Abstract
The effect of skin temperature on the ion reabsorption capacity of sweat glands during exercise in humans is unknown. In this study, eight healthy subjects performed a 60-min cycling exercise at a constant intensity (60% VO2max) under moderate (25°C) and cool (15°C) ambient temperatures at a constant relative humidity of 40%. The sweating rate (SR), index of sweat ion concentration (ISIC) by using sweat conductivity, esophageal temperature (Tes), mean skin temperature, and heart rate (HR) were measured continuously under both ambient temperatures. The SR and ISIC were significantly lower at the cool ambient temperature versus the moderate temperature. There were no significant differences in the changes in HR and esophageal temperature between these ambient temperature conditions, while the mean skin temperature was significantly lower at the cool ambient temperature by almost 3°C (P<0.05). The slopes of the relationships between Tes and the SR and ISIC were significantly lower and the thresholds of these relationships were significantly higher at the cool ambient temperature (P<0.05). The ion reabsorption capacity of the sweat glands was significantly lower (P<0.05) in a cool environment (0.21±0.04 vs. 0.52±0.06 mg/cm2/min at 15 and 25°C, respectively) as evaluated using the relationships for SR and ISIC. The results suggest that the ion reabsorption capacity of the sweat glands is influenced by skin temperature during exercise in humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During dynamic exercise, eccrine sweat glands secrete large amounts of fluid and electrolytes at different concentrations (Bulmer and Forwell 1956; Kuno 1956; Sato 1977). The capacity for the sweat gland to reabsorb NaCl is limited (Cage and Dobson 1965; Schwartz and Thaysen 1956), so the NaCl concentration increases with the sweating rate (SR) (Allan and Wilson 1971; Sato et al. 1989). Therefore, during dynamic exercise, it is important to evaluate the sweat ion concentration and ion reabsorption capacity of sweat glands, because much more sweat is secreted during dynamic exercise than with passive heating at rest (Kondo et al. 2001).
The effect of skin temperature on SR is well-established (Nadel et al. 1971), but it is not known whether skin temperature influences the reabsorption capacity of electrolytes in the sweat gland. Few studies have investigated the effects of temperature on the sweat ion concentration in vivo and in vitro. In vivo, Robinson et al. (1950) showed that when the two arms are maintained at different temperatures, the sweating rate of the cool arm sometimes exceeds that of the warm arm, but that the latter invariably has the higher salt concentration. They and other investigators concluded that the skin temperature has a direct effect on the sweat ion concentration (Johnson et al. 1944; Robinson et al. 1950; Weiner and Heyningen 1952). Conversely, Bulmer and Forwell (1956) stated that skin temperature has little or no effect on the sweat salt concentration. These contradictory results might have resulted from the methodological deficiencies in the techniques used to measure sweat ion concentration, specifically at low rates of sweating. In addition, it is not clear whether the skin temperature influences the reabsorption capacity of electrolytes in the sweat glands during dynamic exercise.
By contrast, in vitro experiments using the amiloride-sensitive epithelial sodium channel (ENaC), which is related to sodium reabsorption, found that the difference is more pronounced at high temperature and that the ENaC unitary current increases with temperature (Chraïbi and Horisberger 2002, 2003). In addition, Na+ channels at the resting potential were more excitable at 37°C than at 19°C (Ruff 1999). These in vitro experiments imply that higher temperature enhances the ion channel activities. We hypothesized that the skin temperature associated with ambient temperature affects the ion reabsorption capacity as well as the sweat ion concentration in vivo during dynamic exercise in humans and that we could measure sweat ion concentration continuously from low to high levels of sweating.
To determine ductal sodium reabsorption indirectly, a practical method by using the relationship between SR and (SR × sweat ion concentration) has been proposed by the earlier reports (Schwartz and Thaysen 1956; Cage and Dobson 1965). Moreover, recently, we have reported a method that could evaluate the ion reabsorption capacity of sweat glands by the relationship between SR and the index of sweat ion concentration (ISIC; Shamsuddin et al. 2005). Therefore, this study investigated the hypothesis that the skin temperature affects the ion reabsorption capacity of sweat glands during exercise in humans. To test the hypothesis, we measured the ion reabsorption capacity of sweat glands by using the relationship between SR and ISIC during dynamic exercise at two different environmental conditions.
Methods
Subjects
This study examined eight healthy subjects (three females and five males) with the following characteristics: age: 22.5±0.9 year (means ± SEM), height: 169.4±4.6 cm, weight: 58.5±4.2 kg, and maximal O2 uptake (VO2max): 48.3±2.7 ml/kg/min. The male subjects wore shorts only, while the females also wore a sports bra. None of the subjects were taking medication nor were smokers. The subjects were prohibited from eating for at least 2 h before each experiment. Each subject gave informed written consent and the Human Subjects Committee of our department in the Faculty of Human Development at Kobe University approved this study.
Procedures
Before the main study, the VO2max of each subject was measured using a ramped exercise protocol (15–30 W/min, pedaling frequency set at 60 rpm) on a cycle ergometer. To evaluate the VO2max test, at least two of the following criteria were applied: maximal or near-maximal values of the rating of perceived exertion (RPE), respiratory gas exchange ratio >1.05, heart rate (HR) within 10 beats/min of the age-predicted maximal HR, and inability to maintain a pedaling frequency of 60 rpm.
The main experiments were conducted in an environmental chamber (SR-3000, Nagano Science, Osaka, Japan). Before entering the chamber, all monitors (skin temperature sensors, esophageal temperature (Tes) sensor, air ventilation capsule for the SR, and a pure-water perfusion chamber for measuring the ISIC) were attached in another air-conditioned room at a thermo-neutral temperature of 25°C and a relative humidity of 50%. Then, the subjects entered the environmental chamber and rested on a bicycle ergometer in a sitting position for about 15 min. During this time, the instrumental setup and connections were completed. After the 5-min baseline data had been collected, subjects started to perform the cycling exercise for 60 min at a constant intensity (60% VO2max) under different ambient temperatures of 25°C (moderate) and 15°C (cool) at a constant relative humidity of 40%, on two separate days.
Measurements
VO2max was estimated from the ventilation volume and the expired fraction of O2 and CO2 production (Aeromonitor AE-300S, Minato, Osaka, Japan). In the main experiment, the ISIC and SR on the back skin surface, Tes, mean skin temperature (Tsk: mean for lower leg, upper leg, chest, abdomen, back, upper arm, and forearm skin), and HR were measured at both ambient temperatures.
Esophageal temperature and local skin temperature were measured with copper–constantan thermocouples. A thermocouple coated with silicon was inserted in the esophagus from the nose to a distance equal to one-quarter of the subject’s height to measure Tes. Tsk was calculated using the method of Hardy and DuBois (Hardy and DuBois 1938). HR was measured continuously from the R–R interval of the electrocardiogram.
The conductivity of perfused water with sweat was measured continuously using the purified water perfusion chamber method (Shamsuddin and Togawa 1998, 2000) and which is used as ISIC (Shamsuddin et al. 2005). Purified water was perfused through the perfusion chamber continuously at a constant flow rate of 1.5 ml/min (using a withdrawal-type syringe pump, PHD-2000, Harvard Apparatus, Holliston, MA, USA) and the conductivity of the washed out sweat was measured continuously with a conductivity meter (ES-14, Horiba, Kyoto, Japan) connected to the perfusion chamber electrodes. The measured conductivity was corrected using the perfused water temperature. The area of the open space in the perfusion chamber was 0.5 cm2 and Ag-electrodes were installed inside it to measure the conductivity of the inlet and outlet water. The chamber was constructed according to our previous method (Shamsuddin and Togawa 1998) with some modifications in the design and size, and was attached on the back skin surface using two-sided adhesive tape after cutting an open space of the same area in the bottom of the chamber. Pure water was formed constantly by circulating water with a roller pump (RP-2000, Eyela, Tokyo, Japan) through an ion-exchange column (G-5C Organo, Tokyo).
Sweating rate was measured continuously using the ventilated-capsule method (Kondo et al. 2001). Dry nitrogen gas was supplied to the capsule (1.54 cm2) at a rate of 600 ml/min, and the humidity and temperature of the nitrogen gas flowing out of the capsule were measured with a capacitance hygrometer (HMP 133y, Vaisala, Helsinki, Finland). The time delay for measuring SR was 1 s and this was taken into account when calculating SR. Both the purified water-perfusion capsule and the humidity capsule were attached to the skin surface of the back to measure the ISIC and SR simultaneously. A data logger (MP 100A, BioPAC Systems Inc., Santa Barbara, CA, USA) was used to record the conductivity of the perfused water and HR measurements on a computer (Macintosh, Apple Computer Inc., USA) at a sampling frequency of 200 Hz. The different temperatures and SR were recorded and stored every second in another personal computer (PC9801RA, NEC Co., Tokyo) using a data logger (HR2300, Yokogawa Co., Tokyo). All values were used to calculate the average response for each minute.
Data processing and statistical analysis
Linear regression analyses were applied for ISIC versus SR and Tes and for SR versus Tes for both the moderate and cool environments. Two regression lines were plotted for ISIC versus SR: for low and for moderate to high levels of sweat. The SR threshold for ISIC was calculated by determining the point of intersection of the two regression lines, which indicates the ion reabsorption capacity of sweat glands (Shamsuddin et al. 2005). The Tes thresholds for ISIC and SR were calculated by determining the increases in each value above the resting value.
The values of the Tes thresholds and the slopes of ISIC and SR, the ion reabsorption capacities at moderate and cool ambient temperatures, were compared statistically using a paired t test. The difference in the time courses of each parameter between the conditions was compared using one-way ANOVA with repeated measurements. The P value for significance was set at 0.05.
Results
Figure 1 shows the time courses of HR, Tes, Tsk, SR, and ISIC at rest and during the cycling exercise at constant intensity in the cool and moderate environments. HR was slightly higher at 25°C, but not significantly different from the value at 15°C. Tes rose sharply until 20 min and continued to increase gradually until the end of the exercise in both environments. Tes was slightly higher in the cool ambient temperature, but not significantly so. Tsk decreased with time and was significantly lower in the cool ambient temperature, by about 3°C. The changes in SR and ISIC were concomitant. At a low SR, ISIC increased more gradually than SR; once SR reached a critical value, ISIC increased linearly and steeply with SR. Both SR and ISIC were significantly lower in the cool environment.
Changes in the heart rate (HR), esophageal temperature (Tes), sweating rate (SR), mean skin temperature (Tsk), and the index of sweat ion concentration (ISIC) at ambient temperatures of 15°C (cool) and 25°C (moderate) during a 60-min cycling exercise. Data are the means ± SEM for eight subjects. *P<0.001, **P<0.01
The relationships between Tes and SR and ISIC are shown in Fig. 2. The Tes thresholds for SR and ISIC did not differ significantly, while the Tes thresholds, slopes, and intercepts between the cool (15°C) and moderate (25°C) environments for both SR and ISIC differed significantly (P<0.05, Table 1).
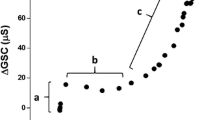
Figure 3 illustrates the relationship between SR and ISIC for both the cool and moderate ambient temperatures. SR and ISIC increased linearly and gradually at low SRs, but after reaching a critical value they increased steeply at moderate to high SRs for both ambient temperatures. The ion reabsorption capacity was evaluated from the relationship between SR and ISIC, and was significantly lower at the cool ambient temperature. The slope of this relationship was less steep under the cool ambient condition, but the difference was not significant (Table 2).
Discussion
The novel findings of this study are that skin temperature profoundly affects the ion reabsorption capacity of the sweat glands, and the capacity is significantly lower at a cool ambient temperature than under moderate conditions.
The changes in SR and ISIC concurred. At a low SR, ISIC increased more gradually than did SR. SR increased sharply before the ISIC. Once it reached a critical value, ISIC also increased linearly and steeply with SR (Fig. 1). The ISIC was significantly lower in the cool environment. This difference might result from the differences in the SR between the two conditions, since the sweat ion concentration increased linearly with SR (Allan and Wilson 1971). Therefore, we evaluated the effect of skin temperature on ISIC versus a changing SR, as shown in Fig. 3. This figure was also confirmed by the results that determined ductal sodium reabsorption by using the relationship between SR and SR×sweat ion concentration, those that have been proposed by the earlier reports (Schwartz and Thaysen 1956; Cage and Dobson 1965). Thus, Fig. 3 shows that the ion reabsorption capacity was significantly lower in a cool environment than in a moderate one. This implies that the reabsorption capacity of the sweat glands is influenced by skin temperature and that a low skin temperature inhibits the capacity.
There are several possible mechanisms for the alteration of ion reabsorption capacity in sweat glands by skin temperature: (1) direct or local effect of temperature; (2) the effect of sudomotor activity through thermoregulatory centers; and (3) responsive ability of sweat glands at a given aldosterone level. Temperature strongly affects the Na+ channels: 30% of the Na+ channels are excitable at the resting potential at 19°C as compared to 93% of the channels at 37°C (Ruff 1999). Since the reabsorption of ion in sweat glands is related to the Na+ channels (Sato 1977), temperature may lower the reabsorption capacity of Na+ channels at cold. In addition, the effects of temperature on the single-channel conductance and channel open probability explain the changes in the macroscopic current that are observed with temperature changes, and the effect of temperature on the current carried by ENaCs (Chraïbi and Horisberger 2003).
At lower (cooler) environmental temperature, the sudomotor activity through thermoregulatory centers was lower (Sugenoya et al. 1990; Ogawa and Sugenoya 1993). This is also confirmed by the upward shift of Tes threshold for sweating and ISIC in the cool environment were reportedly caused by reduced sudomotor activity. Thus, the lower sudomotor activity through thermoregulatory centers induces to decrease the sweat gland activity and then this causes the reabsorption capacity of the sweat glands that was inhibited at low skin temperature.
Furthermore, the interpretation of the increased sweat gland responsiveness to aldosterone following exercise and heat acclimation, as suggested by the elevated ion reabsorption, is based on the assumption that aldosterone is the primary effector of the sodium exchange mechanism in sweat glands (Allsopp et al. 1998; Kirby and Convertino 1986; Orenstein et al. 1984). While the sweat Na+ concentration decreases with heat acclimation, there was no marked change in the aldosterone concentration, suggesting that acclimation enhances the dependence of the sweat glands at a given aldosterone level (Falk et al. 1991; Francois 1999; Melin 1980). No comparative study has examined the effect of temperature on aldosterone during exercise, although no significant change in aldosterone occurred during a cold pressor test (Mizushima et al. 2003) or cold-water immersion (Pääkkönen and Leppäluoto 2002). These results suggest that higher skin temperature might enhance the responsive ability of sweat glands at a given aldosterone level.
Although the ion reabsorption capacity was significantly lower in the cool environment than in the moderate one, the slope of the relationship between SR and ISIC was smaller in the former, although the difference between these ambient temperature conditions was not significant. This may be owing to the Tsk differences between the moderate and cool ambient temperature conditions before and after the onset of sweating. We calculated Tsk before and after the onset of sweating until the end of the exercise session. The difference in Tsk between the 15°C and 25°C conditions before the onset of sweating was 3.52±0.37°C, while after the onset it was 3.14±0.37°C (P=0.09). This might explain why the slopes differed significantly between the cool and moderate ambient temperature conditions.
Control of sweating is assessed by measuring the threshold temperature for the onset of sweating and the sensitivity of the response, which is determined by the slope of the relationship between the change in internal temperature and the change in sweating (Kondo et al. 2001; Nadel et al. 1971). This relationship between internal temperature and SR is influenced by skin temperature; a rise in skin temperature causes the relation to shift to the left (Nadel et al. 1971). In our study, the relationships between Tes and SR and ISIC are shown in Fig. 2. The Tes thresholds for SR and ISIC did not differ significantly, while the Tes thresholds for both SR and ISIC were shifted more significantly to the left at the moderate temperature (25°C vs. 15°C), and the slope was significantly steeper in the moderate environment (P<0.05, Table 1). Since Tes was very similar under these two conditions, the differences in conditions affected the skin temperature differences directly.
In conclusion, we demonstrated that the ion reabsorption capacity of the sweat gland is significantly lower at a cool ambient temperature than at a moderate one during exercise. Lowering the skin temperature may inhibit the ion reabsorption capacity of the sweat gland more at cool temperatures during dynamic exercise in humans.
References
Allan JR, Wilson CG (1971) Influence of acclimatization on sweat sodium concentration. J Appl Physiol 30:708–712
Allsopp AJ, Sutherland R, Wood P, Wootton SA (1998) The effect of sodium balance on sweat sodium secretion and plasma aldosterone concentration. Eur J Appl Physiol 78:516–521
Bulmer MG, Forwell GD (1956) The concentration of sodium in thermal sweat. J Physiol (Lond) 132:115–122
Cage G, Dobson RL (1965) Sodium secretion and reabsorption in the human sweat gland. J Clin Invest 44:1270–1276
Chraïbi A, Horisberger JD (2002) Na self-inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J Gen Physiol 120:133–145
Chraïbi A, Horisberger JD (2003) Dual effect of temperature on the human epithelial Na+ channel. Pflugers Arch Eur J Physiol 447:316–320
Falk B, Bar OR, MacDougall JD (1991) Aldosterone and prolactin response to exercise in the heat in circumpubertal boys. J Appl Physiol 71:1741–1745
Francois V (1999) Early aldosterone action: toward filling the gap between transcription and transport. Am J Physiol 277 (Renal Physiol 46), F319–F327
Hardy JD, DuBois EF (1938) The technique of measuring radiation and convection. J Nutr 15:461–475
Johnson RE, Pitts GC, Consolazio FC (1944) Factors influencing chloride concentration in human sweat. Am J Physiol 141:575–589
Kirby CR, Convertino VA (1986) Plasma aldosterone and sweat sodium concentrations after exercise and heat acclimatization. J Appl Physiol 61:967–970
Kondo N, Shibasaki M, Aoki K, Koga S, Inoue Y, Crandall CG (2001) The function of human eccrine sweat gland during passive heat stress and dynamic exercise. J Appl Physiol 90:1877–1881
Kuno Y (1956) Human perspiration. Thomas, Springfield
Melin B, Eclache JP, Geelen G, Annat G, Allevard AM, Jarsaillon E, Zebidi A, Legros JJ, Gharib C (1980) Plasma AVP, neurophysin, renin activity, and aldosterone during submaximal exercise performed until exhaustion in trained and untrained men. Eur J Appl Physiol Occup Physiol 44:141–151
Mizushima T, Tajima F, Okawa H, Umezu Y, Furusawa K, Ogata H (2003) Cardiovascular and endocrine responses during the cold pressor test in subjects with spinal cord injuries. Arch Phys Med Rehabil 84:112–118
Nadel ER, Bullard RW, Stolwijk JAJ (1971) Importance of skin temperature in regulation of sweating. J Appl Physiol 31:80–87
Ogawa T, Sugenoya J (1993) Pulsatile sweating and sympathetic sudomotor activity. Jpn J Physiol 43:275–289
Orenstein DM, Henke KG, Green CG (1984) Heat acclimation in cystic fibrosis. J Appl Physiol Respirat Environ Exerc Physiol 57:408–412
Pääkkönen T, Leppäluoto J (2002) Cold exposure and hormonal secretion: a review. Int J Circumpolar Health 61:265–276
Robinson SID, Gerking SD, Turrell ES, Kincaid RK (1950) Effect of skin temperature on salt concentration of sweat. J Appl Physiol 2:654–662
Ruff RL (1999) Effects of temperature on slow and fast inactivation of rat skeletal muscle Na+ channels. Am J Physiol 277 (Cell Physiol 46), C937–C947
Sato K (1977) The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev Physiol Biochem Pharmacol 79:51–131
Sato K, Kang WH, Saga K, Sato KT (1989) Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol 20:713–726
Schwartz IL, Thaysen JH (1956) Excretion of sodium and potassium in human sweat. J Clin Invest 34:114–120
Shamsuddin AKM, Togawa T (1998) Continuous monitoring of sweating by electrical conductivity measurement. Physiol Meas 19:375–382
Shamsuddin AKM, Togawa T (2000) Continuous monitoring of single-sweat-gland activity. Physiol Meas 21:535–540
Shamsuddin AKM, Yanagimoto S, Kuwahara T, Zhang Y, Nomura C, Kondo N (2005) Changes in the index of sweat ion concentration with increasing sweat during passive heat stress in humans. Eur J Appl Physiol (in press)
Sugenoya J, Iwase S, Mano T, Ogawa T (1990) Identification of sudomotor activity in cutaneous sympathetic nerves using sweat expulsion as the effector response. Eur J Appl Physiol Occup Physiol 61:302–308
Weiner JS, Heyningen RE (1952) Relation of skin temperature to salt concentration of general body sweat. J Appl Physiol 4:725–733
Acknowledgments
We sincerely thank our volunteer subjects. This study was supported by a grant-in-aid from the Japan Society for the Promotion of Science in the form of a post-doctoral fellowship from the Ministry of Education, Culture, Sport, Science and Technology, Japan (P01344).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shamsuddin, A.K.M., Kuwahara, T., Oue, A. et al. Effect of skin temperature on the ion reabsorption capacity of sweat glands during exercise in humans. Eur J Appl Physiol 94, 442–447 (2005). https://doi.org/10.1007/s00421-005-1354-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-005-1354-z