Abstract
Purpose
Salivary antimicrobial proteins (sAMPs) protect the upper respiratory tract (URTI) from invading microorganisms and have been linked with URTI infection risk in athletes. While high training volume is associated with increased URTI risk, it is not known if fitness affects the sAMP response to acute exercise. This study compared the sAMP responses to various exercising workloads of highly fit experienced cyclists with those who were less fit.
Methods
Seventeen experienced cyclists (nine highly fit; eight less fit) completed three 30-min exercise trials at workloads corresponding to −5, +5 and +15 % of the individual blood lactate threshold. Saliva samples were collected pre- and post-exercise to determine the concentration and secretion of α-amylase, human neutrophil proteins 1–3 (HNP1–3) lactoferrin, LL-37, lysozyme, and salivary SIgA.
Results
The concentration and/or secretion of all sAMPs increased post-exercise, but only α-amylase was sensitive to exercise workload. Highly fit cyclists had lower baseline concentrations of α-amylase, HNP1–3, and lactoferrin, although secretion rates did not differ between the groups. Highly fit cyclists did, however, exhibit greater post-exercise increases in the concentration and/or secretion of a majority of measured sAMPs (percentage difference between highly fit and less fit in parentheses), including α-amylase concentration (+107 %) and secretion (+148 %), HNP1–3 concentration (+97 %) and secretion (+158 %), salivary SIgA concentration (+181 %), lactoferrin secretion (+209 %) and LL-37 secretion (+138 %).
Conclusion
We show for the first time that fitness level is a major determinant of exercise-induced changes in sAMPs. This might be due to training-induced alterations in parasympathetic and sympathetic nervous system activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The upper respiratory tract (URTI) is protected by an array of salivary antimicrobial proteins (sAMPs) that act as a first line of defense against invading microorganisms. sAMPs range from small cationic peptides to larger polypeptides and proteins and are typically secreted by phagocytes, epithelial cells and salivary glands (Bartlett et al. 2008; Davison et al. 2009; Doss et al. 2010; Radek and Gallo 2007; West et al. 2006). The sAMPs play a pivotal role in maintaining mucosal immune integrity (Allgrove et al. 2008; Bals and Hiemstra 2004; Papacosta and Nassis 2011; West et al. 2006) by working synergistically to curtail invading bacteria, viruses, and fungi (De Smet and Contreras 2005; Radek and Gallo 2007; West et al. 2006) and are vital in both the prevention and clearance of many types of infection (Bowdish et al. 2005; Davison et al. 2009; West et al. 2006). In particular, infections of the oral cavity and URTI are associated with depressed levels of sAMPs (Table 1) (Bartlett et al. 2008; Bishop and Gleeson 2009; Dale et al. 2006; Doss et al. 2010; Laube et al. 2006; Putsep et al. 2002; Tanida et al. 2003; Tao et al. 2005; Ueta et al. 2000; West et al. 2006), highlighting their prominent role in first-line immune defense.
Maintaining mucosal immune integrity is of particular interest to elite-level endurance athletes to limit the incidence of URTIs that can have detrimental effects on competition and training. Indeed, periods of intense, frequent, endurance-based training have consistently been associated with self-reported symptoms of URTI in elite-level athletes (Gleeson 2000; Gleeson et al. 1999; Gleeson and Pyne 2000; Nieman 2000). Low levels of salivary secretory immunoglobulin A (SIgA) have been linked with an increased risk of URTI (Fahlman and Engels 2005; Gleeson et al. 1999, 2012; Hanson et al. 1983; Neville et al. 2008) and prolonged, high-intensity exercise bouts lower both the concentration and secretion of salivary SIgA (Fahlman et al. 2001; Nieman et al. 2006; Steerenberg et al. 1997; Tomasi et al. 1982; Usui et al. 2011). On the other hand, shorter exercise bouts, or low to moderate-intensity exercise, may actually increase salivary SIgA concentration and secretion (Allgrove et al. 2008; Li and Gleeson 2004), indicating that mucosal immune integrity, and indeed URTI risk, is dependent on the duration and intensity of the exercise performed. Although the effects of exercise on salivary SIgA have been well studied, relatively little is known about the effects of exercise on other sAMPs. Small cationic peptides present in saliva are primarily classified into two groups: the human defensins, which include human neutrophil peptides (HNP) 1–4 and human defensins 5 and 6, and the cathelicidins, which include LL-37 (Davison et al. 2009; Doss et al. 2010; Radek and Gallo 2007). Lactoferrin and lysozyme are larger sAMPs and are some of the most highly concentrated proteins found in saliva (Bartlett et al. 2008; Bishop and Gleeson 2009; West et al. 2006), while α-amylase, in addition to its antimicrobial properties, is considered to be a marker of sympathetic nervous system activation and has been shown to correlate with anaerobic threshold (Allgrove et al. 2008; Bishop and Gleeson 2009; Calvo et al. 1997; Chatterton et al. 1996).
Davison et al. (2009) found that the concentration and secretion rates of LL-37 and HNP1–3 were significantly greater following a single bout of prolonged exercise at an intensity corresponding to 55–65 % of \(\dot{V}{\text{O}}_{2\hbox{max} }\). Additional studies examining the relationship between exercise and sAMPs have yielded similar results, demonstrating significant increases in the concentration and/or secretion of total salivary protein, α-amylase, salivary SIgA, lactoferrin, lysozyme, human β-defensin-2, and LL-37 following acute bouts of exercise (Allgrove et al. 2008; de Oliveira et al. 2010; Gillum et al. 2014; Usui et al. 2011; Walsh et al. 1999; West et al. 2010); however, this exercise-induced response appears to be dependent on exercise intensity and/or duration. While Allgrove et al. (2008) showed significant increases in α-amylase and lysozyme secretion following ~22 min of exercise at 75 % \(\dot{V}{\text{O}}_{2\hbox{max} }\) and after an incremental effort test to exhaustion, no change in these sAMPS was found after 22 min of exercise at 50 % \(\dot{V}{\text{O}}_{2\hbox{max} }\). West et al. (2010) also reported significant increases in concentrations of lactoferrin and lysozyme following maximal exercise, but not submaximal exercise. Taken together, these findings would indicate that the secretion rates of sAMPs following an acute bout of exercise are intensity-dependent; however, no studies have examined the effects of various workloads on the concentrations and secretion of HNP1–3 or LL-37, nor have any studies specifically examined the effects of exercise on sAMPs at intensities that would typically be practiced by experienced cyclists. Moreover, although previous studies have compared resting sAMP levels between trained and untrained participants (West et al. 2010), it is not known how fitness affects the changes in the concentration and secretion of sAMPs in response to various intensities of exercise.
Using a cohort of experienced cyclists and intensities of exercise typically practiced by competitive cyclists during training, the aim of this study was to determine the impact of fitness on the concentration and secretion of a variety of sAMPs (Table 1) in response to a single bout of exercise at varying workloads. It was hypothesized that higher workloads would elicit greater sAMP responses to exercise in an intensity-dependent manner and that the magnitude of this response would be affected by subjects’ fitness levels.
Methods
Subjects
Seventeen healthy, experienced cyclists were recruited for this study. Subject characteristics are provided in Table 2. All subjects reported participating in road cycling events (i.e., group rides and organized races) at least three times per week for a minimum period of 12 months. Cyclists were required to be non-smokers between the ages of 18 and 44 and were asked to abstain from alcohol, caffeine, and strenuous physical activity for 24 h prior to the trials. To ensure proper hydration, subjects were asked to drink plenty of water the day before the trial and 250–350 mL of water the morning of each trial (Sawka et al. 2007). Further, subjects did not report to be taking any medications known to affect the immune system and were instructed to discontinue vitamin/mineral supplementation at least 4 weeks prior to participating in the study. Compliance was confirmed verbally with subjects upon arrival to the laboratory. Questionnaires including a medication log, a brief medical history questionnaire, cycling history and the ACSM/AHA pre-exercise readiness questionnaire were administered to determine eligibility and adequate cycling experience. Written procedures and participation risks were provided to the subject and were explained orally prior to enrollment. Written informed consent was obtained from each subject. All procedures were approved by the Committee for the protection of human subjects at the University of Houston.
Experimental design
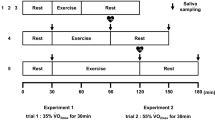
Subjects performed four exercise trials on separate days, beginning with a lactate threshold test and followed by three 30-min steady-state trials at −5, +5, and +15 % of blood lactate threshold (BLT). All tests were performed at the same time of day and the steady-state trials were performed in randomized order. Each trial was performed using subjects’ personal road bicycles mounted to an indoor cycle ergometer (Computrainer Lab, Racermate, Inc., Seattle, WA). A minimum of 2 days and a maximum of 3 weeks were allowed between trials.
Blood lactate threshold test
All subjects completed an incremental discontinuous cycling test to determine the individual BLT. Prior to the test, resting capillary blood samples were collected from the earlobe to determine resting blood lactate concentration. All blood lactate concentrations were determined in duplicate using an automated pre-calibrated analyzer (P-GM7 Micro-Stat, Analox Instruments, London, UK). After a 10-min warm-up (cycling against no resistance) and calibration of the ergometer, subjects began the protocol, which consisted of 3-min incremental stages. Initial workload and increments were dependent on subjects’ fitness levels and ranged from starting workloads of 50–100 W and increments of 20–25 W. Subjects were instructed to maintain steady, comfortable revolutions per minute (RPM) throughout the test. Heart rate and breath-by-breath metabolic data (\(\dot{V}{\text{O}}_{2}\), \(\dot{V}{\text{CO}}_{2}\), ventilation and respiratory exchange ratio) were measured continuously using a metabolic cart (Quark CPET, Cosmed, Rome, Italy). Rating of perceived exertion [RPE; Borg, 6–20 scale, (Borg 1973)] was recorded during the last 30 s of each stage and subjects were asked to stop pedaling at the end of the 3-min stage to allow a capillary blood sample to be collected from the earlobe. The blood sample was processed immediately for the determination of blood lactate concentration. The test was terminated one incremental stage after attaining a blood lactate concentration of >4 mM. The power output corresponding to the BLT was determined visually using the breakpoint method defined by Weltman (1995). Briefly, blood lactate concentration (mM) was plotted against power (watts), with the threshold being determined as the power output corresponding to the breakpoint where a curvilinear rise in blood lactate concentration is first observed (Weltman 1995).
Main exercise trials
Three steady-state continuous exercise trials of differing workloads were completed on separate days in randomized order. Subjects were blinded to the order of trials and their power output and physiological responses during each exercise trial. The steady-state exercise protocols required subjects to maintain a workload (power output) corresponding to −5, +5, and +15 % of his/her previously identified BLT for 30 min. Subjects began the test after a 10-min warm-up and calibration of the ergometer (set to the same calibration as was used for the BLT test). Subjects were instructed to cycle at the same cadence maintained during the BLT test and the RPM were recorded every 5 min. Heart rate and breath-by-breath metabolic data were measured continuously, and RPE was reported every 5 min. Capillary blood samples were collected from the earlobe every 10 min for the analysis of blood lactate concentration. Values for heart rate, \(\dot{V}{\text{O}}_{2}\), RPE, and blood lactate concentration were averaged over each trial. The percent of maximum heart rate for each trial was calculated using the average heart rate and the equation 191.5–0.007 × age2 proposed by Gellish et al. (2007) for predicting maximum heart rate.
Saliva collection
Saliva was collected before and immediately after (within 5 min) each of the three main exercise trials. Subjects rinsed their mouths with a small cup of water 2–5 min prior to collection. Subjects were then instructed to passively drool into a sterile collection cup for 2 min, tilting the head slightly forward while minimizing facial movements. Every 20 s, the subjects were instructed to expectorate any remaining saliva that had pooled in the mouth. Samples were immediately stored at −80 °C for subsequent analysis. For determination of flow rate, each empty collection cup was weighed to the nearest mg prior to saliva collection. Following collection, the collection cup was reweighed. The density of saliva was assumed to be 1.00 g/mL. The difference between the pre- and post-collection weight was then divided by the collection time to yield the amount of saliva (in mL) produced per minute (salivary flow rate).
Determination of salivary α-amylase, HNP1–3, lactoferrin, LL-37, lysozyme, and salivary SIgA
Saliva samples were thawed once, centrifuged at 15,000 g for 2 min and the supernatants removed for determination of analytes. ELISA kits were used to measure the concentrations of HNP1–3, lactoferrin, LL-37, lysozyme, and salivary SIgA, and a kinetic enzyme assay kit was used to measure α-amylase activity. HNP1–3, lactoferrin, and LL-37 kits were purchased through Hycult Biotech, Uden, Netherlands (HK317 Human HNP1–3; HK329 Human Lactoferrin; HK321 Human LL-37); salivary SIgA and α-amylase kits were purchased through Salimetrics, State College, PA, USA (salivary SIgA enzyme immunoassay kit; salivary α-amylase assay kit); and the lysozyme kit was purchased through Abcam, Cambridge, MA, USA (ab10880 Lysozyme Human ELISA kit). Samples were diluted according to the manufacturers’ instructions for salivary kits (salivary SIgA 1:5; α-amylase 1:200). For those kits designed for plasma, dilutions were determined following spike recovery and linearity of dilutions assays (HNP1–3 1:1,000; Lactoferrin 1:500; LL-37 1:5; Lysozyme 1:8,000). All samples were tested in duplicate and in accordance with the manufacturers’ instructions. Absorbances were read according to manufacturer’s instructions (SpectraMax190, Molecular Devices, Sunnyvale, CA, USA) and concentrations were calculated from the absorbance based on the generated standard curves. Intra-assay coefficients of variation were 3.8, 9.7, 5.5, 8.7, 9.6, and 9.6 % for α-amylase, HNP1–3, lactoferrin, LL-37, lysozyme, and salivary SIgA, respectively. Secretion rates were determined by multiplying the concentration of each analyte by the flow rate (mL min−1). For determination of α-amylase activity, the change in absorbance over 2 min at 37 °C was used to calculate α-amylase activity (U mL−1, α-amylase activity = ∆ absorbance/minutes × total assay volume × dilution factor).
Fitness level determination
Subjects were divided into high (Hi) and low (Lo) fitness groups based on their watts per kilogram body mass at BLT. A median split was taken and a clear division between the top nine and the bottom eight was observed, with the top nine subjects above 2.8 W/kg at BLT and the other eight falling below 2.6 W/kg and BLT (Table 2). All subjects reported cycling at least three times per week, but no other subjective measures of training volume were recorded.
Statistical analysis
Data analysis was performed using the SPSS statistical software program (version 21, SPSS Inc., Chicago, IL, USA). The data were tested for normality, and non-normal data were log-transformed to achieve a normal distribution. A repeated-measures analysis of variance (ANOVA) was used to examine differences in the physiological response to exercise over the three exercise intensities. The sAMP response to exercise was analyzed using a 2 (pre- vs. post-exercise) × 3 (intensity) repeated-measures ANOVA, which examined main effects for exercise, main effects for intensity, and the interaction between time and intensity. Post hoc paired t tests with Bonferonni adjustments for multiple comparisons were utilized to assess significant differences identified in the ANOVA. The change in sAMP concentration and secretion from pre- to post-exercise was calculated for each intensity by subtracting the pre-exercise values from the post-exercise values. Given the variability in the measurement of the sAMPs, absolute change was used in place of percent change, as low starting values created unreasonably high percent changes, resulting in multiple outliers. A repeated-measures ANOVA examining the main effects of intensity was performed on these calculated differences.
To examine the effects of fitness on the sAMP response to exercise, independent samples t tests were performed to compare differences in baseline values between groups. Both repeated-measures ANOVAs were repeated with Hi and Lo fitness level included as a between-subjects factor. Main effects for fitness were examined, as were the interaction effects between fitness and exercise. Post hoc independent samples Student’s t tests were performed to analyze between-group differences at each of the three intensities. All data are expressed as means and standard deviations unless stated otherwise. The F statistics for the main effects and for the interactions effects from the repeated-measures ANOVAs are reported, and statistical significance was set at p < 0.05.
Results
Physiological response
All subjects successfully completed the exercise protocols. The physiological responses to the three trials are presented in Table 3. A significant (p < 0.05) main effect of intensity was found for all physiological variables. As expected, significant (p < 0.05) increases in \(\dot{V}{\text{O}}_{2}\), percent of maximum age-predicted heart rate, ventilation, blood lactate concentration, and RPE were observed with increasing workloads across the three trials. While the respiratory exchange ratio was not significantly different between the −5 and +5 % trials, it was significantly (p < 0.05) higher during the +15 % trial. Significant main effects for fitness were observed for ventilation (L min−1) and relative (mL min−1 kg−1) and absolute (mL min−1) \(\dot{V}{\text{O}}_{2}\) (F = 8.678, p = 0.011; F = 26.706, p < 0.001; F = 5.942, p = 0.029, respectively), as Hi had significantly higher ventilation and \(\dot{V}{\text{O}}_{2}\) values for the three trials combined. In examining the trials separately, Hi had significantly (p < 0.05) higher ventilation (L min−1) during the +5 and +15 % trials and significantly (p < 0.05) higher relative \(\dot{V}{\text{O}}_{2}\) (mL min−1 kg−1) values at each of the three workloads, but the absolute \(\dot{V}{\text{O}}_{2}\) between the two groups did not significantly differ (p > 0.05). Blood lactate levels, RPE, respiratory exchange ratio, and percentage of maximum heart rate, were not significantly different between the two groups.
Effects of exercise
Main effects of exercise (i.e., pre-exercise vs. post-exercise) were observed for flow rate, indicating a significant decrease following exercise (Table 4). Main effects of exercise were also observed in the concentration and secretion of a majority of the sAMPs (Table 4). Both the concentrations and secretions of α-amylase, HNP1–3, lactoferrin and LL-37 were significantly greater (p < 0.05) following exercise when all three trials were examined together (main effects of exercise). While the concentration of lysozyme and salivary SIgA in the saliva increased post-exercise, when accounting for flow rate, the secretion of these proteins was not significantly greater post-exercise (p > 0.05). Post hoc paired t tests were performed to determine whether significant changes in flow rate, concentration, and secretion were observed at each of the three intensities. Results of these post hoc tests are shown in Table 4.
Intensity effects
No significant main effects for intensity were observed when examining the pre- and post-exercise concentrations and secretions, nor were there any significant exercise × intensity interaction effects (Table 4); however, main effects of intensity were found when examining the pre- to post-exercise change in concentration for α-amylase (F = 5.759, p = 0.007) (Fig. 1a). Pairwise comparisons between the three trials demonstrated a significant difference in the change in α-amylase concentration pre- to post-exercise between the −5 % trial and the +15 % trial (p = 0.008). No effects of intensity were found for the changes in concentration of the other five sAMPs studied (Fig. 1); however, a trend toward greater increases in salivary SIgA concentration at higher workloads (F = 2.040, p = 0.164 for main effects of intensity) was observed (Fig. 1f). After accounting for flow rate, no significant effects of intensity were found in the change in secretion from pre- to post-exercise; however, trends toward greater changes in secretion rates at higher workloads were observed for α-amylase (F = 2.343, p = 0.113) and salivary SIgA (F = 2.175, p = 0.131) (data not shown).
Mean change in concentrations of a α-amylase, b HNP1-3, c lactoferrin, d LL-37, e lysozyme, and f SIgA from pre- to post-exercise at each of the three exercise intensities. A significantly (p < 0.05) different change in concentration from the −5 % trial is denoted by asterisk. Error bars represent standard error
Effects of fitness
Significant main effects of fitness were observed for flow rate (F = 5.024, p = 0.043), with Hi exhibiting greater salivary flow rates. Post hoc tests revealed that the flow rate of Hi was significantly greater (p < 0.05) than Lo, both pre- and post-exercise. The change in flow rate from pre- to post-exercise, however, was not significantly different between the two groups (p > 0.05). In comparing the baseline values between Hi and Lo, Hi had significantly lower concentrations of α-amylase (t = 2.302, p = 0.026), HNP1–3 (t = 2.633, p = 0.013) and lactoferrin (t = 3.818, p < 0.001). While not significant, the baseline values of LL-37 (t = 1.057, p = 0.297), lysozyme (t = 1.266, p = 0.212), and salivary SIgA (t = 0.444, p = 0.582) were also lower in Hi; however, when accounting for flow rate, the Hi and Lo groups did not differ in the secretion rates of any sAMPs at baseline (p > 0.05) (Fig. 2). Fitness also impacted the exercise response of the sAMPs. Significant (p < 0.05) exercise × fitness interactions were observed for the secretion of lactoferrin and lysozyme and for both the concentration and secretion of α-amylase, HNP1–3, and LL-37, indicating a difference in the exercise response between the two groups. Upon examining change from pre- to post-exercise, it was found that Hi had a much greater exercise response. Significant main effects of fitness (p < 0.05) were found for the secretion of lactoferrin and LL-37, the concentration of salivary SIgA, and for both the concentration and secretion of α-amylase and HNP1–3 (Fig. 3). As no intensity × fitness interactions were found, data from the three trials were collapsed to show main effects of fitness on the sAMP response to exercise, regardless of intensity (Fig. 3).
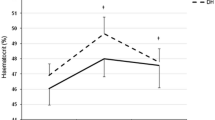
Baseline values combined from all three trials for the concentration and secretion of a α-amylase, b HNP1-3, c lactoferrin, d LL-37, e lysozyme, and f SIgA for the Hi and Lo fitness groups. Significantly (p < 0.05) different values from Lo are denoted by asterisk. Error bars represent standard error
Mean change from pre- to post-exercise in the concentration and secretion of a α-amylase, b HNP1-3, c lactoferrin, d LL-37, e lysozyme, and f SIgA for Hi and Lo fitness groups for the three trials combined. Significantly (p < 0.05) different Δ values for concentration or secretion in the Hi fitness group compared to Lo are denoted by asterisk. Error bars represent standard error
Discussion
The primary aim of this study was to investigate how fitness impacts the concentration and secretion of sAMPs in response to 30 min of steady-state cycling at varying workloads. We show for the first time that fitness is a major determinant of sAMP responses to acute exercise. Highly fit cyclists demonstrated a greater exercise-induced change in the secretion and/or concentration of LL-37, lactoferrin, salivary SIgA, α-amylase and HNP1–3 compared to the less fit cyclists. Although resting concentrations of all sAMPs tended to be lower in the more fit cyclists, secretion rates did not differ, indicating that the lower concentrations of sAMPs in the highly fit cyclists were due to increased salivary flow rates. The concentration and secretion of all sAMPs increased with exercise, however, only α-amylase was sensitive to the intensity of the bout. These findings indicate that mucosal immune responses to a single bout of exercise are largely impacted by individual fitness, which may be due to training-induced alterations in parasympathetic and sympathetic nervous system activation.
We found that the fitness of experienced cyclists impacts sAMP levels and their response to exercise. Baseline concentrations of all sAMPs measured tended to be lower in highly fit cyclists, and α-amylase, HNP1–3 and lactoferrin levels were found to be significantly lower. Despite these lower concentrations, salivary flow rate in the highly fit cyclists was higher, so no group differences were found for the secretion of any sAMP. These results are in accordance with West et al. (2010), who found that elite rowers had lower salivary concentrations of lactoferrin and lysozyme than untrained controls, although they did not take flow rate into consideration. It should be noted that the concentrations of sAMPs reported here, both pre and post-exercise for both fitness groups, are within normal, healthy ranges. Concentrations of α-amylase and SIgA are within the normal ranges provided by the assay manufacturer (Salimetrics, State College, PA, USA), and concentrations of lysozyme were within the normal ranges reported by Tenovuo (1989). Values for LL-37 and HNP1–3 were similar to those obtained in previous exercise and dental health studies (Dale et al. 2006; Davison et al. 2009). While the lactoferrin concentrations reported here are slightly lower than those reported in other studies (Tanida et al. 2003; Ueta et al. 2000; West et al. 2010), we do not deem this to be of clinical significance and is more likely due to variations in the assay system. The differences in flow rate and sAMP concentrations observed in the present study may be related to differences in parasympathetic nervous system activity as saliva flow rate and protein secretion are primarily controlled by the autonomic nervous system (Bishop and Gleeson 2009). Parasympathetic nervous system stimulation results in the production of large amounts of dilute saliva, while sympathetic nervous system activation results in a lower volume of saliva containing higher protein concentrations (Bishop and Gleeson 2009; Chicharro et al. 1998). Exercise training has been shown to increase vagal tone and parasympathetic nervous system activity (Coote 2010; Raczak et al. 2006), which may account for the low concentrations but greater flow rate observed in the highly fit cyclists. Therefore, while the lower baseline sAMP concentrations seen with a high fitness level in the present study may at first seem indicative of depressed mucosal immunity, their higher salivary flow rates may act as a compensatory mechanism to maintain adequate rates of sAMP secretion and may, in fact, be indicative of greater parasympathetic nervous system activity and vagal tone. Hydration may also affect the ability to maintain adequate sAMP secretion rates despite lower concentrations, as Fortes et al. (2012) found that dehydration decreased saliva flow rate and the secretion of α-amylase and lysozyme without affecting the concentrations of either of these sAMPs. Maintaining hydration may, therefore, be an important factor in maintaining mucosal immune integrity during heavy training loads. Unfortunately, while subjects were asked to hydrate prior to each exercise session, hydration status was not assessed.
This is the first study, to our knowledge, to report the effects of fitness and exercise capacity on the sAMP response to a single bout of exercise. We found that acute dynamic exercise increased the secretion and/or concentration of a wide range of sAMPs, from the small cationic peptides LL-37 and HNP1–3, to larger polypeptides and proteins such as lactoferrin, lysozyme and salivary SIgA. These findings are consistent with previous findings that a single bout of exercise enhances the concentration and secretion of a number of sAMPs (Allgrove et al. 2008; Davison et al. 2009; de Oliveira et al. 2010; Gillum et al. 2014; Usui et al. 2011; Walsh et al. 1999; West et al. 2010), but this study shows that fitness level significantly impacts this response. The secretion and/or concentration of all measured sAMPs tended to increase with exercise at a greater magnitude in the highly fit compared to the less fit cyclists, with significant fitness effects being observed for α-amylase, HNP1–3, LL-37, lactoferrin, and salivary SIgA. While these group differences might be partially explained by the lower baseline sAMP concentrations in the highly fit cyclists, the exercise-induced change in α-amylase, HNP1–3, lactoferrin and LL-37 secretion rates was also higher with higher fitness despite the two groups having similar baseline values. This indicates that better fitness, at least in healthy athletes, elicits transient upturns in mucosal immunity in response to a single exercise bout that may be related to autonomic nervous system alterations associated with more fit individuals. The mechanisms for acute exercise-induced increases in sAMPs are not well known, but both sympathetic nervous system activation and epithelial cell damage with concomitant neutrophil activation have been proposed as potential mechanisms (Allgrove et al. 2008; Dorschner et al. 2001; West et al. 2010). Sympathetic nervous system stimulation, which would occur during exercise, results in higher salivary protein concentrations, while the withdrawal of the vasodilatory effects of the parasympathetic nervous system results in decreased flow rate (Bishop and Gleeson 2009; Chicharro et al. 1998). Although the highly fit group had higher flow rate both pre- and post-exercise, the change in flow rate was similar between the groups, indicating equivalent parasympathetic withdrawal in both groups. The highly fit group, however, may have had greater sympathetic nervous system activation, given the greater exercise-induced changes in sAMPs. The catecholamine response to intense exercise has been shown to be higher in endurance-trained men compared to untrained, further indicating an increased sympathetic response (Zouhal et al. 2008). The high levels of α-amylase observed post-exercise in the more fit group are also evidence of sympathetic activation, as α-amylase has been used as an indicator of saliva secretion controlled via adrenergic stimulation (Chicharro et al. 1998). It is possible, therefore, that increased fitness associated with regular training elicits an adaptive and potentially preemptive effect on the mucosal immune response to a single bout of exercise that is the result of greater exercise-induced sympathetic nervous system activation, although more empirical work is required to test this hypothesis. It is possible that exercise training, by increasing parasympathetic stimulation at rest, allows for greater sympathetic nervous system activation during exercise. It would also be illuminating to examine the sAMP and the sympathetic nervous system response to acute exercise in illness-susceptible athletes. Indeed, the sAMP response to a single exercise bout may provide a better indication on the robustness of the immune system to withstand periods of heavy training compared to similar measures taken at rest. Given the potential link between sAMPs and the autonomic nervous system, sAMPs may prove to be a useful tool for determining URTI susceptibility or overtraining, as alterations in the autonomic nervous system are thought to contribute to overtraining (Purvis et al. 2010).
A unique feature of the present study was to determine if sAMP responses to acute exercise were sensitive to the intensity of the bouts and, rather than using vastly contrasting exercising workloads, we elected to use workloads corresponding to various percentages of the individual BLT as realistic training zones that are typically used by competitive cyclists. While exercise increased the concentration and/or secretion of all sAMPs, only α-amylase was sensitive to the workload encountered. This was expected as salivary α-amylase has been used as a biomarker of sympathetic nervous system activation (Papacosta and Nassis 2011), with a number of studies showing increased α-amylase levels in response to a variety of acute stressors such as exercise, academic examinations, medical procedures, and laboratory-based stress tasks (Nater and Rohleder 2009). Further, salivary α-amylase correlates with the anaerobic threshold (Calvo et al. 1997), indicating that blood lactate and α-amylase responses to exercise are tightly linked. As heart rate, ventilation, \(\dot{V}{\text{O}}_{2}\), RPE, and blood lactate also responded to increasing exercise workloads in a step-wise manner, this study supports the use of salivary α-amylase as a sensitive biomarker capable of discriminating between various levels of overall sympathetic nervous system activity. However, using α-amylase as a marker of sympathetic nervous system activation should be interpreted with caution. Leicht et al. (2011) found that exercise induced increases in α-amylase in tetraplegic individuals, who lack centrally mediated sympathetic neural drives. A spinal reflex partially compensates for the loss of a sympathetic central drive, but the resultant sympathetic activation is altered. Therefore, the authors caution against using α-amylase as a marker of central sympathetic nervous system activation, and instead suggest using it as a marker of overall sympathetic drive (Leicht et al. 2011).
Unlike α-amylase, exercise-induced changes in the concentration and/or secretion of LL-37, HNP1–3, lactoferrin and lysozyme were not sensitive to the exercise intensities used in this study. Although there was a trend for the change in salivary SIgA concentration/secretion to be greatest after the high-intensity cycling protocol, this did not reach statistical significance. While Allgrove et al. (2008) and West et al. (2010) did find effects of intensity on salivary SIgA, lysozyme, and lactoferrin, this is perhaps due to the vastly contrasting intensities of exercise that were used in these studies. Allgrove et al. (2008) found the post-exercise secretion of lysozyme to be significantly greater when subjects exercised either at 75 % of their \(\dot{V}{\text{O}}_{2\hbox{max} }\) or to exhaustion than when the subjects exercised at 50 % of their \(\dot{V}{\text{O}}_{2\hbox{max} }\). However, there were no differences in lysozyme or salivary SIgA secretion between the 75 % and exhaustive trials (Allgrove et al. 2008). Similarly, West et al. (2010) found significant increases in the concentration of lactoferrin and lysozyme following a bout of exercise at maximal exertion, but did not observe any significant changes in concentrations following a submaximal bout of exercise. The lack of an intensity effect in our study may be due to the more subtle differences in exercising workload compared to these previous studies. We chose these workloads as BLT intensities that would commonly be used by cyclists in training (Bourdon 2013), whereas exercise at 50 % of \(\dot{V}{\text{O}}_{2\hbox{max} }\), as used in the study by Allgrove et al. (2008) would be very light and fall well below the BLT for a trained cyclist. The findings of the present study, along with those of Allgrove et al. (2008) and West et al. (2010), seem to point to a threshold intensity, above which similar sAMP responses are elicited, but below which the concentration and secretion are not altered.
In conclusion, this study presents two major findings. First, it was demonstrated that a single bout of exercise increases the concentration and/or secretion of the sAMPs α-amylase, HNP1–3, lactoferrin, LL-37, lysozyme, and salivary SIgA, but only α-amylase was sensitive to the intensity of the bout when exercising workloads typically practiced by experienced cyclists are used. Second, fitness was found to be a major determinant of the sAMP response to a single bout of exercise, with more fit individuals exhibiting greater post-exercise increases in concentration and/or secretion in most of the measured sAMPs. These findings indicate that α-amylase is a sensitive biomarker to discriminate between subtle differences in exercise intensity, and that fitness is a stronger determinant of sAMP responses to a single bout of exercise than workload. Future studies should seek to examine mechanistic links between sAMP responses to acute exercise and the effects of fitness on the autonomic nervous system.
Abbreviations
- sAMP:
-
Salivary antimicrobial protein
- URTI:
-
Upper respiratory tract infection
- SIgA:
-
Secretory Immunoglobulin A
- HNP:
-
Human neutrophil peptide
- BLT:
-
Blood lactate threshold
- RPM:
-
Revolutions per minute
- RPE:
-
Rating of perceived exertion
- ANOVA:
-
Analysis of variance
References
Allgrove JE, Gomes E, Hough J, Gleeson M (2008) Effects of exercise intensity on salivary antimicrobial proteins and markers of stress in active men. J Sports Sci 26(6):653–661. doi:10.1080/02640410701716790
Bals R, Hiemstra PS (2004) Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J 23(2):327–333
Bartlett JA, Fischer AJ, McCray PB Jr (2008) Innate immune functions of the airway epithelium. Contrib Microbiol 15:147–163. doi:10.1159/000136349
Bishop NC, Gleeson M (2009) Acute and chronic effects of exercise on markers of mucosal immunity. Front Biosci (Landmark Ed) 14:4444–4456
Borg GA (1973) Perceived exertion: a note on “history” and methods. Med Sci Sports 5(2):90–93
Bourdon P (2013) In: Tanner RK, Gore CJ (eds) Physiological tests for elite athletes, 2nd edn. Human Kinetics, Champaign, pp 77–102
Bowdish DM, Davidson DJ, Hancock RE (2005) A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci 6(1):35–51
Calvo F, Chicharro JL, Bandres F, Lucia A, Perez M, Alvarez J, Mojares LL, Vaquero AF, Legido JC (1997) Anaerobic threshold determination with analysis of salivary amylase. Can J Appl Physiol 22(6):553–561
Chatterton RT Jr, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA (1996) Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol 16(4):433–448
Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, Okumura K, Ogawa H (2005) Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci 40(2):123–132. doi:10.1016/j.jdermsci.2005.03.014
Chicharro JL, Lucia A, Perez M, Vaquero AF, Urena R (1998) Saliva composition and exercise. Sports Med 26(1):17–27
Coote JH (2010) Recovery of heart rate following intense dynamic exercise. Exp Physiol 95(3):431–440. doi:10.1113/expphysiol.2009.047548
Dale BA, Tao R, Kimball JR, Jurevic RJ (2006) Oral antimicrobial peptides and biological control of caries. BMC Oral Health 6(Suppl 1):S13. doi:10.1186/1472-6831-6-S1-S13
Davison G, Allgrove J, Gleeson M (2009) Salivary antimicrobial peptides (LL-37 and alpha-defensins HNP1-3), antimicrobial and IgA responses to prolonged exercise. Eur J Appl Physiol 106(2):277–284. doi:10.1007/s00421-009-1020-y
de Oliveira VN, Bessa A, Lamounier RP, de Santana MG, de Mello MT, Espindola FS (2010) Changes in the salivary biomarkers induced by an effort test. Int J Sports Med 31(6):377–381. doi:10.1055/s-0030-1248332
De Smet K, Contreras R (2005) Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 27(18):1337–1347. doi:10.1007/s10529-005-0936-5
Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, Gallo RL (2001) Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol 117(1):91–97. doi:10.1046/j.1523-1747.2001.01340.x
Doss M, White MR, Tecle T, Hartshorn KL (2010) Human defensins and LL-37 in mucosal immunity. J Leukoc Biol 87(1):79–92. doi:10.1189/jlb.0609382
Fahlman MM, Engels HJ (2005) Mucosal IgA and URTI in American college football players: a year longitudinal study. Med Sci Sports Exerc 37(3):374–380
Fahlman MM, Engels HJ, Morgan AL, Kolokouri I (2001) Mucosal IgA response to repeated wingate tests in females. Int J Sports Med 22(2):127–131
Fortes MB, Diment BC, Di Felice U, Walsh NP (2012) Dehydration decreases saliva antimicrobial proteins important for mucosal immunity. Appl Physiol Nutr Metab 37(5):850–859. doi:10.1139/h2012-054
Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK (2007) Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc 39(5):822–829. doi:10.1097/mss.0b013e31803349c6
Gillum T, Kuennen M, Miller T, Riley L (2014) The effects of exercise, sex, and menstrual phase on salivary antimicrobial proteins. Exerc Immunol Rev 20:23–38
Gleeson M (2000) Mucosal immunity and respiratory illness in elite athletes. Int J Sports Med 21(Suppl 1):S33–S43
Gleeson M, Pyne DB (2000) Special feature for the Olympics: effects of exercise on the immune system: exercise effects on mucosal immunity. Immunol Cell Biol 78(5):536–544. doi:10.1111/j.1440-1711.2000.t01-8-.x
Gleeson M, McDonald WA, Pyne DB, Cripps AW, Francis JL, Fricker PA, Clancy RL (1999) Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc 31(1):67–73
Gleeson M, Bishop N, Oliveira M, McCauley T, Tauler P, Muhamad AS (2012) Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports 22(3):410–417. doi:10.1111/j.1600-0838.2010.01272.x
Hanson LA, Ahlstedt S, Andersson B, Carlsson B, Cole MF, Cruz JR, Dahlgren U, Ericsson TH, Jalil F, Khan SR, Mellander L, Schneerson R, Eden CS, Soderstrom T, Wadsworth C (1983) Mucosal immunity. Ann NY Acad Sci 409:1–21
Laube DM, Yim S, Ryan LK, Kisich KO, Diamond G (2006) Antimicrobial peptides in the airway. Curr Top Microbiol Immunol 306:153–182
Leicht CA, Bishop NC, Goosey-Tolfrey VL (2011) Mucosal immune responses to treadmill exercise in elite wheelchair athletes. Med Sci Sports Exerc 43(8):1414–1421. doi:10.1249/MSS.0b013e31820ac959
Li TL, Gleeson M (2004) The effect of single and repeated bouts of prolonged cycling and circadian variation on saliva flow rate, immunoglobulin A and alpha-amylase responses. J Sports Sci 22(11–12):1015–1024
Markkanen H, Syrjanen SM, Alakuijala P (1986) Salivary IgA, lysozyme and beta 2-microglobulin in periodontal disease. Scand J Dent Res 94(2):115–120
McCormick TS, Weinberg A (2010) Epithelial cell-derived antimicrobial peptides are multifunctional agents that bridge innate and adaptive immunity. Periodontology 54(1):195–206. doi:10.1111/j.1600-0757.2010.00373.x
Nater UM, Rohleder N (2009) Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 34(4):486–496. doi:10.1016/j.psyneuen.2009.01.014
Neville V, Gleeson M, Folland JP (2008) Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med Sci Sports Exerc 40(7):1228–1236. doi:10.1249/MSS.0b013e31816be9c3
Nieman DC (2000) Is infection risk linked to exercise workload? Med Sci Sports Exerc 32(7 Suppl):S406–S411
Nieman DC, Henson DA, Dumke CL, Lind RH, Shooter LR, Gross SJ (2006) Relationship between salivary IgA secretion and upper respiratory tract infection following a 160-km race. J Sports Med Phys Fit 46(1):158–162
Orsi N (2004) The antimicrobial activity of lactoferrin: current status and perspectives. Biometals 17(3):189–196
Papacosta E, Nassis GP (2011) Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J Sci Med Sport 14(5):424–434. doi:10.1016/j.jsams.2011.03.004
Purvis D, Gonsalves S, Deuster PA (2010) Physiological and psychological fatigue in extreme conditions: overtraining and elite athletes. PM R 2(5):442–450. doi:10.1016/j.pmrj.2010.03.025
Putsep K, Carlsson G, Boman HG, Andersson M (2002) Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet 360(9340):1144–1149. doi:10.1016/S0140-6736(02)11201-3
Raczak G, Danilowicz-Szymanowicz L, Kobuszewska-Chwirot M, Ratkowski W, Figura-Chmielewska M, Szwoch M (2006) Long-term exercise training improves autonomic nervous system profile in professional runners. Kardiol Pol 64(2):135–140 discussion 141-132
Radek K, Gallo R (2007) Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol 29(1):27–43
Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS (2007) American college of sports medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39(2):377–390. doi:10.1249/mss.0b013e31802ca597
Steerenberg PA, van Asperen IA, van Nieuw Amerongen A, Biewenga A, Mol D, Medema GJ (1997) Salivary levels of immunoglobulin A in triathletes. Eur J Oral Sci 105(4):305–309
Tanida T, Okamoto T, Okamoto A, Wang H, Hamada T, Ueta E, Osaki T (2003) Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. J Oral Pathol Med 32(10):586–594
Tao R, Jurevic RJ, Coulton KK, Tsutsui MT, Roberts MC, Kimball JR, Wells N, Berndt J, Dale BA (2005) Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob Agents Chemother 49(9):3883–3888. doi:10.1128/AAC.49.9.3883-3888.2005
Tenovuo JO (1989) Human saliva : clinical chemistry and microbiology. CRC Press, Boca Raton
Tomasi TB, Trudeau FB, Czerwinski D, Erredge S (1982) Immune parameters in athletes before and after strenuous exercise. J Clin Immunol 2(3):173–178
Ueta E, Tanida T, Doi S, Osaki T (2000) Regulation of Candida albicans growth and adhesion by saliva. J Lab Clin Med 136(1):66–73. doi:10.1067/mlc.2000.107304
Usui T, Yoshikawa T, Orita K, Ueda SY, Katsura Y, Fujimoto S, Yoshimura M (2011) Changes in salivary antimicrobial peptides, immunoglobulin A and cortisol after prolonged strenuous exercise. Eur J Appl Physiol 111(9):2005–2014. doi:10.1007/s00421-011-1830-6
Walsh NP, Blannin AK, Clark AM, Cook L, Robson PJ, Gleeson M (1999) The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J Sports Sci 17(2):129–134. doi:10.1080/026404199366226
Weltman A (1995) The blood lactate response to exercise. Human Kinetics, Champaign
West NP, Pyne DB, Renshaw G, Cripps AW (2006) Antimicrobial peptides and proteins, exercise and innate mucosal immunity. FEMS Immunol Med Microbiol 48(3):293–304. doi:10.1111/j.1574-695X.2006.00132.x
West NP, Pyne DB, Kyd JM, Renshaw GM, Fricker PA, Cripps AW (2010) The effect of exercise on innate mucosal immunity. Br J Sports Med 44(4):227–231. doi:10.1136/bjsm.2008.046532
Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A (2008) Catecholamines and the effects of exercise, training and gender. Sports Med 38(5):401–423
Acknowledgments
This work was supported by NASA Grant NNX12AB48G to R.J. Simpson.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Flueck.
Rights and permissions
About this article
Cite this article
Kunz, H., Bishop, N.C., Spielmann, G. et al. Fitness level impacts salivary antimicrobial protein responses to a single bout of cycling exercise. Eur J Appl Physiol 115, 1015–1027 (2015). https://doi.org/10.1007/s00421-014-3082-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-3082-8