induces a significant reduction in HR, and core and skin temperatures during exercise, as well as a significant increase in SR (Gisolfi and Cohen 1979; Eichna et al. 1950; Mitchell et al. 1976; Wyndham et al. 1976). As we did not observe a significant increase in whole-body SR throughout exercise heat acclimation, or an increase in local SR during passive heating, it is likely that the magnitude of the adaptation induced by heat acclimation was mild in the present study. Nevertheless, the maximum rate for reabsorbing the sweated ion in eccrine sweat duct, evaluated by the ΔGSC-ΔSR relationship, may improve with exercise, as shown in the present study. This suggests that improvement of the sweat gland capacity to reabsorb sweated ions would occur prior to an increase in the overall SR. Further studies are warranted to confirm at which point the improvement of sweat gland capacity to reabsorb sweat ions occurs, compared with other physiological adaptations to heat acclimation.
Limitations
It is possible that sweat electrolytes may accumulate on the skin surface or stratum corneum during passive heating, which could potentially affect the GSC response. It is assumed that the slope of the GSC plotted against changes in SR during passive heating would be modified by such accumulation, especially if the test was protracted. However, the SR threshold for increasing GSC should not be affected by any accumulation of electrolytes on the skin surface as such an accumulation would only take place after the reabsorption limit, and thus the threshold, would have been surpassed. Therefore, it is believed that the change in SR threshold upon increasing GSC during passive heating, before and after exercise heat acclimation in the present study, is a reliable phenomenon.
Although we found a significant correlation between GSC and sweat electrolytes concentration in a technical study, it is considered that the actual continuous measurement of sweat sodium concentration during passive heating would be needed to completely validate our proposed new method of using the GSC–SR relationship. To our knowledge, such a method to measure sweat sodium concentration continuously is not available. Instead of a direct measurement of sweated sodium concentration, we thought that we could evaluate a validity of our method by comparing the SR threshold for increasing GSC with two criteria of sweat ion reabsorption in earlier studies. The GSC–SR relationship in the present study was in good agreement with the characteristics of sweat gland ion reabsorption as reported previously (Buono et al. 2007; Allan and Wilson 1971; Shamsuddin et al. 2005a, b), indicating that the GSC–SR relationship would reflect the characteristics of sweated ion reabsorption of the sweat glands. However, future development of continuous measurement of actual sweated ion concentration is expected to precisely validate the GSC–SR relationship in the present study.
Based on the reproducibility of the predicted characteristics of sweat gland reabsorption of sweated ions using the GSC-SR relationship of the present study, we conclude that the relationship can serve as a new index for the determination of the maximum rate of sweat glands ion reabsorption. The method is useful, and of interest to thermal and exercise physiologists, and, given that it is simple, can be performed easily in a basic laboratory, and may add reliable information on sweat gland capacities to reabsorb sweated ions.
Abbreviations
- ANOVA:
-
Analysis of variance
- BWR:
-
Body weight reduction
- GSC:
-
Galvanic skin conductance
- HR:
-
Heart rate
- MAP:
-
Mean arterial blood pressure
- NaCl:
-
Sodium chloride
- RPE:
-
Rate of perceived exhaustion
- SR:
-
Sweat rate
- T b :
-
Mean body temperature
- T es :
-
Oesophageal temperature
- \(\overline{T}_{\text{sk}}\) :
-
Mean skin temperature
- \({{\dot{\text{V}}}\text{O}}_{{ 2 {\text{max}}}}\) :
-
Maximum oxygen uptake
References
Allan JR, Wilson CG (1971) Influence of acclimatization on sweat sodium concentration. J Appl Physiol 30(5):708–712
Araki T, Matsushita K, Umeno K, Tsujino A, Toda Y (1981) Effect of physical training on exercise-induced sweating in women. J Appl Physiol Respir Environ Exerc Physiol 51(6):1526–1532
Boisvert P, Candas V (1994) Validity of the Wescor’s sweat conductivity analyzer for the assessment of sweat electrolyte concentrations. Eur J Appl Physiol 69(2):176–178
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, Filion DL (2012) Publication recommendations for electrodermal measurements. Psychophysiology 49(8):1017–1034. doi:10.1111/j.1469-8986.2012.01384.x
Bulmer MG, Forwell GD (1956) The concentration of sodium in thermal sweat. J Physiol 132(1):115–122
Buono MJ, Ball KD, Kolkhorst FW (2007) Sodium ion concentration vs. sweat rate relationship in humans. J Appl Physiol 103(3):990–994. doi:10.1152/japplphysiol.00015.2007
Buono MJ, Claros R, Deboer T, Wong J (2008) Na + secretion rate increases proportionally more than the Na + reabsorption rate with increases in sweat rate. J Appl Physiol 105(4):1044–1048. doi:10.1152/japplphysiol.90503.2008
Cheuvront SN, Bearden SE, Kenefick RW, Ely BR, Degroot DW, Sawka MN, Montain SJ (2009) A simple and valid method to determine thermoregulatory sweating threshold and sensitivity. J Appl Physiol 107(1):69–75. doi:10.1152/japplphysiol.00250.2009
Darrow CW (1964) The rationale for treating the change in galvanic skin response as a change in conductance. Psychophysiology 1:31–38
Eichna LW, Park CR, Nelson N, Horvath SM, Palmes ED (1950) Thermal regulation during acclimatization in a hot, dry (desert type) environment. Am J Physiol 163(3):585–597
Fowles DC (1986) The eccrine system and electrodermal activity. In: Coles GH, Donchin E, Porges SW (eds) Psychophysiology: systems, processes, and applications. Guilford Press, New York, pp 51–96
Gerrett N, Redortier B, Voelcker T, Havenith G (2013) A comparison of galvanic skin conductance and skin wettedness as indicators of thermal discomfort during moderate and high metabolic rates. J Therm Bio 38:530–538
Gisolfi CV, Cohen JS (1979) Relationships among training, heat acclimation, and heat tolerance in men and women: the controversy revisited. Med Sci Sports 11(1):56–59
Gutrecht JA (1994) Sympathetic skin response. J Clin Neurophysiol 11(5):519–524
Hamouti N, Del Coso J, Ortega JF, Mora-Rodriguez R (2011) Sweat sodium concentration during exercise in the heat in aerobically trained and untrained humans. Eur J Appl Physiol 111(11):2873–2881. doi:10.1007/s00421-011-1911-6
Hardy JD, DuBois EF (1938) Basal metabolism, radiation, convection and vaporization at temperature of 22 to 35 degree C. J Nutr 15:477–497
Inoue Y, Nakao M, Ishizashi H, Tsujita J, Araki T (1998) Regional differences in the Na + reabsorption of sweat glands. Appl Human Sci 17(5):219–221
Kuno Y (1956) Human perspiration. Charles C. Thomas, Springfield
Machado-Moreira CA, Taylor NA (2012) Psychological sweating from glabrous and nonglabrous skin surfaces under thermoneutral conditions. Psychophysiology 49(3):369–374. doi:10.1111/j.1469-8986.2011.01309.x
Mitchell D, Senay LC, Wyndham CH, van Rensburg AJ, Rogers GG, Strydom NB (1976) Acclimatization in a hot, humid environment: energy exchange, body temperature, and sweating. J Appl Physiol 40(5):768–778
Sato K, Kang WH, Saga K, Sato KT (1989) Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol 20(4):537–563
Shamsuddin AK, Kuwahara T, Oue A, Nomura C, Koga S, Inoue Y, Kondo N (2005a) Effect of skin temperature on the ion reabsorption capacity of sweat glands during exercise in humans. Eur J Appl Physiol 94(4):442–447. doi:10.1007/s00421-005-1354-z
Shamsuddin AK, Yanagimoto S, Kuwahara T, Zhang Y, Nomura C, Kondo N (2005b) Changes in the index of sweat ion concentration with increasing sweat during passive heat stress in humans. Eur J Appl Physiol 94(3):292–297. doi:10.1007/s00421-005-1314-7
Stolwijk JA, Hardy JD (1966) Partitional calorimetric studies of responses of man to thermal transients. J Appl Physiol 21(3):967–977
Thomas PE, Korr IM (1957) Relationship between sweat gland activity and electrical resistance of the skin. J Appl Physiol 10(3):505–510
Verde T, Shephard RJ, Corey P, Moore R (1982) Sweat composition in exercise and in heat. J Appl Physiol Respir Environ Exerc Physiol 53(6):1540–1545
Vetrugno R, Liguori R, Cortelli P, Montagna P (2003) Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res 13(4):256–270. doi:10.1007/s10286-003-0107-5
Wang GH (1957) The galvanic skin reflex; a review of old and recent works from a physiologic point of view. Am J Phys Med 36(5):295–320
Wyndham CH, Rogers GG, Senay LC, Mitchell D (1976) Acclimization in a hot, humid environment: cardiovascular adjustments. J Appl Physiol 40(5):779–785
Acknowledgments
We would like to thank our volunteer subjects for participating in this study. NK is supported by Grants-in-Aid for Scientific Research (No. 23300231) from the Japan Society for the Promotion of Science (JSPS) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. TA is supported by a JSPS fellowship (No. 244185) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. TN is supported by a Grant-in-Aid for Scientific Research (no. 25242061) from the Japan Society for the Promotion of Science (JSPS) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Communicated by Guido Ferretti.
Rights and permissions
About this article
Cite this article
Amano, T., Gerrett, N., Inoue, Y. et al. Determination of the maximum rate of eccrine sweat glands’ ion reabsorption using the galvanic skin conductance to local sweat rate relationship. Eur J Appl Physiol 116, 281–290 (2016). https://doi.org/10.1007/s00421-015-3275-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3275-9