Abstract
Purpose
Fatigue in one limb can decrease force production in the homologous muscle as well as other muscles of the non-fatigued limb affecting balance. The objective of the study was to examine the effect of unilateral knee extensor fatigue on the non-fatigued limb’s standing balance, muscle force and activation.
Method
Sixteen healthy male subjects performed pre-fatigue balance trials, warm-up exercises, maximum voluntary isometric contractions, a knee extensors fatigue protocol, and post-fatigue balance trials. The fatigue protocol consisted of sets of 15 consecutive isometric contractions of 16 s each with 4 s recovery between repetitions, which were performed at 30 % peak force for the dominant knee extensor muscles. Additional sets of contractions continued until a 50 % decrease in MVIC knee extensor force was observed. Pre- and post-fatigue balance assessment consisted of transition from double to single leg standing and also single leg standing trials, which were performed bilaterally and in randomized order.
Result
The peak force and F100 were significantly decreased by 44.8 % (ES = 2.54) and 39.9 % (ES = 0.59), respectively, for the fatigued limb post-fatigue. There were no significant changes in the non-fatigued limb’s muscle force, activation, muscle onset timing or postural stability parameters.
Conclusion
While the lack of change in non-fatigued limb force production is in agreement with some of the previous literature in this area, the lack of effect on postural measures directly contradicts earlier work. It is hypothesized that discrepancies in the duration and the intensity of the fatigue protocol may have accounted for this discrepancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Balance can be affected by Parkinson disease (Schoneburg et al. 2013), multiple sclerosis (Dibble et al. 2013), injuries (Hertel 2000), aging (Vellas et al. 1997) and during muscle fatigue (Kanekar et al. 2008; Bellew and Fenter 2006). Muscular fatigue, which has been defined as “any exercise-induced reduction in the ability to exert muscle force or power, regardless of whether or not the task is sustained” (Bigland-Ritchie and Woods (1984), page 691) is one of the many factors that can impair balance (Yaggie and Armstrong 2004). Muscle fatigue is also believed to affect joint position sense by increasing the threshold of muscle firing rate and by disrupting the afferent feedback, thereby impairing proprioceptive and kinesthetic feedback (Macefield et al. 1990; Gribble and Hertel 2004).
Muscle fatigue can be further characterized as peripheral or central. Peripheral fatigue occurs due to changes occurring distal to the peripheral nerve. On the other hand, central fatigue has been defined as an exercise-induced attenuation in the ability of the central nervous system (CNS) to drive muscle maximally or as a decline in voluntary muscle activation (Taylor et al. 2006). The effects of fatigue can either be localized or global (Rattey et al. 2006). Some research has shown that local fatigue occurring in one limb can result in decreased force production in the homologous muscle (Martin and Rattey 2007; Halperin et al. 2014a; Kawamoto et al. 2014) as well as in heteronymous muscles (Takahashi et al. 2011; Kennedy et al. 2013; Halperin et al. 2014b) of a non-fatigued limb. This crossover or non-local fatigue effect has been identified for upper (Humphry et al. 2004; Halperin et al. 2014a, b) and lower limbs (Rattey et al. 2006; Martin and Rattey 2007; McLean and Samorezov 2009; Paillard et al. 2010; Halperin et al. 2014a, b). Despite this evidence of crossover fatigue, controversy about its existence remains with many studies being unable to confirm its occurrence (Zijdewind et al. 1998; Grabiner and Owings 1999; Todd et al. 2003; Regueme et al. 2007; Strang et al. 2009; Place et al. 2004; Ross et al. 2007, 2010; Elmer et al. 2013; Halperin et al. 2014a, b). Hence there is a conflict in the literature that needs further exploration.
Aside from the crossover effect of fatigue on muscle force, fatigue has also shown to negatively influence balance. There is only one study that has examined such effects of unilateral fatigue (Paillard et al. 2010). Paillard et al. (2010) demonstrated crossover fatigue effects on balance reporting increased sway area of center of pressure (CoP), although no decrease in the non-fatigued quadriceps femoris maximum voluntary isometric contractions (MVIC) was noted.
Although Paillard et al. (2010) provided initial insight into the effects of unilateral fatigue on contralateral limb balance, it remains unknown whether the balance changes in the non-fatigued limb were due to alterations in quadriceps femoris function or whether the effects of the fatigue were more global, affecting the activation patterns and force production of many other lower limb muscles. Also, it is not clear how fatigue in a non-postural muscle such as the quadriceps femoris (Masani et al. 2013) could have such a profound effect on non-fatigue limb balance. Based on these questions, the main objective of the current study was to examine the effect of unilateral knee extensor fatigue on non-fatigued limb’s standing balance, muscle force and activation. From the results obtained by Paillard et al. (2010), it was hypothesized that knee extensors fatigue would result in reduced balance while standing on the non-fatigued limb and would also affect muscle force and activation patterns in lower limb muscles.
Methodology
Participants
Sixteen healthy male subjects with a mean age of 24.9 ± 5 years, height 183 ± 7.7 cm and weight 86.4 ± 10 kg were recruited for the study. Only individuals who engaged in lower body resistance exercise for at least 2 days/week for a minimum of 20 min were recruited. Additionally, participants who had no history of balance disorders over the past 2 years, or neurological or musculoskeletal impairment, injury or medical conditions that might affect their postural stability were eligible to participate. This information was determined from the Physical Activity and Medical Questionnaire. The study was approved by the Interdisciplinary Committee on Ethics in Human Research (#20131842-HK).
Experimental design
Procedure
Participants came to the laboratory for a single testing session. They were asked to complete the consent form and two questionnaires (Physical Activity Readiness Questionnaire and Physical Activity and Medical Questionnaire) to determine if they were able to take part in this study. Then the dominant leg of the participants was determined by asking them which leg they would use to kick a ball. In this study, 15 out of 16 participants were right leg dominant.
Electromyography (EMG)
Participants were then fitted with bipolar surface electromyography (EMG) electrodes on their non-dominant leg. These electrodes were used to record muscle activity from eight lower limb muscles: tibialis anterior (TA), peroneus longus (PL), gastrocnemius medialis (GM), biceps femoris, vastus lateralis (VL), vastus medialis (VM), gluteus maximus (Gmax) and gluteus medius (Gmed). Before electrode placement, skin surfaces were shaved, abraded using sand paper and cleaned with alcohol to decrease the resistance offered by dead surface skin and tissue oils. Disposable Ag/AgCl disc electrode (3 cm in diameter) pairs (Kendall Medi-trace 100 series, Chikopee, MA) were placed on the muscle with an inter-electrode distance of 2 cm. Electrodes were placed according to the recommendation of Criswell (2011). As Criswell did not describe electrode placement for PL, these electrodes were placed over the muscle belly at the sight of the strongest signal intensity (4 cm lateral to the shin of the tibia and approximately one-third to one-fourth proximally the distance between the knee and the ankle). Tape was applied over the electrodes to minimize any movement of the electrodes during the contractions. The ground electrode was secured at the distal one-third of the iliac crest on the dominant side. A Biopac Systems MEC 100 amplifier (Santa Barbara, CA), with an input impedance of 2 m MΩ and common mode rejection ratio of >110 dB minimum (50/60 Hz) was used to collect all EMG. The signals were sampled at a rate of 2000 Hz and then digitized using a 12-bit analog-digital converter (BIOPAC MP 150).
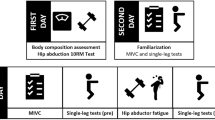
The experimental protocol consisted of pre-fatigue balance trials, warm-up exercises, MVIC, fatigue protocol, and post-fatigue MVIC and balance trials (see protocol outline in Fig. 1). Prior to beginning this protocol, participants performed familiarization trials to become acquainted with the two-legged and single leg standing trials that would be used in the study. The stance width during natural two-legged standing was determined and was marked with surgical tape for reference. This stance was used as the starting point for all subsequent balance trials performed.
Balance tests
Pre- and post-fatigue balance assessment consisted of transition from double to single leg standing and also single leg standing trials. All balance trials were performed pre- and post-fatigue on both right and left sides using a randomized selection order (both legs to single left leg × 2, both legs to single right leg × 2, single left leg standing × 2, single right leg standing × 2). During all balance tests, subjects stood on a force platform (AMTI, Watertown, MA, USA) which was connected to a six channel amplifier and an analog-to-digital converter. The force plate recorded ground reaction forces (GRF) and moment of force along X, Y and Z axis. The signals were sampled at a rate of 2000 Hz and synchronized with the EMG signals. The initial stance position was the same for both the pre- and post-fatigue balance test: barefooted and weight evenly distributed across both feet. Participants were asked to stand in such a way that a single foot was placed on the force plate. Also, they were instructed to stand naturally and try to maintain their balance. As stated above, two types of balance trials were performed: single leg standing and a transition from double to single leg standing. For the single leg standing balance trials, the participants were asked to stand on a single leg and once they were balanced they were asked to close their eyes. Once their eyes were closed, they were asked to maintain their single leg stance for a period of 30 s. Data collection for these trials did not begin until the person was stable with their eyes closed. Similar to previously published procedures from this laboratory (Penney et al. 2014), subjects were also asked to place their little finger on the side of their non-supporting limb, on the edge of a chair placed immediately adjacent to the force plate. They were instructed to use the chair for minimal support. This procedure was done to ensure participants could maintain the full 30 s stance required for this condition. The position of the chair was marked with tape for reference and remained in the same position for all balance testing during that session. The participants were asked to stand such that the knee of the non-stance leg was flexed at an angle of about 45°. During trials where participants transitioned from double to single leg stance, participants were asked to maintain an initial 3 s of double leg stance and then shift as quickly as possible to single leg stance. Once they achieved a single leg stance, they were asked to maintain their balance for 5 s. For these double to single transition trials, they were asked to keep their eyes open and rest both hands on their hips. The trials where participants touched their foot to the floor or otherwise lost their balance were discarded and another trial was conducted. Force plate and EMG data were recorded throughout all balance tests.
Maximum voluntary isometric contractions (MVIC)
Following completion of the pre-fatigue balance test, participants were asked to perform a 5-min warm-up on a cycle ergometer (1 kilo pound resistance at 70 revolutions per minute). Next, the MVICs for all eight muscles on the non-dominant side and knee extensors on the dominant side (MVIC PRE) were performed. For all MVICs, participants were asked to contract maximally against the resistance provided. For TA, participants were in a supine position with their arms across the chest and were asked to perform a maximum dorsiflexion contraction against the researcher’s resistance. While in the same position, participants were asked to produce maximal ankle eversion against researcher’s resistance to elicit an MVIC from PL. For the GM, subjects were asked to stand with a single limb stance on the non-dominant leg. Participants were then asked to perform a heel raise while using a chair to maintain balance. The researcher provided resistance to this motion by applying a downward force on the participant’s shoulders. For the MVIC of Gmax, participants lay prone and produced maximum hip extension against the resistance of the researcher while keeping their knee in flexion. For the Gmed, participants were in a side lying position and were asked to produce an MVIC of their hip abductors against the resistance of the researcher. Muscle activation data recorded from these MVICs was used to normalize EMG collected during balance trials.
In addition to the MVICs described above, MVICs were also performed for knee extension (quadriceps) and flexion (hamstrings). As force production in these muscles was used to assess the extent of fatigue in both dominant and non-dominant limbs, both muscle force and EMG were recorded during these trials. For the knee flexors, only data from the non-dominant limb were collected, since the biceps femoris was not directly targeted with the dominant side knee extensor fatiguing protocol. Participants stood facing a bench with the non-dominant knee slightly bent and their foot touching the ground. Their ankle was inserted into a padded strap, which was then attached to a Wheatstone bridge load cell (LCCA 500 pounds; sensitivity = 3 mV/V, OEI, Canada) through a high-tension wire. They were asked to flex their knee as hard as possible against the strap for a period of 4 s. Knee extension MVICs were performed for both the dominant and non-dominant leg. Participants sat on a bench with their hips and knees flexed at 90° and their chest, hips and upper legs restrained with straps. The participants were then asked to perform an isometric knee extension as hard as possible against the strap attached to a load cell for 4 s (while placing their arms across their chest). All the MVICs were randomized into two trials, each lasting 4 s and performed with a 2-min rest between each trial. Verbal encouragement was provided throughout the collection of MVICs. MVC reliability measures from this laboratory have been previously reported at r = 0.99 (Button and Behm 2008).
Fatigue protocol
Following completion of the MVIC trials, the fatigue protocol was performed. Participants remained in the position used for the knee extension MVIC with the same padded strap and load cell affixed to their dominant leg. Participants were then asked to perform an isometric knee extension contraction at 30 % of peak MVIC force. To ensure force was maintained at the desired magnitude, subjects were provided with visual feedback of the generated force during their fatiguing tasks, based on a previously published fatigue protocol from this laboratory (Behm and St-Pierre 1997). The fatigue protocol consisted of 15 consecutive 16 s isometric knee extension contractions. Each contraction was followed by a 4 s recovery. Following the 15 contractions, an MVIC (MVIC POST I) was performed to assess the fatiguing effects. If force production had dropped by at least 50 % of MVIC, then fatigue was considered to have occurred and the isometric contractions were stopped. If fatigue had not occurred, then another set of 15 repetitions was repeated until a 50 % reduction in force was observed. If volitional fatigue occurred during the 15 contractions (i.e., participants could not maintain the 30 % MVIC force required), then participants’ contractions were stopped. A knee extension MVIC (MVIC POST I) was then performed on the non-fatigued contralateral side to record any potential crossover effect of the fatiguing contractions on the dominant side. Two strain gauges were attached, so separate knee extension MVICs were performed for both the dominant and non-dominant leg without the need for moving and adjusting the strain gauges. Since the two strain gauges were already attached to each leg, this MVIC POST I was performed almost immediately (approximately. 10 s) after the completion of the fatigue trials.
Similar to procedures previously published (Behm et al. 2004; Kibele and Behm 2009), participants immediately did the post-fatigue balance trials, which were performed similar to the pre-fatigue balance trials. Following these balance trials, another series of non-fatigued contralateral limb knee extension (MVIC POST II) and flexion MVICs (MVIC POST II) were performed. These were performed approximately 10 min from the end of the fatiguing protocol.
Data analysis
Force
Force data from the load cell was assessed to determine the effect of fatigue on both the fatigued and contralateral non-fatigued leg. This analysis was performed by calculating peak force (N) and F100 (N) (MacDonald et al. 2014) during the knee extension MVIC trials. F100 was the force developed in the first 100 ms of MVIC and was calculated for the time period where the first deflection of the baseline activity of force (visual inspection) was observed to the period of initial 100 ms of force developed. The MVIC with the highest peak force was used to assess both the effects of fatigue on force production and for the calculation of F100.
Ground reaction force (GRF) data
All GRF data were processed using custom-designed software (MATLAB 2013a; Visual Basic 6.0). Initial processing was done to determine CoP location in both the anterio-posterior (AP) and medio-lateral (ML) directions. This was done using a formula provided by Robertson et al. (2004). For the single leg standing trials, a variety of sway parameters were determined to help quantify the effect of fatigue on postural stability. Details of the sway parameters can be found in Table 1. Briefly, CoP velocity and length in the ML and AP directions were determined as were the total CoP length and mean sway velocity over the duration of the 30 s trial. In addition, CoP range (AP and ML) and standard deviation (SD) were determined as was the total sway area. All measures were determined as per Bigelow (2008) with the exception of total sway area, which was calculated based on Duarte and Zatsiorsky (2002).
Data collection for the 30 s balance trial was started once the individual became stable in the one-legged stance. This was judged in two ways: (1) the participant was instructed to close their eyes once they felt stable; (2) the researcher also watched the participant to determine when there was no longer any excessive movement occurring on one leg. At this time, the researcher used a hand signal to tell the assistant to begin the data.
For trials where participants were asked to move from double to single leg standing, GRF data were used to determine when participants began to transition from double to single support. Motion onset was determined as per Sims and Brauer (2000). The start of motion was considered as the point of time when the vertical forces underneath the stepping leg dropped below the mean force—3 SD for more than 0.05 s (Sims and Brauer 2000). Mean and SD were determined over a 2 s period during the double leg stance portion of the trial (Fig. 2). This movement start time was considered time zero and was used as a reference point for all muscle onset times determined during this task as described below.
The vertical force during the double to single stance trials. Black horizontal and dotted horizontal line represent the mean − 3 SD of the vertical force during double limb standing portion of the trial. a Start of the motion as determined by the vertical force was less than the (mean − 3 SD), b point at which the force becomes minimum and c point at which the transition from double to single limb stance was considered complete
Electromyography
All EMG data were analyzed using custom-designed software written in Visual Basic 6.0. Prior to analysis, all EMG data were high pass filtered at 20 Hz to eliminate motion artifacts produced because of cable movement (De Luca et al. 2010). Following this filtering, all raw EMG signals were first normalized using data collected during the MVIC trials. A 50 ms moving window was used to determine root mean square (RMS) of MVIC EMG for each muscle. The peak RMS for each muscle was determined and used to normalize all EMG data collected during the study. This amplitude-normalized EMG was used for all calculations of EMG amplitude and onset timing described below (Burden et al. 2003).
During the single leg standing trials, EMG amplitude was quantified by calculating RMS EMG (Behm et al. 2001) for all muscles over the full 30 s duration of the trial. In addition, RMS EMG was determined for hamstrings, VL and VM, during the non-fatigued limb knee extensor and hamstring MVIC trials. The RMS EMG was calculated over a 1 s period starting 0.5 s before and ending 0.5 s following the peak force attained during the MVIC trial. For trials where peak force occurred at the end (i.e., no 0.5 s window existed after the peak was reached), RMS EMG was calculated using the 1 s prior to the time when the peak force occurred. RMS EMG activity was also analyzed during the 100 ms period of the F100.
Muscle activation timing EMG collected during the double to single leg trials was used primarily to assess the effects of the fatiguing contractions on muscle onset timing. Muscle onset was determined based on the protocol established by Hodges and Bui (1996). In accordance with these authors, all data were first full-wave rectified and low pass filtered at 50 Hz. The mean and SD of the EMG during the double leg stance portion of the trial was first determined over a 2 s period. This 2 s period was considered to represent quiet stance and therefore minimal muscle activity was observed. Muscle onset was determined to have occurred once the level of muscle activity exceeded the mean +1 SD for at least 100 ms. As per Hodges and Bui (1996), muscle activity was determined using a 100 ms moving average of the full-wave rectified and filtered signal (Fig. 3). All onset times were expressed with respect to the onset of motion as described above. Due to the potential for errors in the estimation of muscle onset times by the automated computer process, all onset times were subsequently checked manually to ensure their accuracy. The individual doing the manual checking was blind to the trial condition to prevent any bias in assigning an onset time to each muscle.
The EMG onset for TA during the transition from double to single limb stance on the non-dominant limb. Horizontal dotted line represents the mean + 1 SD of the double limb stance TA muscle activation. EMG depicted was first full-wave rectified and high pass filtered. A 100 ms moving window was then use to calculate a moving average of the rectified and filtered EMG signal. Muscle onset was determined to have occurred once EMG exceeded the mean + 1 SD (see text for more details) and has been represented as a vertical dotted line
Statistical analysis
Based on prior studies (Halperin et al. 2014a, b; Paillard et al. 2010), a priori statistical power analysis was conducted which determined that approximately 16 subjects would provide an alpha of p < 0.01 with a power of 0.8. The data were examined to assess differences in force, EMG, muscle onsets and sway parameters prior to and following the fatigue protocol. Separate repeated measures ANOVAs were used for the contralateral non-exercised MVIC and F100 force and EMG (pre-, post-MVIC I, post-MVIC II). If significant differences were detected, an adjusted Bonferroni post hoc test was used. Paired t tests were used for the fatigued limb (pre- vs. post-MVIC I). Significant differences were detected at p < 0.05. To infer the magnitude of the outcomes, effect sizes (ES) were calculated (Cohen 1990). The following formula was used to calculate the ES as per Cohen (1969): Pre–post ES = post mean − pre mean/pre standard deviation. Cohen (1969) considered an ES of less than 0.2 as trivial, 0.2–0.41 as small, 0.41–0.70 as moderate and greater than 0.70 as large. As some of the data were not normally distributed, a Wilcoxon signed rank test was also performed.
Results
Force
Significant changes pre- and post-fatigue were detected for the fatigued side peak force (p < 0.0001 and F100 (p = 0.04) (Fig. 4a). The peak force and F100 were significantly decreased by 44.8 % (ES = 2.54, large) and 39.9 % (ES = 0.59, moderate), respectively, for the fatigued limb post-fatigue protocol (MVIC POST I). There were no significant changes in peak force and F100 for the knee extensors on the contralateral non-fatigued limb (Fig. 4b) when pre- and post-MVIC I and II extensor forces were compared. Non-dominant limb F100 forces represented 26.54, 29.38 and 28.56 % of peak MVC force at pre-test, MVIC post I and post-II, respectively. The dominant limb F100 forces represented 19.59 and 21.31 % at pre-test and MVIC post I, respectively.
EMG
With both knee extensors and hamstrings MVICs on the contralateral non-fatigued side, fatigue had no effect on the magnitude of hamstrings, VL or VM EMG activation during peak MVIC force or F100 at post-MVIC I or II. Similarly, no significant changes were observed in EMG of these muscles during the pre- and post-fatigue single leg standing trials on the non-fatigued side (see Table 2).
Double to single leg standing parameters
There were no significant changes with the pre- to post-fatigue muscle onsets during the transition from double to single leg standing on the contralateral non-fatigued side (Fig. 5). Furthermore, prior to fatigue, it took participants 0.43 s to transition from double to single leg stance. This did not differ statistically from the 0.47 s post-fatigue (p = 0.4).
Muscle onset time estimated during the transition from double to single limb standing on the non-fatigued side. All times are reported with respect to the start of motion with positive times indicating that muscle onset occurred after the start of motion. See text for details on how motion start was determined. The muscle onsets for TA, PL, GM, hamstrings, Gmax and Gmed were not normally distributed and were analyzed using Wilcoxon rank test. The test revealed no significant results
Stability during single leg standing
On the fatigued side, the fatigue protocol led to an increase in the total length covered by CoP, CoPAP, CoP velocity and the total sway area. CoP length increased by 1.6 % (p = 0.002), although the effect size (0.12) was trivial (Table 3). CoPAP length (Table 3) and CoP velocity (Table 3) demonstrated similar trivial effect sizes (0.14 and 0.12, respectively) as they increased by 2.4 % (p = 0.007) and 1.6 % (p = 0.002) following the fatiguing contractions. Sway area demonstrated the largest increase at 27.9 % (p = 0.01, ES = 0.66, moderate, Table 3). The only significant change observed on the contralateral non-fatigued side was the CoPML range which was 15.4 % greater (p = 0.04, ES = 0.38, small) post-fatigue than pre-fatigue (Table 3). All other postural sway measures values remained unchanged following the fatigue protocol (see Table 3).
Discussion
The most important findings of this study were the absence of any crossover fatigue effects. More specifically, the contralateral non-fatigued limb showed no significant reductions in muscle force, EMG or muscle onset timing and no disturbances in the postural stability parameters except for a small effect with one of the measures (CoPML range).
The aforementioned results contradict the study hypothesis. The significant decrease in the peak force and F100 with the fatigued limb indicated that the fatigue protocol did lead to unilateral fatigue. However, the unilateral fatigue did not produce crossover fatigue in the contralateral non-fatigued limb. In the literature, crossover fatigue effects have been observed as a decrease in voluntary muscle activation (Rattey et al. 2006), force (Martin and Rattey 2007) and an increase in postural sway (Paillard et al. 2010) of the contralateral non-fatigued limb. The findings of the present study are similar to published studies that have found no evidence of crossover force deficits associated with unilateral fatigue (Zijdewind et al. 1998; Grabiner and Owings 1999; Todd et al. 2003; Regueme et al. 2007; Strang et al. 2009; Place et al. 2004; Ross et al. 2007, 2010; Elmer et al. 2013). The study by Zijdewind et al. (1998) used a similar protocol with 30 % MVICs regularly interrupted with MVICs and brief rest periods in the right first dorsal interosseus muscle until failure and found no evidence of crossover force deficits.
However, the present findings are in opposition to several studies that have reported crossover fatigue effects (Rattey et al. 2006; Martin and Rattey; 2007; Halperin et al. 2014a, b; Kawamoto et al. 2014). Rattey’s et al. (2006); Martin and Rattey (2007) and Halperin et al. (2014a) used 100 s sustained MVICs of the dominant limb knee extensors to induce fatigue. While Rattey et al. (2006) found a decrease in voluntary activation (interpolated twitch technique) and EMG of the contralateral limb but a non-significant MVIC decrease, Martin and Rattey (2007) and Halperin et al. (2014a) found significant reductions in both force and EMG activity in the contralateral limb. One possible reason for the lack of agreement between Rattey et al.’s (2006) and Halperin et al.’s (2014a) works and the present research may be related to the intensity of the fatiguing contraction. In contrast to the aforementioned work (Rattey et al. 2006; Martin and Rattey 2007; Halperin et al. 2014a) that used maximal contractions, fatiguing contractions were maintained at 30 % MVIC in the present study. Crossover fatigue may depend upon the intensity at which the isometric contractions are maintained (Kennedy et al. 2013) and is also thought to depend on the occurrence of central fatigue (Enoka and Duchateau 2008). Kawamoto et al. (2014) fatigued the non-dominant knee extensors with heavier (70 % MVC) and lighter (40 % MVC) loads to task failure. Compared to the lighter intensity protocol, the higher intensity protocol induced greater force (7.1 vs. 4.4 %) and time to exhaustion (8 vs. 2 %) crossover fatigue in the non-exercised knee extensors. Bigland-Ritchie et al.’s (1986) work suggests that the contraction intensity used in the present study might not have been sufficient to create the central changes required for crossover effects. Specifically, Bigland-Ritchie et al. (1986) used repeated voluntary submaximal contractions which were maintained at 30 % MVIC to identify neuromuscular fatigue. Their study reported decreases in force; however, central activation was preserved suggesting that submaximal contractions, like those used in the present study, may not be sufficient to induce central activation changes. Similarly, Kennedy et al. (2013) concluded that the energy required to drive the muscle (forearm) maximally might lead to more severe central alterations as compared to the submaximal contractions. While the contraction intensity rationale concurs with the work of Bigland-Ritchie et al. (1986), another research suggests that the relationship may not be as simple. For example, Place et al. (2009) reported that low intensity sustained contractions are often related to central alterations, whereas maximal contractions have been related to peripheral changes. Clearly, more work is necessary to delineate contraction intensities and durations related to central or global fatigue effects.
Furthermore, contralateral single MVICs may not be as sensitive to crossover fatigue as repeated fatiguing contractions. Halperin et al. (2014b) reported no crossover effects with a single MVIC, but force decrements were found in the last five MVICs of a 12 MVIC (5 s contraction/10 s recovery) repetition protocol. Amann et al. (2013) tested the contralateral knee extensors with two different tests following a unilateral fatiguing protocol: an MVIC and a constant load knee extension to failure. While the MVIC was unaffected, time to exhaustion was ~50 % shorter compared to the control conditions. Additionally, after subjects performed a unilateral fatiguing protocol with the elbow flexors, the contralateral arm MVC remained unaffected, but significant decrements were found in the time to exhaustion tests (Triscott et al. 2008).
To expand upon the work of Paillard et al. (2010), the present study was designed to allow for a more detailed examination of contralateral non-fatigued limb muscle activation changes, in an effort to better understand the mechanism underlying postural control changes. With the exception of a slight increase in the M/L range of the CoP in the non-fatigued limb, the present study failed to show crossover effects of fatigue on standing balance, contradicting the results of Paillard et al. (2010). Evidence of crossover fatigue was supported in the Paillard study (2010) by the increased CoP sway area, though there was no decrease in the non-fatigued quadriceps femoris MVIC force. The present study found no effect of unilateral fatigue on almost all sway measures, force or muscle activation. These contradictory findings between Paillard et al. (2010) and the present study may be related to differences between the fatigue protocols. Contraction intensity differences between the protocols (10 vs. 30 % in the present study) might have been a factor. In the previous paragraph, it was suggested that higher intensity contraction were needed to produce crossover fatigue. Based on this, and the contraction used in the present study and the work of Paillard et al. (2010), it is surprising that Paillard et al. (2010) found crossover effects. This suggests that there is likely some other mechanism, other than contraction intensity that needs to be explored. Examining the protocols used in this study and the Paillard et al. (2010), an additional factor that has to be considered is the duration of the fatiguing contractions. In the present study, 15 consecutive contractions of 16 s each were executed at 30 % peak force until a 50 % decrease in force occurred. Paillard et al. (2010) utilized 10 sets of 50 repetitions at 10 % peak torque resulting in a longer duration of contractions. Specifically, it was estimated that Paillard’s et al. (2010) protocol would have resulted in individuals contracting for approximately 33 min at 10 % MVIC. In the present study, on average, individuals contracted for 3.5 min at 30 % MVIC. It is possible that the difference in fatigue duration could be a factor that led to the contradictory results. Behm and St-Pierre (1997) examined the effects of fatigue protocol duration on quadriceps femoris muscle activation properties. They reported that longer duration protocols (~19 min at 25 % MVIC) differentially affected muscle activation properties when compared to shorter duration fatiguing contractions (~4 min of 50 % MVIC isometric contractions). Behm and St-Pierre (1997) suggested their results were indicative of greater central inhibitory (muscle inactivation) responses for the longer duration contractions. Rattey et al. (2006) have shown that such central responses can lead to reflex impulses at the medullar level, which can potentially affect contralateral function. In addition, the prolonged Paillard protocol may have elicited greater muscular pain or discomfort, which can adversely affect muscular force through central mechanisms that can affect both local and generalized (non-local) responses (Graven-Nielsen et al. 2002). Collectively these results suggest that it is possible that the more prolonged contractions in the Paillard et al. (2010) study had a greater capacity to alter contralateral muscle function, leading to the alterations in posture on the contralateral non-fatigued limb post-fatigue.
Although not measured in the present study, it is possible that there are both inhibitory and excitatory effects on the contralateral muscle. Unilateral motor activity has been proposed to activate excitatory paths interconnecting the ipsilateral and contralateral primary motor cortex, referred to as motor irradiation (Zijdewind et al. 1998). However, the theory of default bilateral interaction states that throughout the unilateral contractions, the activation of the non-targeted muscle group can be actively inhibited (Post et al. 2008). In the present study, it is possible that these competing inhibitory and facilitating effects might have balanced contralateral responses resulting in no significant changes in the force and postural sway parameters on the non-fatigued limb. Clearly, further research is needed to determine the exact mechanisms underlying the crossover effects of fatigue.
As with all studies, there were certain limitations, which included the use of only male participants that contributed to group homogeneity, but may not have adequately represented female responses. Also, the placement of the finger on the chair during the single leg standing balance trials might have contributed to the lack of change in non-fatigued limb balance. Work by Bolton et al. (2011) has shown that even light finger pressure has the ability to alter postural sway. Despite these potential effects, fatigued leg standing balance was still negatively impacted by the fatiguing trials. It is possible, however, that deficits in non-fatigued limb balance may have been masked by use of the chair. In other words, if the chair had not been used, contralateral effects of fatigue on balance may have been observed. Unfortunately during pilot testing, many participants could not complete the full 30 s single leg stance trial on the fatigued leg once they were fatigued. We therefore had to include the chair in the testing protocol to ensure that testing could be completed as outlined in methods.
Conclusion
No evidence of reductions in force, muscle activation, muscle co-ordination or disturbance in the postural stability parameters was noted for the contralateral non-fatigued limb. However, significant changes were noted for the fatigued limb with MVIC, CoP sway area, total length covered by CoP, CoPAP length as well as the mean velocity covered by CoP. Posture can be dependent upon the extent and onset of muscle forces to compensate for disruptions to the center of gravity. It can be concluded that the lack of changes in non-fatigued force, F100 and stability parameters contributed to the lack of change in non-dominant postural sway parameters. A briefer fatigue duration than that in a previously published similar study (Paillard et al. 2010) or the use of submaximal intensity contractions as compared to MVIC (Rattey et al. 2006; Martin and Rattey 2007; Halperin et al. 2014a) may have contributed to the lack of crossover fatigue effects.
Abbreviations
- AP:
-
Antero-posterior
- CNS:
-
Central nervous system
- CoP:
-
Center of pressure
- EMG:
-
Electromyography
- ES:
-
Effect size
- GM:
-
Gastrocnemius medialis
- Gmax:
-
Gluteus maximus
- Gmed:
-
Gluteus medius
- GRF:
-
Ground reaction force
- Hz:
-
Hertz
- ML:
-
Medio-lateral
- MVIC:
-
Maximal voluntary isometric contraction
- PL:
-
Peroneus longus
- RMS:
-
Root mean square
- SD:
-
Standard deviation
- TA:
-
Tibialis anterior
- VL:
-
Vastus lateralis
- VM:
-
Vastus medialis
References
Amann M, Venturelli M, Ives SJ (2013) Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol 115:355–364
Behm DG, St-Pierre DM (1997) Effects of fatigue duration and muscle type on voluntary and evoked contractile properties. J Appl Physiol 82:1654–1661
Behm DG, Button DC, Butt JC (2001) Factors affecting force loss with prolonged stretching. Can J Appl Physiol 26:261–272
Behm D, Bambury A, Cahill F, Power K (2004) The effect of acute static stretching on force, balance, reaction time and movement time. Med Sci Sports Exerc 36(8):1397–1402
Bellew JW, Fenter PC (2006) Control of balance differs after knee or ankle fatigue in older women. Arch Phys Med Rehabil 87:1486–1489
Bigelow KE (2008) Identification of key traditional and fractal postural sway parameters to develop a clinical protocol for fall risk assessment in older adults. Dissertation, Ohio State University
Bigland-Ritchie B, Woods JJ (1984) Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7:691–699
Bigland-Ritchie B, Cafarelli E, Vollestad NK (1986) Fatigue of submaximal static contractions. Acta Physiol Scand Suppl 556:137–148
Bolton DA, McIlroy WE, Staines WR (2011) The impact of light fingertip touch on haptic cortical processing during a standing balance task. Exp Brain Res 212:279–291
Burden AM, Trew M, Baltzopoulos V (2003) Normalization of gait EMGs: a re-examination. J Electromy Kinesiol 13(6):519–532
Button DC, Behm DG (2008) The Effect of Stimulus Anticipation on the Interpolated Twitch Technique. J Sport Sci Med 7:520–524
Cohen J (1969) Statistical power analysis for the biomechanical sciences. L. Erbraum Associates, New York, USA
Cohen J (1990) Things I have learned (so far). Am Psych 45:1304–1312
Criswell E (2011) Cram’s introduction to surface electromyography. Jones and Bartlett Publishers, USA
De Luca CJ, Gilmore LD, Kuznetsov M (2010) Filtering the surface EMG signal: movement artifact and baseline noise contamination. J Biomech 43:1573–1579
Dibble LE, Lopez-Lennon C, Lake W et al (2013) Utility of disease-specific measures and clinical balance tests in prediction of falls in persons with multiple sclerosis. J Neuro Phys Ther 37:99–104
Duarte M, Zatsiorsky VM (2002) Effects of body lean and visual information on the equilibrium maintenance during stance. Exp Brain Res 146:60–69
Elmer SJ, Amann M, McDaniel J et al (2013) Fatigue is specific to working muscles: no crossover with single-leg cycling in trained cyclists. Eur J Appl Physiol 113:479–488
Enoka RM, Duchateau J (2008) Muscle fatigue: what, why and how it influences muscle function. J Physiol 586:11–23
Grabiner MD, Owings TM (1999) Effects of eccentrically and concentrically induced unilateral fatigue on the involved and uninvolved limbs. J Electromyogr Kinesiol 9:185–189
Graven-Nielsen T, Lund H, Arendt-Nielsen L (2002) Inhibition of maximal voluntary contraction force by experimental muscle pain: a centrally mediated mechanism. Muscle Nerve 26:708–712
Gribble PA, Hertel J (2004) Effect of lower-extremity muscle fatigue on postural control. Arch Phys Med Rehabil 85:589–592
Halperin I, Copithorne D, Behm DG (2014a) Unilateral isometric muscle fatigue decreases force production and activation of contralateral knee extensors but not elbow flexors. Appl Physiol Nutr Metab 39:1338–1344
Halperin I, Aboodarda SJ, Behm DG (2014b) Knee extension fatigue attenuates repeated force production of the elbow flexors. Eur J Sport Sci 14:823–829
Hertel J (2000) Functional instability following lateral ankle sprain. Sports Med 29:361–371
Hodges PW, Bui BH (1996) A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol 101:511–519
Humphry AT, Lloyd-Davies EJ, Teare RJ et al (2004) Specificity and functional impact of post-exercise depression of cortically evoked motor potentials in man. Eur J Appl Physiol 92:211–218
Kanekar N, Santos MJ, Aruin AS (2008) Anticipatory postural control following fatigue of postural and focal muscles. Clin Neurophysiol 119:2304–2313
Kawamoto JE, Aboodarda SJ, Behm DG (2014) Effect of differing intensities of fatiguing dynamic contractions on contralateral homologous muscle performance. J Sports Sci Med 13:836–845
Kennedy A, Hug F, Sveistrup H (2013) Fatiguing handgrip exercise alters maximal force-generating capacity of plantar-flexors. Eur J Appl Physiol 113:559–566
Kibele A, Behm DG (2009) Seven weeks of instability and traditional resistance training effects on strength, balance and functional performance. J Strength Cond Res 23(9):2443–2450. doi:10.1519/JSC.0b013e3181bf0328
MacDonald GZ, Button DC, Drinkwater EJ, Behm DG (2014) Foam rolling as a recovery tool following an intense bout of physical activity. Med Sci Sports Exerc 46:131–142
Macefield G, Gandevia SC, Burke D (1990) Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol 429:113–129
Martin PG, Rattey J (2007) Central fatigue explains sex differences in muscle fatigue and contralateral crossover effects of maximal contractions. Pflugers Arch 454:957–969
Masani K, Sayenko DG, Vette AH (2013) What triggers the continuous muscle activity during upright standing? Gait Posture 37:72–77
McLean SG, Samorezov JE (2009) Fatigue-induced ACL injury risk stems from a degradation in central control. Med Sci Sports Exerc 41:1661–1672
Paillard T, Chaubet V, Maitre J et al (2010) Disturbance of contralateral unipedal postural control after stimulated and voluntary contractions of the ipsilateral limb. Neurosci Res 68:301–306
Penney T, Ploughman M, Behm DG, Byrne JM (2014) Determining the activation of gluteus medius and the validity of the single leg stance test in chronic non-specific low back pain. Arch Physical Med Rehab 95:1969–1976
Place N, Lepers R, Deley G (2004) Time course of neuromuscular alterations during a prolonged running exercise. Med Sci Sports Exerc 36:1347–1356
Place N, Duclay J, Lepers R, Martin A (2009) Unchanged H-reflex during a sustained isometric submaximal plantar flexion performed with an EMG biofeedback. J Electromyogr Kinesiol. 19:e395–e402
Post M, Bayrak S, Kernell D et al (2008) Contralateral muscle activity and fatigue in the human first dorsal interosseous muscle. J Appl Physiol 105:70–82
Rattey J, Martin PG, Kay D et al (2006) Contralateral muscle fatigue in human quadriceps muscle: evidence for a centrally mediated fatigue response and cross-over effect. Pflugers Arch 452:199–207
Regueme SC, Barthelemy J, Nicol C (2007) Exhaustive stretch-shortening cycle exercise: no contralateral effects on muscle activity in maximal motor performances. Scand J Med Sci Sports 17:547–555
Robertson DGE, Caldwell GE, Hamill J et al (2004) Research methods for biomechanics. Human Kinetics Publishers, Champaign III USA
Ross EZ, Middleton N, Shave R et al (2007) Corticomotor excitability contributes to neuromuscular fatigue following marathon running in man. Exp Physiol 92:417–426
Ross EZ, Goodall S, Stevens A et al (2010) Time course of neuromuscular changes during running in well-trained subjects. Med Sci Sports Exerc 42:1184–1190
Schoneburg B, Mancini M, Horak F et al (2013) Framework for understanding balance dysfunction in Parkinson’s disease. Mov Disord 28:1474–1482
Sims KJ, Brauer SG (2000) A rapid upward step challenges medio-lateral postural stability. Gait Posture 12:217–224
Strang AJ, Berg WP, Hieronymus M (2009) Fatigue-induced early onset of anticipatory postural adjustments in non-fatigued muscles: support for a centrally mediated adaptation. Exp Brain Res 197:245–254
Takahashi K, Maruyama A, Hirakoba K et al (2011) Fatiguing intermittent lower limb exercise influences corticospinal and corticocortical excitability in the non-exercised upper limb. Brain Stimul 4:90–96
Taylor JL, Todd G, Gandevia SC (2006) Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol 33:400–405
Todd G, Petersen NT, Taylor JL et al (2003) The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Exp Brain Res 150:308–313
Triscott S, Gordon J, Kuppuswamy A et al (2008) Differential effects of endurance and resistance training on central fatigue. J Sports Sci 26:941–951
Vellas BJ, Wayne SJ, Romero L et al (1997) One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc 45:735–738
Yaggie J, Armstrong WJ (2004) Effects of lower extremity fatigue on indices of balance. J Sport Rehabil 13:312–322
Zijdewind I, Zwarts MJ, Kernell D (1998) Influence of a voluntary fatigue test on the contralateral homologous muscle in humans? Neurosci Lett 253:41–44
Acknowledgments
This study was partially funded by the Natural Science and Engineering Research Council (NSERC) of Canada. We thank Dr. Thamir Alkanani for his technical support and the subjects for their enthusiastic cooperation.
Conflict of interest
None of the authors have a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fausto Baldissera.
Rights and permissions
About this article
Cite this article
Arora, S., Budden, S., Byrne, J.M. et al. Effect of unilateral knee extensor fatigue on force and balance of the contralateral limb. Eur J Appl Physiol 115, 2177–2187 (2015). https://doi.org/10.1007/s00421-015-3198-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3198-5