Abstract
There are many factors in mucosal secretions that contribute to innate immunity and the ‘first line of defence’ at mucosal surfaces. Few studies, however, have investigated the effects of exercise on many of these ‘defence’ factors. The aim of the present study was to determine the acute effects of prolonged exercise on salivary levels of selected antimicrobial peptides (AMP) that have not yet been studied in response to exercise (HNP1–3 and LL-37) in addition to immunoglobulin A (IgA). A secondary objective was to assess the effects of exercise on saliva antibacterial capacity. Twelve active men exercised on a cycle ergometer for 2.5 h at ~60% of maximal oxygen uptake. Unstimulated whole saliva samples were obtained before and after exercise. There was a significant decrease (P < 0.05) in salivary IgA:osmolality ratio, following exercise, but IgA concentration and secretion rate were unaltered. Salivary HNP1–3 and LL-37 concentrations (P < 0.01 and P < 0.05, respectively), concentration:osmolality ratios (P < 0.01) and secretion rates (P < 0.01) all increased following exercise. Salivary antibacterial capacity (against E. coli) did not change. The increased concentration of AMPs in saliva may confer some benefit to the ‘first line of defence’ and could result from synergistic compensation within the mucosal immune system and/or airway inflammation and epithelial damage. Further study is required to determine the significance of such changes on the overall ‘defence’ capacity of saliva and how this influences the overall risk for infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the effects of exercise on mucosal immunity have been extensively studied over the past 20 years many previous investigations have focused only on salivary immunoglobulins, especially immunoglobulin A (IgA). This is not surprising given that IgA is the predominant immunoglobulin in saliva (Gleeson and Pyne 2000) and the relationship between this aspect of mucosal immunity and the incidence of upper respiratory illness (URI) symptoms that is often reported in the literature (Fahlman and Engels 2005; Gleeson et al. 1999; Gleeson 2000; Mackinnon and Jenkins 1993; Neville et al. 2008). However, there are many other factors present in mucosal secretions (including saliva), which also serve to protect mucosal surfaces and contribute to innate host defence, such as the antimicrobial peptides and proteins (AMPs) (Allgrove et al. 2008; Singh et al. 2000; West et al. 2006). Salivary AMPs have attracted considerable interest in recent dentistry and oral hygiene research, in which associations have been observed between AMP levels and oral health and infection (Dale et al. 2006; Pütsep et al. 2002; Tanida et al. 2003; Tao et al. 2005). However, the relationships between exercise, salivary AMPs, IgA and URI risk have not yet been explored. Therefore, research into the acute effects of exercise on salivary AMP responses may serve as an important step in developing our understanding of such relationships.
In general, AMPs have broad-spectrum antimicrobial, antifungal and antiviral properties (Radek and Gallo 2007) and are mainly produced by phagocytes and epithelial cells. Two major classifications of AMPs are the cathelicidins and defensins. There are six human α-defensins subtypes; human neutrophil peptides 1–6 (HNP 1–6). HNPs 1–4 are found in the azurophilic granules of neutrophils and HNP 1–3 make up 50% of the azurophilic protein content [and are also found in B cells and natural killer (NK) cells]. In neutrophils, the α-defensins play a role in the oxygen-independent killing of phagocytosed microorganisms (De Smet and Contreras 2005). Human cathelicidin is a 37 amino acid peptide, starting with 2 leucine residues (hence the name, LL-37), that is expressed in neutrophils, epithelial cells (Bals 2000; De Smet and Contreras 2005), and salivary glands (Murakami et al. 2002); it can be detected in whole but not parotid saliva (Bachrach et al. 2006). LL-37 may contribute to host defence due to both antimicrobial and immunomodulatory functions.
The AMPs are major effector substances of innate immunity (Bals 2000) contributing to overall innate immunity, working synergistically with each other and other aspects of the innate defence system (De Smet and Contreras 2005). Indeed, a recent review by Radek and Gallo (2007) concluded that AMPs form an important part of the “synergistic arsenal” of substances that act as a first line of host defence against infection. Animal models have indicated that AMPs are crucial for both the prevention and clearance of infection (Bowdish et al. 2005a). However, there is limited research regarding the way that exercise affects the salivary concentration and/or secretion of most AMPs (Allgrove et al. 2008; West et al. 2006). Generally, the magnitude and direction of any exercise-induced alteration in immunity is dependent on the intensity, duration, frequency and chronicity of the exercise (Hoffman-Goetz and Pedersen 1994). A few recent studies have examined salivary lysozyme, chromogranin A and lactoferrin responses to acute exercise of up to ~40 min duration (Allgrove et al. 2008; West et al. 2008) and to chronic endurance training (West et al. 2008). Allgrove et al. (2008) observed a significant increase in salivary lysozyme and chromogranin A secretion rate immediately after approximately 22 min of incremental ‘exhaustive’ exercise and ‘high-intensity’ exercise (75% \( \dot{V} \)O2max) but not after approximately 22 min of ‘lower-intensity’ exercise (50% \( \dot{V} \)O2max), whereas salivary IgA secretion rate was increased only after the exhaustive incremental exercise bout. West et al. (2008) compared a group of 17 elite rowers with a relatively sedentary control group, over a 5-month period from the beginning of their ‘competitive’ season. They observed lower salivary lactoferrin in the rowers at the beginning and mid-point (~60% lower), but not the end of the study period (at this time salivary lactoferrin was ~50% lower in the rowers but this failed to reach statistical significance). Salivary lysozyme concentration was only lower by trend in the rowers, compared with the control group. West et al. (2008) also assessed the acute responses of salivary lysozyme and lactoferrin, in a subset of 11 subjects. The exercise was a discontinuous, submaximal, incremental rowing ergometer test (up to a maximum duration of 28 min of exercise) followed, after 20 min of rest, by a 2,000 m maximal effort ‘time-trial’. Salivary lysozyme and lactoferrin did not increase significantly after the submaximal part of the test but increased significantly after the ‘maximal’ time-trial, which is in agreement with the findings of Allgrove et al. (2008).
As mentioned previously, the acute exercise-induced alterations in immunity are largely influenced by the intensity and duration of exercise, but no previous research (to our knowledge) has investigated the effects of prolonged (i.e. >40 min duration) acute exercise, which may pose a greater ‘threat’ to immunity, on salivary AMP responses. Therefore, the main aim of the present study was to investigate the acute effects of prolonged exercise on salivary responses of the neutrophil α-defensins, HNP1–3, the human cathelicidin, LL-37 and IgA. A secondary objective was to assess the antibacterial capacity of saliva at rest and following exercise.
Methods
Ethics approval was obtained from the University Research Ethics Committee. Subjects were informed of the experimental procedures (verbally and in writing) and gave their written informed consent. Subjects also completed a medical questionnaire before participating in each test. Pilot data were used to estimate that a sample of 12 has >80% power to detect differences between pre- and post-exercise measures, using a 2-tailed paired t test, for the primary variables: salivary LL-37 and HNP 1–3 concentration, concentration:osmolality ratio, and secretion rate. That is, assuming a difference of 319 ± 168 μg L−1 (>99% power), 3.73 ± 2.4 μg mOsmol−1 (>99% power), and 75.3 ± 73.3 ng min−1 (89% power), for HNP1–3 concentration, concentration:osmolality ratio, and secretion rate, respectively and 4.33 ± 1.40 μg L−1 (>99% power), 0.053 ± 0.024 μg mOsmol−1 (>99% power), and 1.25 ± 0.98 ng min−1 (98% power), for LL-37 concentration, concentration:osmolality ratio, and secretion rate, respectively. The pilot study (n = 6) also revealed no significant diurnal variation, in any of the measures, over a 2.5 h period in the morning (commencing at 09:00) coinciding with the pre- and post-exercise measures during the main study trials. For example, salivary IgA concentration was 189 ± 55 and 159 ± 41 mg L−1; HNP1–3 concentration was 186 ± 214 and 190 ± 212 μg L−1; LL-37 concentration was 1.35 ± 0.44 and 1.45 ± 0.52 μg L−1; and antibacterial activity (percentage inhibition of E. coli) was 41.4 ± 20.2 and 45.7 ± 15.9% inhibition at the measurement times coinciding with the pre- and post-exercise times, respectively.
Subjects
Twelve healthy recreationally active men (age 23.9 ± 7.6 years, body mass 71.5 ± 6.9 kg, \( \dot{V} \)O2max 54.1 ± 7.6 mL min−1 kg−1, power output at \( \dot{V} \)O2max 345 ± 50 W; means ± standard deviation) participated in this study.
Testing protocols
All subjects completed two exercise bouts; a preliminary trial (\( \dot{V} \)O2max determination) and a main trial. For \( \dot{V} \)O2max determination, subjects performed a continuous incremental exercise test, with a 30 W min−1 ramp rate (ramp test), to volitional exhaustion on an electromagnetically braked cycle ergometer (Lode Excalibur Sport, The Netherlands) to determine maximal oxygen uptake and the gas exchange threshold (GET), as previously described (Vanhatalo et al. 2007). Expired gas was analyzed throughout the ramp test using a Jaeger Oxycon Pro (Hoechberg, Germany) online breath-by-breath gas analysis system.
Main trials
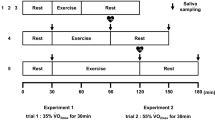
Subjects were instructed to consume 500 mL of water 2 h pre-exercise before arrival at the laboratory at ~08:45, on the morning of the main trial, after an overnight fast of at least 10 h. Subjects sat quietly in the laboratory before beginning exercise at ~09:00 for 2.5 h. Subjects exercised at GET plus 15% of the difference between GET and maximal oxygen uptake (i.e. 15% delta, see Eq. 1), which was equivalent to between 55 and 65% \( \dot{V} \)O2max for the subjects in this study.
e.g.
Expired gas was analyzed, using an online gas analysis system as previously described, for 2-min periods starting from the 13th, 28th, 43rd, 58th, 88th, 118th and 143rd min of exercise. Heart rate, measured with a telemetric heart rate monitor (Polar S610, Polar Electro Oy, Kempele, Finland), and rating of perceived exertion (RPE) were recorded every 15 min during exercise. Subjects were given 2 mL kg−1 body mass of a beverage (a low calorie lemon flavoured squash with artificial sweetener, providing 21 kJ energy, 0.1 g protein, 1.1 g carbohydrate, trace fat and trace sodium per L of solution) on commencement of, and every 15 min during, exercise. This was equivalent to ~1.6 L of beverage, for the average subject, over the course of the 2.5 h trial.
Subjects were all non-smokers and were required to abstain from alcohol, caffeine and strenuous activity for 48 h prior to the main trial. It was also stipulated that subjects should not take any mineral or vitamin supplement or any other dietary supplements during and for the 4 weeks before the study.
Saliva samples
Saliva samples were collected as previously described (Li and Gleeson 2004), immediately before (Pre) and within 5 min after completing the exercise bout (Post). Briefly, timed, unstimulated whole saliva was obtained whilst subjects were seated, with the head tilted slightly forward and making minimal orofacial movement. They were asked to swallow to empty the mouth, before whole saliva was collected by passive dribble into a pre-weighed sterile tube for a period of 2 min. Subjects were not allowed to drink for at least 10 min prior to each sample. The tube was re-weighed after collection of the sample so that saliva volume (and hence flow rate) could be estimated. Tubes were weighed to the nearest 0.1 mg (saliva density was assumed to be 1.00 g ml−1) and aliquots were frozen, at −80°C, for later analysis.
Analytical methods
All samples were thawed only once prior to analysis. After thawing, at room temperature, samples were centrifuged at 15,000×g for 2 min to pellet debris and mucins (which are precipitated from the solution after a freeze–thaw cycle) and obtain a clear supernatant. Saliva osmolality was determined using a freezing point depression osmometer (Osmomat 030, Gonotec, GbBH, Berlin, Germany) calibrated with 300 mOsmol kg−1 saline solution, in accordance with the manufacturer’s instructions, and checked with a reference solution (50 mOsmol kg−1) to ensure measurements were accurate.
Saliva IgA, LL-37 and HNP1–3
Aliquots of saliva were analyzed for the determination of IgA (Salimetrics, USA), LL-37 and HNP1–3 concentrations (Hycult biotechnology, The Netherlands) in duplicate using commercially available enzyme-linked immunosorbent assay (ELISA) kits. HNP1, 2 and 3 are structurally similar and the ELISA method does not discriminate between them and so simply measures the total concentration of all three together (HNP1–3). The intra-assay coefficient of variation was 7.4, 4.1 and 3.4% for the IgA, LL-37 and HNP1–3 assays, respectively. The minimum detection limit for the IgA, LL-37 and HNP1–3 assays is 2.5 mg L−1, 0.14 μg L−1, and 50 ng L−1, respectively. However, samples were diluted prior to analysis (5× for IgA and LL-37 and 1,000× for HNP1–3) giving an actual minimum detection of 12.5 mg L−1, 0.7 and 50 μg L−1 for the IgA, LL-37 and HNP1–3 assays, respectively.
In vitro antibacterial assay against E. coli
Salivary antibacterial capacity against E. coli, before and after exercise, was assessed in a subset of eight subjects. This analysis was carried out by a commercial laboratory (BluScientific Test Data, Glasgow Caledonian University, Scotland, UK). Thawed and cleared saliva (as previously described) was used in the assays, with a contact time of 60 min at 37°C. The challenged bacteria (E. coli) were in the range of 1.5–5.0 × 106 colony forming units (CFU). Following contact with the saliva (or isotonic saline as the control), samples were diluted to 10−2 and 10−4, in tryptone saline, plated out onto tryptone soya agar in duplicate for each dilution, incubated overnight at 37°C then counted. Results were expressed as percentage mortality, in comparison to the isotonic saline control (i.e. % inhibition).
Data analysis
Statistical analysis was carried out using the statistical computer software package SPSS (v16.00; SPSS Inc., Chicago, IL, USA). All data, except salivary IgA secretion rate, HNP1–3 concentration, secretion rate and concentration:osmolality ratio, were normally distributed. Data that were not normally distributed were normalised with log transformation before analysis. Pre- and post-exercise measures were compared with 2-tailed paired t tests. All results are presented as mean ± standard deviation.
Results
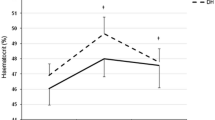
The mean physiological responses over the 2.5 h exercise bout are illustrated in Table 1. Absolute saliva IgA concentration and the estimated secretion rate (Table 2) were unaffected by the exercise bout (P > 0.05). There was a significant post-exercise decrease in the salivary IgA:osmolality ratio (P < 0.05).
There were significant post-exercise increases in salivary HNP1–3 concentration (P < 0.01), HNP1–3:osmolality ratio (P < 0.01), and HNP1–3 secretion rate (P < 0.01). There were significant post-exercise increases in salivary LL-37 concentration (P < 0.05), LL-37:osmolality ratio (P < 0.01), and LL-37 secretion rate (P < 0.01) (Table 2; Fig. 1).
The saliva samples exhibited a greater inhibitory effect against E. coli in the CFU assay (42.5 ± 10.2% inhibition of CFU) compared to the saline control, but there was no difference between the inhibitory effects of the pre- (42.5 ± 10.2% inhibition) and post-exercise (48.1 ± 20.8% inhibition) saliva, regardless of whether the values were expressed with (P = 0.710) or without (P = 0.371) normalisation for changes in saliva osmolality.
Discussion
The main findings of this study were that salivary IgA:osmolality ratio decreased immediately after prolonged exercise, although the concentration and secretion rate did not change significantly. Salivary HNP1–3 and LL-37, on the other hand, increased following exercise regardless of whether values were expressed as an absolute concentration, relative to osmolality or as the secretion rate. Salivary antibacterial capacity (against E. coli) did not change significantly after exercise.
The salivary IgA:osmolality ratio results are in accordance with previous studies suggesting depressed mucosal immunity induced by prolonged exercise (Krzywkowski et al. 2001; Nieman et al. 2002; Steerenberg et al. 1997), although others have reported no change in salivary IgA responses following prolonged exercise of up to 2 h or more in duration (e.g. Blannin et al. 1998; Li and Gleeson 2004). The post-exercise increases in salivary HNP1–3 and LL-37 are in line with the findings from previous studies that have employed shorter duration acute exercise, on other salivary antimicrobial peptides and proteins (Allgrove et al. 2008; West et al. 2008). The fact that some aspects of mucosal (salivary) innate immunity were seen to increase following exercise may indicate that some innate immune factors (i.e. AMPs) increase to counteract or ‘compensate’ for a decrease in other salivary immune factors. Similar synergistic compensation is evident in patients with low salivary IgA, whereby (high) salivary IgM can ‘compensate’ for an IgA deficiency (meaning URI risk/incidence is normal), whereas those who are also deficient in IgM suffer a high rate of URI (Mellander et al. 1986).
It has been demonstrated that lactoferrin and lysozyme work synergistically in airway surface liquid (Singh et al. 2000), whereby the combined antimicrobial activity is greater than the sum of the individual components. Likewise, lactoferrin and IgA in breast milk have been shown to work synergistically against enteric bacteria that are not killed by either substance alone (Rogers and Synge 1978). Singh et al. (2000) observed that some AMPs in combination, such as lysozyme and LL-37, demonstrate additive effects, when used in optimal concentrations, although when sub-inhibitory concentrations of LL-37 were tested there was synergy between LL-37 and lysozyme. This is relevant because the concentration of many AMPs, such as LL-37 and HNP1–3, in mucosal secretions such as saliva, are below minimum antimicrobial concentrations reported from in vitro studies using isolated peptides, supporting the idea that the compensation may occur in mucosal fluids due to synergism between substances. Further study is warranted to determine whether similar ‘compensation’ is provided by the AMPs in saliva and if so, under which circumstances. Recent studies have shown that a decrease in salivary AMPs is associated with increased infection incidence/oral disease (Bals 2000; Dale et al. 2006; Pütsep et al. 2002; Tanida et al. 2003; Tao et al. 2005), supporting the idea that changes in salivary levels may have an important impact on mucosal immunity. Whether this is relevant to protection against URI is yet to be determined. In general, AMPs do have broad-spectrum antimicrobial and antiviral properties and have been shown, in relatively high concentrations, to be effective against some pathogens which can potentially cause respiratory tract infection such as Staphylococcus aureus, Legionella pneumophila, adenovirus and respiratory syncytial virus (De Smet and Contreras 2005; Radek and Gallo, 2007; Tenovuo 2002). However, the idea that an increased concentration of these factors would enhance host protection against infection (such as URI) is not supported by previous research that has reported an increased risk for URI after prolonged or strenuous exercise (Fahlman and Engels 2005; Gleeson et al. 1999; Gleeson 2000; Mackinnon and Jenkins 1993; Neville et al. 2008). However, to fully understand the relationships between exercise, salivary AMPs, IgA and URI risk, further study is required on the effects of different intensities and durations of exercise (which may cause greater effects on other immune parameters, such as IgA, than in the present study) on a wider variety of AMPs and with measurements also made during the post-exercise recovery period.
Bals (2000) has noted that it is difficult to determine the contribution of individual AMPs to host defence (or extrapolate in vivo function from in vitro assays with isolated substances), and recommends against functional ‘antimicrobial’ analysis of purified AMPs, as this does not reflect the complexity of component interactions and synergism between substances. Additionally, many AMPs have an immunomodulatory role, in addition to possessing direct antimicrobial activity (Tjabringa et al. 2005). For example, AMPs have been shown to induce leukocyte cytokine secretion, stimulate chemotaxis and participate in the remodelling of injured epithelia (Bowdish et al. 2005a; Ganz 2003). The immunomodulatory properties of LL-37 were demonstrated in a study by Bowdish et al. (2005b) in which synthetically synthesised LL-37 peptides (that do not possess direct antimicrobial activity) still provided protection against infection in animal models, suggesting an additional or alternative role for LL-37 in immunity (Bowdish et al. 2005b). Alpha-defensins also possess immunomodulatory properties; inducing the chemotaxis of monocytes and T cells, the modulation of cell proliferation and an antibody response, and the inhibition of complement activation, of fibrinolysis and of the activity of serpin family members (Bals 2000; Ganz 2003).
The observation of a post-exercise increase in salivary HNP1–3 and LL-37 may be related, to some extent, to an exercise-induced inflammatory response. It is clear that prolonged strenuous dynamic exercise may induce airway inflammation and damage to airway epithelial cells. This has been suggested as one mechanism to account for increased URI symptoms, in the absence of detectable pathogens (the non-infective hypothesis of URI) in some athletes (Bermon 2007; Gleeson 2007). Airway inflammation may induce an increase in neutrophil infiltration into the oral mucosa. West et al. (2008) have proposed this as a possible explanation for their observation of an exercise-induced increase in salivary lysozyme and lactoferrin. It is possible that these mechanisms also contribute to the increase in salivary AMPs observed in the present study. Although it was not possible, in the present study, to determine precisely from where the increased salivary LL-37 and HNP1–3 were derived following exercise it is likely that neutrophils and airway epithelial cells are major sources. Preformed α-defensins are stored in azurophilic granules of neutrophils (Radek and Gallo 2007) and contribute about 50% of the protein content in these granules. LL-37 is derived by cleavage from the human CAP18 protein and is also expressed in neutrophils, monocytes, NK cells, some lymphocytes, and epithelial cells (De Smet and Contreras 2005) but has also been identified in salivary glands (Murakami et al. 2002). Neutrophils can be detected in many mucosal secretions, including human saliva (e.g. Tanida et al. 2001). Müns (1994) demonstrated prolonged strenuous exercise to induce a significant (twofold) increase in the number of neutrophils in mucosal secretions (nasal lavage fluid in this case). Additionally, an increase in blood neutrophil count can cause an increase in the salivary concentration of some AMPs, for example, neutrophilia was shown to lead to increased levels of α-defensins in saliva (Shiomi et al. 1993). It is well known that neutrophilia occurs immediately after prolonged exercise; in previous studies from our laboratory that have employed the same exercise protocol as in the present study, we have consistently observed up to a sixfold post-exercise increase in blood neutrophil count (Davison and Gleeson 2005, 2006, 2007; Davison et al. 2007). It seems reasonable, therefore, to assume similar responses would have occurred in the present study. Neutrophils continually migrate into the saliva/oral fluids from the blood, predominantly via gingival crevices (Bender et al. 2006; Lukac et al. 2003) so an increase in neutrophil numbers in saliva is likely to occur when blood neutrophils counts are increased to such an extent although further study is required to confirm this. Furthermore, exercise has also been shown to induce neutrophil priming and activation (Pyne 1994; Smith and Pyne 1997), which could result in increased secretion, into saliva, of HNP1–3 and LL-37 from local neutrophils.
As the variety of AMPs is so numerous (i.e. at least 700 have been identified to date), and some are difficult to measure, it may be more appropriate to assess the net effects of exercise on the protective functions of the mucosal immune system. We attempted to gain a preliminary insight into this by assessing the effects of exercise on total antimicrobial capacity (against E. coli) of saliva, in a subset of eight subjects. There was a significant inhibition by saliva of E. coli CFU, but no difference between pre- and post-exercise saliva samples. This would seem to support the synergistic compensation theory. However, this method may require some minor modification to give a better indication of overall ‘defence’ capacity of the saliva. Modifications that should be considered in any future study employing similar methods could include: the use of a target pathogen that is more relevant to URI; and the use of fresh saliva in the assay. Saliva samples were frozen prior to analysis, in the present study, meaning that the mucins were precipitated from the sample upon thawing. The antimicrobial capacity that was measured, therefore, was the mucin-independent antimicrobial capacity. It must be acknowledged, however, that mucins also have antimicrobial properties and contribute to the synergistic mucosal defences (often working in complex with AMPs) (Amerongen and Veerman 2002), so fresh saliva may be more appropriate. Nevertheless, the assay employed in the present study could provide a useful indication of salivary antimicrobial capacity and may be sensitive to any changes in overall salivary antimicrobial capacity, that are caused by changes in the other salivary ‘defence’ factors (such as AMPs for example). It will be interesting, therefore, to see whether assessment of overall ‘defence’ capacity (using pathogens more relevant to URI in the assay) in future studies can enhance the assessment of URI risk in athletes (i.e. in combination with other, established, risk factors such as salivary IgA).
Study limitations
The addition of a resting control trial would have been beneficial, although no significant changes were observed over the same time period in a resting control pilot study (n = 6). We are confident, therefore, that the comparisons between pre- and post-exercise measures in the present study are worthwhile and real. Larger (or different) changes may be apparent following higher intensity exercise and/or in the recovery period immediately after exercise. However, the aim of this study was to first determine whether exercise alters the salivary levels of these AMPs. As we have established this here, it is anticipated that future studies which address these potential limitations (i.e. with the inclusion of additional points of measurement during recovery, and/or using various intensities of exercise) may provide useful in determining the relationships between salivary AMPs and URI risk. Furthermore, the use of ‘fresh’ saliva and pathogens relevant to URI in the antimicrobial capacity assay may be more appropriate (as previously discussed).
Conclusions
We have observed a decrease in salivary IgA:osmolality ratio and an increase in the concentration and secretion of HNP1–3 and LL-37 into saliva following acute prolonged exercise. The increase in salivary AMPs may result from synergistic compensation within the mucosal immune system, and/or exercise-induced airway inflammation and epithelial damage, or may be part of the normal stress response. Regardless of the mechanism or reasons for these effects, it is possible that the post-exercise increase in salivary AMPs confers some benefit to the ‘first line of defence’ of the mucosal immune system. However, further study is required in order to determine the effects of prolonged exercise on a wider variety of immune parameters within the saliva and how this influences the overall ‘defence’ capacity of the saliva and/or URI risk.
References
Allgrove JE, Gomes E, Hough J, Gleeson M (2008) Effect of exercise intensity on salivary antimicrobial proteins and markers of stress in active men. J Sports Sci 26:653–661. doi:10.1080/02640410701716790
Amerongen AV, Veerman EC (2002) Saliva––the defender of the oral cavity. Oral Dis 8:12–22. doi:10.1034/j.1601-0825.2002.1o816.x
Bachrach G, Chaushu G, Zigmond M, Yefenof E, Stabholz A, Shapira J, Merrick J, Chaushu S (2006) Salivary LL-37 secretion in individuals with Down syndrome is normal. J Dent Res 85:933–936. doi:10.1177/154405910608501012
Bals R (2000) Epithelial antimicrobial peptides in host defence against infection. Respir Res 1:141–150. doi:10.1186/rr25
Bender JS, Thang H, Glogauer M (2006) Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. J Periodontal Res 41:214–220. doi:10.1111/j.1600-0765.2005.00861.x
Bermon S (2007) Airway inflammation and upper respiratory tract infection in athletes: is there a link? Exerc Immunol Rev 13:6–14
Blannin AK, Robson PJ, Walsh NP, Clark AM, Glennon L, Gleeson M (1998) The effect of exercising to exhaustion at different intensities on saliva immunoglobulin A, protein and electrolyte secretion. Int J Sports Med 19:547–552. doi:10.1055/s-2007-971958
Bowdish DM, Davidson DJ, Hancock RE (2005a) A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci 6:35–51. doi:10.2174/1389203053027494
Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE (2005b) Impact of LL-37 on anti-infective immunity. J Leukoc Biol 77:451–459. doi:10.1189/jlb.0704380
Dale BA, Tao R, Kimball JR, Jurevic RJ (2006) Oral antimicrobial peptides and biological control of caries. BMC Oral Health 6:S13. doi:10.1186/1472-6831-6-S1-S13
Davison G, Gleeson M (2005) Influence of acute vitamin C and/or carbohydrate ingestion on hormonal, cytokine, and immune responses to prolonged exercise. Int J Sport Nutr Exerc Metab 15:465–479
Davison G, Gleeson M (2006) The effect of 2 weeks vitamin C supplementation on immunoendocrine responses to 2.5 h cycling exercise in man. Eur J Appl Physiol 97:454–461. doi:10.1007/s00421-006-0196-7
Davison G, Gleeson M (2007) The effects of acute vitamin C supplementation on cortisol, interleukin-6, and neutrophil responses to prolonged cycling exercise. Eur J Sport Sci 7:15–25. doi:10.1080/17461390701197734
Davison G, Gleeson M, Phillips S (2007) Antioxidant supplementation and immunoendocrine responses to prolonged exercise. Med Sci Sports Exerc 39:645–652. doi:10.1249/mss.0b013e318031303d
De Smet K, Contreras R (2005) Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 27:1337–1347. doi:10.1007/s10529-005-0936-5
Fahlman MM, Engels HJ (2005) Mucosal IgA and URTI in American collage football players: a year longitudinal study. Med Sci Sports Exerc 37:374–380. doi:10.1249/01.MSS.0000155432.67020.88
Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720. doi:10.1038/nri1180
Gleeson M (2000) Salivary mucosal immunity and respiratory illness in elite athletes. Int J Sports Med 21:S33–S43. doi:10.1055/s-2000-1450
Gleeson M (2007) Immune function in sport and exercise. J Appl Physiol 103:693–699. doi:10.1152/japplphysiol.00008.2007
Gleeson M, Pyne DB (2000) Special feature for the olympics: effects of exercise on the immune system: exercise effects on mucosal immunity. Immunol Cell Biol 78:536–544. doi:10.1046/j.1440-1711.2000.00956.x
Gleeson M, McDonald WA, Pyne DB, Cripps AW, Francis JL, Fricker PA, Clancy RL (1999) Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc 31:67–73. doi:10.1097/00005768-199901000-00012
Hoffman-Goetz L, Pedersen BK (1994) Exercise and the immune system: a model of the stress response? Immunol Today 15:382–387. doi:10.1016/0167-5699(94)90177-5
Krzywkowski K, Petersen EW, Ostrowski K, Link-Amster H, Boza J, Halkjaer-Kristensen J, Pedersen BK (2001) Effect of glutamine and protein supplementation on exercise-induced decreases in salivary IgA. J Appl Physiol 91:832–838
Li TL, Gleeson M (2004) The effect of single and repeated bouts of prolonged cycling and circadian variation on saliva flow rate, immunoglobulin A and alpha-amylase responses. J Sports Sci 22:1015–1024. doi:10.1080/02640410410001716733
Lukac J, Mravak-Stipetić M, Knezević M, Vrcek J, Sistig S, Ledinsky M, Kusić Z (2003) Phagocytic functions of salivary neutrophils in oral mucous membrane diseases. J Oral Pathol Med 32:271–274
Mackinnon LT, Jenkins DG (1993) Decreased salivary IgA after intense interval exercise before and after training. Med Sci Sports Exerc 25:678–683. doi:10.1249/00005768-199306000-00005
Mellander L, Bjørkander J, Carlsson B, Hanson LA (1986) Secretory antibodies in IgA-deficient and immunosuppressed individuals. J Clin Immunol 6:284–291. doi:10.1007/BF00917328
Müns G (1994) Effect of long-distance running on polymorphonuclear neutrophil phagocytic function of the upper airways. Int J Sports Med 15:96–99. doi:10.1055/s-2007-1021027
Murakami M, Ohtake T, Dorschner RA, Gallo RL (2002) Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res 81:845–850. doi:10.1177/154405910208101210
Neville V, Gleeson M, Folland JP (2008) Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med Sci Sports Exerc 40:1228–1236. doi:10.1249/MSS.0b013e31816be9c3
Nieman DC, Henson DA, Fagoaga OR, Utter AC, Vinci DM, Davis JM, Nehlsen-Cannarella SL (2002) Change in salivary IgA following a competitive marathon race. Int J Sports Med 23:69–75. doi:10.1055/s-2002-19375
Pütsep K, Carlsson G, Boman HG, Andersson M (2002) Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet 360:1144–1149. doi:10.1016/S0140-6736(02)11201-3
Pyne DB (1994) Regulation of neutrophil function during exercise. Sports Med 17:245–258. doi:10.2165/00007256-199417040-00005
Radek K, Gallo R (2007) Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol 29:27–43. doi:10.1007/s00281-007-0064-5
Rogers HJ, Synge C (1978) Bacteriostatic effect of human milk on Escherichia coli: the role of IgA. Immunology 34:19–28
Shiomi K, Nakazato M, Ihi T, Kanagawa K, Matsuo H, Matsukura S (1993) Establishment of radioimmunoassay for human neutrophil peptides and their increases in plasma and neutrophil in infection. Biochem Biophys Res Commun 195:1336–1344. doi:10.1006/bbrc.1993.2190
Singh PK, Tack BF, McCray PB Jr, Welsh MJ (2000) Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol 279:L799–L805
Smith JA, Pyne DB (1997) Exercise, training and neutrophil function. Exerc Immunol Rev 3:96–117
Steerenberg PA, van Asperen IA, Nieuw Amerongen A, Biewenga A, Mol D, Medema GJ (1997) Salivary levels of immunoglobulin A in triathletes. Eur J Oral Sci 105:305–309. doi:10.1111/j.1600-0722.1997.tb00245.x
Tanida T, Ueta E, Tobiume A, Hamada T, Rao F, Osaki T (2001) Influence of aging on candidal growth and adhesion regulatory agents in saliva. J Oral Pathol Med 30:328–335. doi:10.1034/j.1600-0714.2001.300602.x
Tanida T, Okamoto T, Okamoto A, Wang H, Hamada T, Ueta E, Osaki T (2003) Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. J Oral Pathol Med 32:586–594. doi:10.1034/j.1600-0714.2003.00015.x
Tao R, Jurevic RJ, Coulton KK, Tsutsui MT, Roberts MC, Kimball JR, Wells N, Berndt J, Dale BA (2005) Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob Agents Chemother 49:3883–3888. doi:10.1128/AAC.49.9.3883-3888.2005
Tenovuo J (2002) Antimicrobial agents in saliva—protection for the whole body. J Dent Res 81:807–809
Tjabringa GS, Rabe KF, Hiemstra PS (2005) The human cathelicidin LL-37: a multifunctional peptide involved in infection and inflammation in the lung. Pulm Pharmacol Ther 18:321–327. doi:10.1016/j.pupt.2005.01.001
Vanhatalo A, Doust JH, Burnley M (2007) Determination of critical power using a 3-min all-out cycling test. Med Sci Sports Exerc 39:548–555. doi:10.1249/mss.0b013e31802dd3e6
West NP, Pyne DB, Renshaw G, Cripps AW (2006) Antimicrobial peptides and proteins, exercise and innate mucosal immunity. FEMS Immunol Med Microbiol 48:293–304. doi:10.1111/j.1574-695X.2006.00132.x
West NP, Pyne DB, Kyd JM, Renshaw GM, Fricker PA, Cripps AW (2008) The effect of exercise on innate mucosal immunity. Br J Sports Med (May 22). doi:10.1136/bjsm.2008.046532
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davison, G., Allgrove, J. & Gleeson, M. Salivary antimicrobial peptides (LL-37 and alpha-defensins HNP1–3), antimicrobial and IgA responses to prolonged exercise. Eur J Appl Physiol 106, 277–284 (2009). https://doi.org/10.1007/s00421-009-1020-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1020-y