Abstract
Purpose
The aim of this study was to investigate the effect of obesity on neuromuscular fatigue in adolescent girls.
Methods
Twelve lean (13.6 ± 0.8 years) and 12 obese (13.9 ± 0.9 years) girls repeated 5-s maximal voluntary contractions (MVC) of the knee extensors until the generated torque reached 55 % of its initial value. Magnetic stimulations were delivered to the femoral nerve every five MVCs to follow the course of voluntary activation (VA) and potentiated twitch torque (Qtwpot).
Results
Torque reached 55 % of its initial value after 52.6 ± 20.4 and 74.9 ± 22.8 repetitions in obese and lean girls, respectively (p < 0.01). Furthermore, the decline of VA was smaller in obese girls (p < 0.001). In contrast, Qtwpot decreased to a greater extent in obese girls (p < 0.05).
Conclusions
Obese girls fatigue faster than their lean counterparts. The peripheral factors mainly account for fatigue in obese girls, whereas central factors are mainly involved in lean girls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Commonly, muscle fatigue is defined as “an exercise-induced reduction in the ability of skeletal muscle to produce power or force irrespective of task completion” (Gandevia 2001). Historically, potential factors involved in muscle fatigue were classified into two categories, i.e., (i) central factors involving the central nervous system and neural pathways, and (ii) peripheral factors occurring within the muscle, beyond the neuromuscular junction (Enoka and Stuart 1992). As a result, it is possible to distinguish between central, i.e., neural, and peripheral fatigue, i.e., muscular fatigue. As the neuromuscular system is highly adaptable (Enoka and Stuart 1992), neuromuscular fatigue varies in response to different conditions. For instance, numerous studies examined the differential effect of growth (Ratel et al. 2002, 2015), aging (Kent-Braun et al. 2002), sex (Hunter et al. 2004), training status (Bogdanis 2012) and the mode of contraction (Callahan et al. 2009) on muscle fatigue. However, less attention has been paid to the impact of pathological conditions such as obesity on the development of neuromuscular fatigue.
This lack of information about obesity-associated neuromuscular fatigue is surprising, since fatigue has important functional implications. Indeed, it is frequently advocated as a potential limitation to daily-living tasks in young and adult obese individuals (Tsiros et al. 2011). Yet, there is currently no consensus on the detrimental effect of obesity on neuromuscular fatigue. For instance, Maffiuletti et al. (2007, 2008) conducted two successive studies regarding the effect of obesity on muscle fatigue of the knee extensor (KE) muscles during 50 repeated isokinetic contractions in adolescent boys and men, but reported different results. While Maffiuletti et al. (2007) reported a greater voluntary torque decrement of the KE muscles in 25-year-old obese men (BMI: 41 kg m−2) as compared to their normal weight counterparts; these authors also showed no significant difference of muscle fatigue between severely obese (BMI: 34 kg m−2) and non-obese adolescent boys (Maffiuletti et al. 2008). Furthermore, other authors showed that manifestations of fatigue over the course of repeated contractions of the lower or upper limb muscles were similar (Cavuoto and Nussbaum 2013; Halvatsiotis et al. 2002; Minetto et al. 2013; Paolillo et al. 2012) or lower (Cavuoto and Nussbaum 2014; Mehta and Cavuoto 2015) in obese compared to their normal weight counterparts. Besides, it is still unclear whether the contribution of central and peripheral factors to fatigue is different between non-obese and obese people, since no objective measurement of central vs. peripheral fatigue has been done so far in obese subjects.
Despite this lack of consensus regarding the rate of fatigue development in obese people, we suggest that obese individuals could fatigue to a greater extent than their normal weight counterparts because of (i) their higher absolute strength/power capacity (Abdelmoula et al. 2012; Maffiuletti et al. 2013; Tsiros et al. 2013) and (ii) their potentially higher amount of fast-fatigable muscle fibers (Kriketos et al. 1997; Wade et al. 1990). Indeed, it has been shown that the higher neuromuscular fatigue of healthy men vs. women was no longer observed when subjects were matched for absolute MVC force (Hunter et al. 2004), suggesting that the higher the MVC force, the higher is the muscle fatigue. In addition, the amount of peripheral fatigue is greater in individuals with predominantly type II fibers (Hamada et al. 2003). Taken together, these factors could promote a faster and/or greater development of peripheral fatigue in obese people during repeated maximal contractions. Conversely, the higher strength/power capacity and the fast-fatigable typology should be associated with a short exercise duration, when exercising until exhaustion. This could limit the development of central fatigue, which is mainly promoted by prolonged exercise durations (Martin et al. 2010).
Therefore, the purpose of the present study was to examine the consequences of obesity on neuromuscular fatigue development and its etiology in obese subjects during repeated maximal contractions. We chose to study more specifically an adolescent girl population given that the prevalence and incidence of obesity are increasing worldwide in this population because of a gradually decreasing level of physical activity during adolescence (Yan et al. 2014). On that basis, contrary to an obese male adolescent population (Maffiuletti et al. 2008), the level of fatigability could be specifically higher in obese adolescent girls compared to their non-obese counterparts.
Materials and methods
Subjects
Twelve obese (12–15 years; BMI: 32.1 ± 4.2 kg m−2) and 12 non-obese (12–15 years; BMI: 18.3 ± 1.3 kg m−2) adolescent girls volunteered to participate in the present study. They were classified as obese and non-obese according to the IOTF criteria (Cole et al. 2000). Both groups were formed so that chronological age, age from peak height velocity (APHV), height and maturation status (Tanner stages) were not significantly different. At first, both groups were formed according to chronological age and maturation status, and in a second time we checked whether APHV and height differed between obese and non-obese adolescents (Table 1). In both groups, all adolescents performed recreational physical activity for ≤4 h/week. The level of physical activity (i.e., variety of physical activities, intensity and sedentary level scores) was determined from a questionnaire validated among French children and adolescents (Tessier et al. 2007). None of the participants had orthopedic or cardiovascular pathology interfering with the execution of the tests. This study was approved by the local ethics committee (Protection Committee of People for Biomedical Research South East VI; Authorization Number AU994). All the volunteers were fully informed of the experimental procedures and gave their written assent before any testing was conducted. In addition, the written consent of the parents/guardians was obtained for every participant.
Experimental procedure (design)
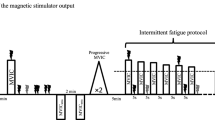
All subjects were tested on three experimental sessions separated by at least 1 week. The first session was dedicated to collecting subjects’ characteristics, familiarization with the experimental procedures and clinical examination by a pediatrician. During the second session, the participants were asked to perform maximal voluntary contractions (MVC) of the KE muscles at different knee angles (20°, 40°, 60°, 70°, 80°, 90°, 100°; 0° = full extension) to determine the optimal angle for maximal torque production. Finally, during the third session, subjects performed an intermittent voluntary isometric fatigue protocol, at the optimal angle, until the voluntary torque reached 55 % of its initial value.
Anthropometrical measurements
Body mass (BM) was measured to the nearest 0.1 kg using a calibrated scale and height was determined to the nearest 0.01 m using a standing stadiometer. Height and BM were measured without shoes and while wearing underwear. Sitting height was also measured while the participants sat on the floor against a wall, using the same stadiometer. Body mass index (BMI) was calculated using a standard formula, i.e., mass divided by height squared (kg m−2). Body fat (BF) and fat-free mass (FFM) were determined using dual-energy X-ray absorptiometry (DXA) (HOLOGIC, QDR-4500, Hologic Inc, Bedford, Massachusetts, USA).
Maturation assessment
Two methods were used to assess adolescent girls’ maturation. Tanner stages were the first method and determined from self-assessment of pubic hair and breast development (Tanner and Whitehouse 1976). The second method was the age from peak height velocity (APHV). APHV was used to assess somatic maturity and determined by using height, sitting height and BM. Its calculation was based on sex-specific regression equations, as proposed by Mirwald et al. (2002).
Metabolic indexes
The metabolic profile of obese girls was determined at the children’s medical center. In fasting conditions, venous blood samples were withdrawn from a prominent superficial vein in the antecubital fossa for the determination of metabolic indexes. Glucose, triglycerides (TG), total cholesterol (TC), HDL-cholesterol, calculated LDL-cholesterol, and C-reactive protein (CRP) were measured using a Konelab analyzer (Konelab, France). Insulin hormone was measured with enzyme-linked immunosorbent assay (ELISA). Insulin sensitivity was assessed by using the homeostasis model assessment-insulin resistance (HOMA-IR) proposed by Matthews et al. (1985).
Intermittent voluntary fatigue protocol
Subjects performed an intermittent voluntary fatigue protocol consisting in a repetition of isometric 5-s MVCs of the KE muscles interspersed with 5-s passive recovery periods until the generated torque reached 55 % of its initial value. The subjects were not informed of this criterion of task failure and had no visual feedback of torque output during the exercise. However, they were strongly encouraged by the experimenters during the entire fatiguing task. To follow the recovery process, the participants performed a 5-s MVC 3, 6 and 15 min after the end of the protocol. Single magnetic stimulations were delivered to the femoral nerve every five MVCs and during the recovery period to determine the maximal level of voluntary activation (VA) by means of the twitch interpolation technique (see below for more details). The electromyographic activity of the KE muscles was recorded during the entire fatigue protocol. The amplitude of the potentiated twitch torque (Qtwpot) and vastus lateralis (VL) and rectus femoris (RF) concomitant compound action potential amplitudes (M max) were considered as indicators of peripheral fatigue. The time course of VA and normalized electromyographic activity (EMG; see below) of the VL and RF muscles throughout the protocol were considered as indexes of central fatigue.
Torque measurements
Voluntary and evoked contractions were assessed in isometric condition with an isokinetic dynamometer (Cybex Norm, Lumex, Ronkonkoma, NY, USA). Subjects were comfortably positioned on an adjustable chair with the hip joint flexed at 30° (0° = neutral position). The dynamometer lever arm was attached 1–2 cm above the lateral malleolus with a Velcro strap. The axis of rotation of the dynamometer was aligned with the lateral femoral condyle of the right femur. During each test, subjects were instructed to grip the seat during the voluntary contractions to stabilize the pelvis. Torque data were corrected for gravity using the Cybex software and acquired and digitized on-line at a rate of 2 kHz by an A/D converter (Powerlab 8/35, ADInstruments, New South Walles, Australia) driven by the Labchart 7.3 Pro software (ADInstruments, Australia).
Femoral nerve stimulation
Evoked contractions of the KE muscles were triggered by a single magnetic stimulus, delivered to the femoral nerve using a 70-mm figure-of-eight coil connected to a magnetic stimulator (peak magnetic field strength 2.2 T, stimulation duration 0.1 ms; MagstimCo, Whiteland, Dyfed, UK). The coil was placed high in the femoral triangle with regard to the femoral nerve. Small spatial adjustments were initially performed to determine the optimal position where the greatest unpotentiated KE twitch amplitude (Qtwunpot) and the greatest VL and RF M max were evoked. The optimal stimulation intensity, i.e., the intensity, where maximal twitch and concomitant M-waves amplitudes started to plateau, was determined from a recruitment curve. Briefly, two single stimulations were delivered every 30 s at 70, 80, 85, 90, 95, 97 and 100 % of the maximal stimulator power output. Qtwunpot plateaued at 88.7 ± 6.0 and 82.1 ± 7.0 % of the stimulator power output in obese and non-obese adolescent girls, respectively. To overcome the potential confounding effect of axonal hyperpolarization, the stimulation intensity was set to 100 % of the stimulator output during the subsequent testing procedures (i.e., voluntary intermittent fatigue protocol). This intensity then corresponded to 113.3 ± 10.0 and 122.7 ± 11.0 % of the optimal intensity in the obese and non-obese groups, respectively. M max evoked at 100 % of the stimulator output corresponded to 119.6 ± 13.8 and 130.1 ± 12.3 % of the optimal intensity for VL and 122.9 ± 7.8 and 129.7 ± 10.1 % of the optimal intensity for RF in obese and non-obese adolescent girls, respectively. Values were not significantly different between groups.
Voluntary activation level
To determine VA, the twitch interpolation technique was used. Briefly, a superimposed (Qtws) and a potentiated (Qtwpot) single twitch were delivered during MVC after the torque had reached a plateau and 3 s after the cessation of the contraction, respectively. This provided the opportunity to obtain a potentiated mechanical response and hence reduce the variability of VA values. These superimposed and potentiated mechanical amplitudes allowed the quantification of VA (%VA) as proposed by Merton (1954):
EMG activity
The EMG signals of the VL, RF and biceps femoris (BF) muscles were recorded using bipolar silver chloride surface electrodes (Blue Sensor N-00-S, Ambu, Denmark) during voluntary and evoked contractions. The recording electrodes were taped lengthwise on the skin over the muscle belly, as recommended by SENIAM (Hermens et al. 2000) with an inter-electrode distance of 20 mm. The reference electrode was attached to the patella. Low impedance (Z < 5 kΩ) at the skin–electrode surface was obtained by shaving, gently abrading the skin with thin sand paper and cleaning with alcohol. EMG signals were amplified (Dual Bio Amp ML 135, ADInstruments, Australia) with a bandwidth frequency ranging from 10 to 500 Hz (common mode rejection ratio >85 dB, gain = 1000) and simultaneously digitized together with the torque signals. The sampling frequency was 2 kHz. During the course of the fatigue protocol, root mean square (RMS) values of the VL and RF EMG activity were calculated during the MVC trials over a 0.5-s period after the torque had reached a plateau and before the superimposed stimulation was evoked. This RMS value was then normalized to the maximal peak-to-peak amplitude of the potentiated VL and RF M-waves (RMS M −1max ).
Antagonist co-activation
The level of antagonist co-activation (%CoActBF) of the BF muscle was computed as the BF EMG activity during knee extensions (KE), normalized to the maximal BF EMG activity recorded during a maximal knee flexion (KF) (Eq. 2). To record this maximal BF RMS value, the participants were asked to perform 3-s maximal voluntary isometric contractions of the knee flexors before the fatigue protocol. This measurement was repeated twice at a 90°-knee angle. The best trial was used for subsequent analysis:
Statistical analysis
Data were screened for normality of distribution and homogeneity of variances using a Shapiro–Wilk normality test and the Bartlett’s test, respectively. Student’s t tests for unpaired samples were used to compare age, APHV and anthropometric characteristics between both groups. Maturation status (Tanner staging) was compared between groups using a Chi squared test for qualitative variables. Differences in absolute values and in percent changes relative to initial values were analyzed with a two-way (group × % of repetitions) ANOVA with repeated measures. When the ANOVA revealed significant effects or interactions between factors, a Fisher’s LSD post hoc test was applied to test the discrimination between means. Moreover, to discriminate the effect of MVC torque on fatigue, we used a mixed general linear model: the initial MVC torque was used as continuous predictor variable (=co-variable), the group as categorical independent variable and the MVC torque during exercise, Qtwpot or VA as dependent variables. Pearson’s correlation coefficients were used to determine linear correlations between the initial MVC torque, the total number of repetitions and relative Qtwpot and VA variations over the fatigue protocol. The limit for statistical significance was set at p < 0.05. Statistical procedures were performed using the Statistica 8.0 software (Statsoft, Inc, USA). Results were presented in the text and tables in absolute values (mean ± SD). For the sake of clarity, data presented in figures were expressed as a percentage of their initial values (mean ± SD).
Results
Participants’ characteristics
As expected, obese adolescent girls showed significantly higher values for BM, BMI, FFM and BF as compared with their normal weight counterparts (p < 0.001) (Table 1). No significant difference was observed between groups for chronological age, height, sitting height, Tanner staging, APHV and physical activity scores (Table 1). Furthermore, all metabolic indexes fell within the normal ranges for this age and sex (Table 2).
MVC torque
The peak MVC torque was produced at a knee angle of 82 ± 8° and 84 ± 5° in non-obese and obese girls, respectively. Task failure, corresponding to the predetermined 55 % MVC decrement was reached, respectively, after 74.9 ± 22.8 and 52.6 ± 20.4 repetitions in normal weight and obese adolescent girls and this difference was significant between groups (p < 0.01). ANOVA revealed a significant interaction of group and percentage of repetitions for the time course of absolute MVC torque (p < 0.001). Obese adolescent girls produced significantly higher absolute MVC torque values than non-obese girls throughout the fatigue protocol and subsequent recovery period (p < 0.001) (Fig. 1a). However, when percent MVC changes (relative to the first MVC torque) were calculated, obese adolescent girls displayed both a similar decline of MVC torque during the fatigue protocol and a similar recovery pattern than non-obese adolescent girls (Fig. 2a). In both groups, the recovery of MVC torque was not complete at the 15th min of recovery compared to the initial MVC value.
Time course of the maximal voluntary contraction (MVC) torque (panel A), potentiated twitch torque (Qtwpot; panel B) and voluntary activation (VA; panel B) of the knee extensor (KE) muscles during the fatigue protocol and subsequent recovery period in the obese (open square) and non-obese (filled square) adolescent girls (mean ± SD). $, $$ and $$$ Significantly different from the first MVC at p < 0.05, p < 0.01 and p < 0.001 respectively. £, ££ and £££ Significantly different from the post-exercise (100 %) measurement at p < 0.05, p < 0.01 and p < 0.001, respectively. *, ** and *** Significantly different between groups at p < 0.05, p < 0.01 and p < 0.001, respectively
Time course of the maximal voluntary contraction (MVC) torque (panel A), potentiated twitch torque (Qtwpot; panel B) and voluntary activation (VA; panel C) of the knee extensor (KE) muscles (expressed as a percentage of the corresponding initial values) during the fatigue protocol and subsequent recovery period in obese (open square) and non-obese (filled square) adolescent girls (mean ± SD). $$ and $$$ Significantly different from the first MVC at p < 0.01 and p < 0.001, respectively. £ and £££ Significantly different from the post-exercise (100 %) measurement at p < 0.05 and p < 0.001, respectively. *, ** and *** Significantly different between groups at p < 0.05, p < 0.01 and p < 0.001, respectively
Furthermore, when the MVC torque was used as co-variable, no main effect or interaction was observed.
Potentiated twitch torque
ANOVA revealed a significant interaction of group and percentage of repetitions for the time course of Qtwpot (p < 0.001). Obese girls showed greater Qtwpot than non-obese girls over the entire protocol (p < 0.05) (Fig. 1b). Furthermore, the percent Qtwpot decrement was significantly higher in obese compared to their normal weight counterparts at the end of the test (p < 0.05). Qtwpot reached 82.7 ± 18.4 and 66.1 ± 14.0 % of initial value in non-obese and obese adolescent girls, respectively (Fig. 2b). During the recovery period, no recovery of Qtwpot was observed in the normal weight group, while the Qtwpot recovered significantly at the 3rd min in obese girls compared to the end of test (p < 0.001). Nevertheless, the Qtwpot recovery was still incomplete in both groups at the 15th min of recovery (Fig. 2b).
However, when the MVC torque was used as co-variable in the statistical analysis, no significant interaction was observed for the Qtwpot variable over the entire fatigue protocol.
Voluntary activation level
Absolute voluntary activation levels of both groups are displayed in the Fig. 1c. ANOVA showed a significant effect of group and percentage of repetitions on absolute VA values (p < 0.001). Furthermore, a significant interaction of group and percentage of repetitions for the relative VA change was observed (p < 0.001). The relative VA loss was significantly smaller in obese compared to non-obese girls between 80 and 100 % of total repetitions (Fig. 2c). At the end of the test, VA reached 74.2 ± 14.3 and 90.6 ± 7.9 % of initial value in non-obese and obese adolescent girls, respectively (p < 0.001). At the 15th min of recovery, VA had fully recovered in obese girls, whereas in controls, VA remained significantly below the initial pre-fatigue value (−8.3 ± 9.0 %, p < 0.01).
However, the time course of VA over the fatigue test was similar between groups when the MVC torque was used as co-variable in the statistical analysis.
Raw data of representative obese and non-obese subjects for the first and last MVC of the fatigue test are displayed in Fig. 3. These raw traces clearly show that the obese subject developed mainly peripheral fatigue, as illustrated by the greater reduction of Qtwpot, whereas the non-obese subject mainly developed central fatigue, as evidenced by the increased Qtws at the end of the fatiguing exercise. The higher torque produced by the obese subject is also clearly visible.
Example of raw data for the first (continuous line) and last (dashed line) MVC of the fatigue test superimposed with a twitch (Qtws) and followed by a potentiated twitch (Qtwpot) for typical non-obese (A) and obese (B) adolescent girls. At the onset of the fatigue protocol, the obese subject produced higher evoked and voluntary torque levels than the non-obese subject (MVC torque: 220.4 vs. 105.3 N.m; Qtwpot: 53.3 vs. 38.8 N.m, respectively). At the end of the exercise, the obese subject mainly developed peripheral fatigue, as illustrated by the greater reduction of Qtwpot (−53 vs. −17 %, respectively), whereas the non-obese subject mainly developed central fatigue, as evidenced by the greater impairment of VA (−40 vs. −5.6 %), translating into an increased Qtws
EMG activity
ANOVA revealed no significant interaction of factors for the RMS M −1max ratio of the VL and RF muscles. However, a main effect of the percentage of repetitions was obtained for both muscles (p < 0.001). More specifically, the RMS M −1max values of the VL and RF muscles declined significantly over the entire fatigue test (−16.2 ± 27.5 and −25.5 ± 20.5 %, respectively) and had fully recovered after 3 min of recovery. Furthermore, the M max of the VL and RF muscles did not vary as a function of the percentage of repetitions, but as a function of group: the M max of VL muscle was significantly higher in non-obese compared to obese girls (p < 0.01). The M max of RF muscle was also higher in non-obese girls, but this difference was not significant.
Antagonist co-activation
ANOVA only revealed a main effect of the percentage of repetitions on %CoActBF (p < 0.01). %CoActBF values declined significantly during the fatigue test from 22.8 ± 8.6 % at the onset of the test to 20.3 ± 5.8 % at the end of the test (p < 0.01).
Correlations
The MVC torque at the beginning of the fatigue test was significantly related to the exercise duration and to the relative VA and Qtwpot changes: the higher the MVC torque, the smaller was the number of repetitions (r = −0.67, p < 0.001; Fig. 4a), the smaller was the VA decrement (r = −0.59, p < 0.01; Fig. 4b) and the higher was the Qtwpot decrement (r = 0.44, p < 0.05; Fig. 4c).
Correlations between initial MVC torque, the number of total repetitions (panel A) and the relative decrements of voluntary activation (VA; panel B) and potentiated twitch torque (Qtwpot; panel C) over the fatigue test. In all panels, both groups were pooled. Initial MVC torque was inversely related to the number of total repetitions (panel A) and the VA decrement (panel B) and positively associated with the Qtwpot decrement (panel C)
Discussion
The aim of the present study was (i) to determine the effect of obesity on neuromuscular fatigue induced by repeated maximal contractions of the KE muscles and (ii) to compare the contribution of central and peripheral factors to fatigue development between normal weight and obese adolescent girls. The main findings revealed that obese girls are more fatigable than their normal weight counterparts. Moreover, the central and peripheral contributions to fatigue development differed as a function of weight status. Peripheral factors mainly accounted for fatigue in obese adolescent girls, whereas central factors were mainly involved in normal weight adolescent girls. However, when MVC was used as a co-variable in the statistical analysis, all the differences reported between groups disappeared.
It is now well acknowledged that muscle fatigue is influenced by growth and maturation (Ratel et al. 2002, 2015) and by some metabolic pathologies associated with obesity (i.e., diabetes mellitus) possibly because of an impaired mitochondrial function (Halvatsiotis et al. 2002). We (Ratel et al. 2002, 2015) and other authors (Zafeiridis et al. 2005) showed that adults are more fatigable than children during high-intensity intermittent exercise. Moreover, it has been reported that the muscle oxidative capacity of the KE muscles, which partly determines fatigue (Bogdanis et al. 1996), is impaired in insulin-resistant obese as compared to insulin-sensitive obese children and normal weight children (Fleischman et al. 2009; Slattery et al. 2014). Consequently, to exclude any confusing effect of growth and maturation on our measurements, we constituted our groups so that chronological age, height and maturation status were not significantly different. Furthermore, given that the metabolic indexes (glycemia, lipidemia, insulinemia and inflammation status) in obese girls fell within the normal ranges for their age and sex (Vukovic et al. 2013), we can reasonably exclude any confusing effect of metabolic disorders on the rate of fatigue development. Thus, other factors should account for the specificity of neuromuscular fatigue in obese. The non-obese group was healthy, so it is unlikely that they had an abnormal metabolic profile. Therefore, we can assume that this confusing effect can also be ruled out in the non-obese group, although we did not measure the metabolic indexes in this group.
The results of the present study highlight that obese girls fatigue faster than non-obese during repeated isometric maximal contractions of the KE muscles. Our data concur with some studies that reported a faster voluntary isometric strength loss and a lower endurance of the upper limb muscles in obese compared to non-obese individuals (Cavuoto and Nussbaum 2014; Mehta and Cavuoto 2015). However, other studies did not observe any difference of fatigue between non-obese and obese people (Maffiuletti et al. 2008; Minetto et al. 2013; Paolillo et al. 2012). For instance, Maffiuletti et al. (2008) showed no significant difference in the voluntary and stimulated torque decrement of the KE muscles between normal weight and obese adolescent boys after repeated concentric contractions. Minetto et al. (2013) also failed to demonstrate any significant difference between normal weight and obese adults after exhausting isometric tasks. Finally, Paolillo et al. (2012) compared the rate of fatigue over the course of a 1-min set of voluntary concentric knee extensions of normal weight and obese women and did not observe any significant difference in work decrement between groups. Such inconsistencies may be ascribed to large experimental differences regarding age, sex, muscle group, mode of contraction (i.e., isometric vs. isokinetic), the severity of obesity and the metabolic profile, which were not reported in the above-mentioned studies.
To gain insight into the origin of neuromuscular fatigue, we assessed the role of central and peripheral mechanisms in both non-obese and obese adolescent girls. While central factors were mainly involved in normal weight adolescent girls, peripheral factors mainly accounted for fatigue in obese girls.
Peripheral mechanisms
The absence of M max alteration in both groups suggests that sarcolemmal excitability was preserved. In contrast, the greater decrement of Qtwpot in obese adolescent girls points out to a greater impairment of the excitation–contraction coupling and/or the contractile processes.
Among the factors that may account for the greater peripheral fatigue in obese girls are the MVC torque and the muscle fiber type composition. Indeed, it is likely that the level of voluntary torque produced during MVC partly accounted for the greater peripheral fatigue and earlier exhaustion in obese girls. As expected (Tsiros et al. 2013), obese girls produced higher absolute KE torque than their normal weight counterparts. Interestingly, we demonstrated here that this higher force production capacity was positively correlated to the peripheral fatigue development (i.e., Qtwpot decrement). Furthermore, when the initial MVC torque was used as co-variable, no significant interaction or main effect was observed. This suggests that the initial MVC torque highly accounted for the amount of peripheral fatigue. This is consistent with other studies, which showed that the greater fatigue seen in men vs. women was no longer observed when subjects were matched for absolute MVC force (Hunter et al. 2004). Furthermore, in a study showing greater fatigue in men vs. women, Russ (2009) found that sub-samples of strength-matched men and women exhibited similar extents of peripheral fatigue. The principal mechanism cited to support the role of strength in the development of fatigue is muscle perfusion. Muscle contraction produces mechanical compression of the vascular supply, with the degree of compression increasing with contraction intensity (Wigmore et al. 2006). Because obese girls have typically higher muscle mass and consequently greater absolute force than their normal weight counterparts (Abdelmoula et al. 2012; Maffiuletti et al. 2013), this could result in a greater intramuscular pressure and, hence, a higher degree of vascular occlusion during intermittent muscular contractions. This greater occlusion could limit energy supply and the removal rate of metabolic by-products and hence accelerate peripheral fatigue in the obese. Muscle fiber type composition could also account for the greater peripheral fatigue in obese. It has been shown that individuals with predominantly type II fibers have a greater decrease in peak twitch torque compared to subjects with a higher proportion of type I fibers (Hamada et al. 2003). Kriketos et al. (1997) reported a higher percentage of type II fibers in VL muscle in obese compared to normal weight adults. Furthermore, a significant negative relationship was observed between body fatness and the percentage of type I fibers in adults (Wade et al. 1990), suggesting a higher amount of type II fibers in obese people. Therefore, it is likely that this higher fast-twitch fiber proportion could account for the higher peripheral fatigue in the obese. However, direct evidence is still lacking to support this assumption.
Central mechanisms
Regarding nervous factors, this study is the first to have objectively evaluated the time course of the maximal level of voluntary activation (VA) throughout a fatigue protocol in obese subjects. We showed that the VA reduction was greater in non-obese than in obese girls. This was associated with a faster initial VA recovery in normal weight girls.
The lower contribution of central factors to the development of fatigue in obese girls could be ascribed to their higher absolute MVC torque and the associated shorter exercise duration. Indeed, it is now well acknowledged that central fatigue is mainly induced by prolonged low-intensity exercise (Martin et al. 2010), and peripheral fatigue mainly occurs during maximal contractions or short-duration high-intensity exercise (Kent-Braun 1999). This assumption concurs with the results of the present study, which reports an inverse relationship between the first MVC of the fatigue test and the relative VA loss. Furthermore, no significant interaction or main effect was observed when the MVC torque was used as co-variable. These results agree with those of Russ (2009), which reported similar changes in neuromuscular fatigue and central activation in strength-matched men and women.
However, these results need to be interpreted cautiously. Despite the fact that the feasibility of surface EMG measurements has been established in the obese (Minetto et al. 2013), we observed a lack of agreement between the time course of the VA and normalized EMG data, the latter being comparable between non-obese and obese girls, whereas the former differed. This inconsistency between the results of EMG and the interpolated twitch method probably originates from the fact that surface EMG is not sufficiently sensitive to measure small differences in voluntary activation level (Kalmar and Cafarelli 1999). Other studies previously reported this lack of consistency between EMG and the twitch interpolation data (Kluka et al. 2015). Another difference between the two methods is that the twitch interpolation technique reflects the activation of the entire KE muscle group, whereas EMG assesses the activity of individual muscles. Therefore, in the present study, we cannot rule out the possibility that a greater reduction of normalized EMG activity could have been observed in normal weight girls on other KE muscles (i.e., on the vastus medialis and intermedius muscles).
Finally, the present study was the first to examine the neural recruitment strategies of antagonist BF muscles during fatigue in obese girls. Nevertheless, the parallel decrease of the level of co-activation of BF muscles during the fatigue test in the experimental groups rules out any contribution of this mechanism to the differences of fatigue reported between non-obese and obese girls.
Conclusions
To conclude, the results of the present study show that obese girls fatigue faster than normal weight girls, but that the relative pattern of torque loss over time is similar in both populations. However, the contribution of central and peripheral mechanisms to the development of fatigue differs between the two groups. Peripheral factors mainly account for fatigue in obese girls, whereas central factors are mainly involved in normal weight girls. The higher absolute MVC torque of the KE muscles and the potentially higher proportion of fast-fatigable fibers in obese adolescent girls could account for their higher peripheral fatigue and lower central fatigue. The faster development of neuromuscular fatigue in obese girls may contribute to the reduction of their functional capacity, but this link remains to be established during daily-living activities.
Abbreviations
- APHV:
-
Age from peak height velocity
- BMI:
-
Body mass index
- BF:
-
Body fat
- DXA:
-
Dual-energy X-ray absorptiometry
- EMG:
-
Electromyography
- FFM:
-
Fat-free mass
- KE:
-
Knee extensors
- MVC:
-
Maximal voluntary contraction
- M-wave:
-
Compound action potential amplitude
- Qtwpot :
-
Potentiated twitch torque
- RF:
-
Rectus femoris
- VA:
-
Voluntary activation
- VL:
-
Vastus lateralis
- VM:
-
Vastus medialis
References
Abdelmoula A, Martin V, Bouchant A, Walrand S, Lavet C, Taillardat M, Maffiuletti NA, Boisseau N, Duche P, Ratel S (2012) Knee extension strength in obese and nonobese male adolescents. Appl Physiol Nutr Metab 37(2):269–275. doi:10.1139/h2012-010
Bogdanis GC (2012) Effects of physical activity and inactivity on muscle fatigue. Front Physiol 3:142. doi:10.3389/fphys.2012.00142
Bogdanis GC, Nevill ME, Boobis LH, Lakomy HK (1996) Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J Appl Physiol 80(3):876–884
Callahan DM, Foulis SA, Kent-Braun JA (2009) Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve 39(5):692–702. doi:10.1002/mus.21278
Cavuoto LA, Nussbaum MA (2013) Obesity-related differences in muscular capacity during sustained isometric exertions. Appl Ergon 44(2):254–260. doi:10.1016/j.apergo.2012.07.011
Cavuoto LA, Nussbaum MA (2014) The influences of obesity and age on functional performance during intermittent upper extremity tasks. J Occup Environ Hyg 11(9):583–590. doi:10.1080/15459624.2014.887848
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320(7244):1240–1243
Enoka RM, Stuart DG (1992) Neurobiology of muscle fatigue. J Appl Physiol 72(5):1631–1648
Fleischman A, Kron M, Systrom DM, Hrovat M, Grinspoon SK (2009) Mitochondrial function and insulin resistance in overweight and normal weight children. J Clin Endocrinol Metab 94(12):4923–4930. doi:10.1210/jc.2009-1590
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81(4):1725–1789
Halvatsiotis P, Short KR, Bigelow M, Nair KS (2002) Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes 51(8):2395–2404
Hamada T, Sale DG, MacDougall JD, Tarnopolsky MA (2003) Interaction of fibre type, potentiation and fatigue in human knee extensor muscles. Acta Physiol Scand 178(2):165–173. doi:10.1046/j.1365-201X.2003.01121.x
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10(5):361–374
Hunter SK, Critchlow A, Shin IS, Enoka RM (2004) Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol 96(1):195–202. doi:10.1152/japplphysiol.00893.2003
Kalmar JM, Cafarelli E (1999) Effects of caffeine on neuromuscular function. J Appl Physiol 87(2):801–808
Kent-Braun JA (1999) Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol 80(1):57–63. doi:10.1007/s004210050558
Kent-Braun JA, Ng AV, Doyle JW, Towse TF (2002) Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 93(5):1813–1823. doi:10.1152/japplphysiol.00091.2002
Kluka V, Martin V, Vicencio SG, Jegu AG, Cardenoux C, Morio C, Coudeyre E, Ratel S (2015) Effect of muscle length on voluntary activation level in children and adults. Med Sci Sports Exerc 47(4):718–724. doi:10.1249/MSS.0000000000000463
Kriketos AD, Baur LA, O’Connor J, Carey D, King S, Caterson ID, Storlien LH (1997) Muscle fibre type composition in infant and adult populations and relationships with obesity. Int J Obes Relat Metab Disord 21(9):796–801
Maffiuletti NA, Jubeau M, Munzinger U, Bizzini M, Agosti F, De Col A, Lafortuna CL, Sartorio A (2007) Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol 101(1):51–59. doi:10.1007/s00421-007-0471-2
Maffiuletti NA, Jubeau M, Agosti F, De Col A, Sartorio A (2008) Quadriceps muscle function characteristics in severely obese and nonobese adolescents. Eur J Appl Physiol 103(4):481–484. doi:10.1007/s00421-008-0737-3
Maffiuletti NA, Ratel S, Sartorio A, Martin V (2013) The impact of obesity on in vivo human skeletal muscle function. Curr Obes Rep 2:251–260
Martin V, Kerherve H, Messonnier LA, Banfi JC, Geyssant A, Bonnefoy R, Feasson L, Millet GY (2010) Central and peripheral contributions to neuromuscular fatigue induced by a 24-h treadmill run. J Appl Physiol 108(5):1224–1233. doi:10.1152/japplphysiol.01202.2009
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Mehta RK, Cavuoto LA (2015) The effects of obesity, age, and relative workload levels on handgrip endurance. Appl Ergon 46:91–5. doi:10.1016/j.apergo.2014.07.007 (Part A)
Merton PA (1954) Voluntary strength and fatigue. J Physiol 123(3):553–564
Minetto MA, Botter A, Sprager S, Agosti F, Patrizi A, Lanfranco F, Sartorio A (2013) Feasibility study of detecting surface electromyograms in severely obese patients. J Electromyogr Kinesiol 23(2):285–295. doi:10.1016/j.jelekin.2012.09.008
Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP (2002) An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc 34(4):689–694
Paolillo FR, Milan JC, Bueno Pde G, Paolillo AR, Borghi-Silva A, Parizotto NA, Arena R, Kurachi C, Bagnato VS (2012) Effects of excess body mass on strength and fatigability of quadriceps in postmenopausal women. Menopause 19(5):556–561. doi:10.1097/gme.0b013e3182364e80
Ratel S, Duche P, Hennegrave A, Van Praagh E, Bedu M (2002) Acid-base balance during repeated cycling sprints in boys and men. J Appl Physiol 92(2):479–485. doi:10.1152/japplphysiol.00495.2001
Ratel S, Kluka V, Garcia-Vicencio S, Jegu AG, Cardenoux C, Morio C, Coudeyre E, Martin V (2015) Insights into the mechanisms of neuromuscular fatigue in boys and men. Med Sci Sports Exerc May 12. (Epub ahead of print)
Russ D (2009) Sex differences in muscle fatigue. In: Williams CA, Ratel S (eds) Human muscle fatigue. Routledge, London and New-York, pp 135–163
Slattery MJ, Bredella MA, Thakur H, Torriani M, Misra M (2014) Insulin resistance and impaired mitochondrial function in obese adolescent girls. Metab Syndr Relat Disord 12(1):56–61. doi:10.1089/met.2013.0100
Tanner JM, Whitehouse RH (1976) Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 51(3):170–179
Tessier S, Vuillemin A, Briançon S (2007) Psychometric properties of a physical activity questionnaire for school children aged between 6 and 10 years: QAPE-week. Sci Sport 22(7):224–231
Tsiros MD, Coates AM, Howe PR, Grimshaw PN, Buckley JD (2011) Obesity: the new childhood disability? Obes Rev 12(1):26–36. doi:10.1111/j.1467-789X.2009.00706.x
Tsiros MD, Coates AM, Howe PR, Grimshaw PN, Walkley J, Shield A, Mallows R, Hills AP, Kagawa M, Shultz S, Buckley JD (2013) Knee extensor strength differences in obese and healthy-weight 10-to 13-year-olds. Eur J Appl Physiol 113(6):1415–1422. doi:10.1007/s00421-012-2561-z
Vukovic R, Mitrovic K, Milenkovic T, Todorovic S, Soldatovic I, Sipetic-Grujicic S, Zdravkovic D (2013) Insulin-sensitive obese children display a favorable metabolic profile. Eur J Pediatr 172(2):201–206. doi:10.1007/s00431-012-1867-5
Wade AJ, Marbut MM, Round JM (1990) Muscle fibre type and aetiology of obesity. Lancet 335(8693):805–808
Wigmore DM, Propert K, Kent-Braun JA (2006) Blood flow does not limit skeletal muscle force production during incremental isometric contractions. Eur J Appl Physiol 96(4):370–378. doi:10.1007/s00421-005-0037-0
Yan AF, Voorhees CC, Beck KH, Wang MQ (2014) A social ecological assessment of physical activity among urban adolescents. Am J Health Behav 38(3):379–391. doi:10.5993/AJHB.38.3.7
Zafeiridis A, Dalamitros A, Dipla K, Manou V, Galanis N, Kellis S (2005) Recovery during high-intensity intermittent anaerobic exercise in boys, teens, and men. Med Sci Sports Exerc 37(3):505–512
Acknowledgments
This project was funded by a grant of the Auvergne Regional Council (New Researcher Program 2012—Vincent Martin).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest. A funding from the Auvergne Regional Council (New Researcher Program 2012—Vincent Martin) was received for this project. This work is known to and agreed by the co-authors identified on the manuscript’s title page. This work required more than six people, because of clinical examination (physician or pediatrician), recruitment of volunteers, experimental procedures, statistical analysis and data analysis.
Additional information
Communicated by Jean-René Lacour.
Rights and permissions
About this article
Cite this article
Garcia-Vicencio, S., Martin, V., Kluka, V. et al. Obesity-related differences in neuromuscular fatigue in adolescent girls. Eur J Appl Physiol 115, 2421–2432 (2015). https://doi.org/10.1007/s00421-015-3222-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3222-9