Abstract
Purpose
This study compared responses to static stretching between eccentrically damaged and non-damaged muscles.
Methods
Twelve young men performed 60 maximum knee flexor eccentric contractions of one leg, and received a 300-s continuous passive static stretching at tolerable intensity without pain to both knee flexors at 2 and 4 days after the eccentric exercise. Range of motion (ROM) and passive stiffness during knee extension, passive torque at onset of pain (PT), maximum voluntary isometric (MVC-ISO) and isokinetic concentric contraction torque (MVC-CON), and visual analogue scale (VAS) for muscle soreness were measured before, immediately after, 60 min, 2 and 4 days after exercise as well as before, immediately after, 20 and 60 min after the stretching. Changes in these variables after eccentric exercise and stretching were compared between limbs.
Results
The eccentric exercise decreased MVC-ISO, MVC-CON, ROM and PT, and increased passive stiffness and VAS (p < 0.05), suggesting that muscle damage was induced to the knee flexors. ROM and PT increased after stretching for both limbs; however, the magnitude of the increase was greater (p < 0.05) for the damaged than non-damaged limb. Passive stiffness decreased for both limbs similarly (4–7 %) at immediately after stretching (p < 0.05). Significant decreases in MVC-ISO torque (7–11 %) after stretching were observed only for the non-damaged limb (p < 0.05), but MVC-CON torque did not change after stretching for both limbs. VAS decreased for the exercised limb after stretching (p < 0.05).
Conclusions
These results suggest that the static stretching at tolerable intensity without pain produced greater positive effects on damaged than non-damaged muscles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Static stretching is performed as a part of warm-up exercise in the belief that it will reduce a risk of injuries and will improve exercise performance (Smith 1994; Woods et al. 2007; Shrier 2005). As stated by Magnusson (1998), static stretching decreases passive stiffness and increases range of motion (ROM), which are considered to be positive effects of the stretching exercise. However, recent articles documented that static stretching does not necessarily reduce injury risks, but could induce detrimental effects on muscle performance (McHugh and Cosgrave 2010; Simic et al. 2013; Kay and Blazevich 2012; Small et al. 2008). For example, Simic et al. (2013) have recently shown based on meta-analysis that pre-exercise static stretching negatively affects muscle performance when the stretch duration is longer than 45 s. Kay and Blazevich (2012) concluded in their systematic review that muscle function was decreased after a long (≥60 s) static stretching.
Possible mechanisms of the stretch-induced force loss include decreases in neural drive and peripheral force-generating capacity such as a decrease in musculotendinous stiffness and/or changes in muscle’s length–tension relationship (Ryan et al. 2008; Fowles et al. 2000). Trajano et al. (2013) have recently reported that the force loss induced by a 5-min continuous passive static stretch is mainly associated with a reduction in central drive.
It is well known that eccentric exercise induces muscle damage that is characterised by a prolonged decrease in muscle strength and ROM, development of delayed onset muscle soreness (DOMS) and swelling, and increases in blood makers such as creatine kinase (CK) activity, especially when the exercise is performed first time, or with a long-time interval from the previous exposure to the same or similar eccentric exercise (Nosaka et al. 2002; Nosaka and Sakamoto 2001; Janecki et al. 2011; Reisman et al. 2009). To attenuate the magnitude of eccentric exercise-induced muscle damage, static stretching is often performed as a prophylactic (Chen et al. 2011a) and/or a therapeutic modality (Torres et al. 2007; Reisman et al. 2009; Johansson et al. 1999); however, its effects on DOMS and other symptoms of muscle damage are equivocal. For example, Herbert et al. (2011) reviewed 12 studies and concluded that effect of stretching on DOMS was minimal. On the other hand, static stretching has been shown to be effective for improving ROM (Torres et al. 2007) and flexibility assessed by passive torque (Reisman et al. 2009) for eccentrically damaged muscle. Reisman et al. (2009) applied a 30-s static stretching at the same intensity (ankle joint was at 40° of dorsiflexion from neutral position) to the damaged and non-damaged muscle at 24 h after eccentric exercise, when DOMS was developed to triceps surae muscles. They found that the magnitude of decrease in passive torque after stretching was the same between the damaged and non-damaged muscles, despite passive torque for the damaged muscle was greater than that of the non-damaged muscle. They also found that muscle soreness for the damaged muscle was reduced by 40 % after stretching. However, no previous studies have investigated the acute effect of static stretching which is performed at the point where pain started to feel, which is a general practice (the same relative intensity between damaged and non-damaged muscles, but the absolute intensity is different—the absolute intensity is greater for the non-damaged than damaged muscle) on passive torque and muscle pain. Moreover, it has not been examined in the previous studies whether the stretch-induced force loss occurs in the same magnitude to damaged muscle as that of non-damaged muscle.
Given the above, the purpose of the present study was to investigate how muscles damaged by eccentric contractions would respond to a long-duration (300-s) static stretching at tolerable intensity without pain, which has been shown to induce force loss, reduce muscle stiffness, and increase ROM (Matsuo et al. 2013), when compared with non-damaged muscles. The responses included force, passive torque, and passive stiffness. It was previously reported that eccentrically damaged muscle became stiffer and less extensible than non-damaged muscle, and experienced DOMS (Janecki et al. 2011; Nosaka and Sakamoto 2001). Thus, it was assumed that the tolerance to passive stretch would be reduced in the muscle that was suffering from eccentric exercise-induced muscle damage, and the stretching intensity would be lower for the damaged than non-damaged muscle. It was also hypothesised that the decreases in maximum voluntary contraction (MVC) force and passive stiffness, and the increase in ROM after static stretching would be smaller for the damaged than non-damaged muscle.
Methods
Participants
Twelve healthy young men participated in this study after being informed of the purpose and protocol of the study, and provided a written informed consent that had been approved by the Human Research Ethics Committee of the Nagoya University School of Health Sciences. Participants were excluded if they had lower-extremity contracture, an operation of their back or lower extremity, or neurological disorders, if they were regularly taking analgesic or anti-inflammatory drugs, if they could completely extend their knee joints (too flexible) at the setting position as described below, and if they engaged in competitive sports or performed resistance, aerobic or flexibility training in the past 6 months. Their mean (±SD) age, height, body mass, and body mass index were 21.0 ± 0.5y, 172.9 ± 6.2 cm, 64.3 ± 6.9 kg, and 21.5 ± 1.3 kg/m2, respectively. They were asked and reminded to refrain from unaccustomed exercise or vigorous physical activity, and maintain their normal dietary habits, and not to take any anti-inflammatory drugs or nutritional supplements during the experimental period. They were instructed not to have any treatments (e.g. massage) other than that given in the study (static stretching) during the experimental period.
The number of subjects was determined by a sample size estimation using the data from a previous study (Matsuo et al. 2013) and a preliminary experiment, using G*Power software (v 3.0.10; Franz Faul, Kiel University, Kiel, Germany). The calculated effect size was 1.3 when the effect of static stretching on the knee extension ROM for the exercised limb was assumed as 50 % of that for the non-exercised limb. On the basis of the effect size, α level of 0.05, and a power (1-β) of 0.80, the minimum number of subjects was estimated to be 11. Considering a possible dropout, 12 participants were recruited.
Experimental design
The experiment was performed in a university’s laboratory, where the room temperature was maintained at 26 °C throughout the study. One leg of all participants was used as the control without eccentric exercise, and the other leg performed a bout of eccentric exercise of the knee flexors as elaborated below, but both legs received a 300-s static stretching at tolerable intensity without pain at 2 and 4 days after the eccentric exercise. The limb assigned for the eccentric exercise (dominant or non-dominant limb) was randomised among the participants. The criterion measures consisted of range of motion of passive knee extension (ROM), passive torque (PT) at onset of pain, passive stiffness of passive knee extension, maximum voluntary isometric contraction torque (MVC-ISO torque), rate of force development (RFD), maximum voluntary concentric contraction torque (MVC-CON torque), optimum angle, electromyography root mean square (RMS) during the torque measures, visual analogue scale (VAS) for muscle soreness, and static passive torque (SPT) during stretching. All participants attended a familiarisation session one day before performing eccentric exercise, in which they experienced all of the measurements. No eccentric contraction was performed in the familiarisation session, but the participants were briefed on the exercise protocol. The measurements except SPT were taken immediately before exercise, immediately after, 60 min, and 2 and 4 days after exercise, as well as before stretching, immediately after, 20 and 60 min after the stretching that was performed at 2 and 4 days after exercise. All measurements were taken at the same time of day (±1 h) for each participant between days. Changes in the dependent variables before and after the eccentric exercise, and before and after the static stretching were compared between exercised and non-exercised (control) limbs.
Eccentric exercise
All participants performed 6 sets of 10 maximal eccentric contractions of the unilateral knee flexors on an isokinetic dynamometer (Primus RS, BTE Technologies, Hanover, MD, USA) after 5 min of ergometer cycling at a load of 90 W. The eccentric exercise protocol was the same as that of a previous study (Chen et al. 2011a). In brief, each subject lay prone on the platform of the dynamometer, and the upper and lower back regions and the thigh of the exercised limb were stabilised with Velcro straps. The knee joint of the exercised limb was aligned with the axis of rotation of the isokinetic dynamometer, and the ankle of the exercised limb was strapped to the lever arm attachment of the dynamometer. The participants were instructed to contract the knee flexors to resist the knee extending action of the dynamometer that moved the knee joint from a flexed (130°) to an extended position (0°) at an angular velocity of 30°/s. After each eccentric contraction, the lever arm passively returned the knee joint to the starting position at 10°/s, which gave a 13-s rest between contractions. This was repeated 10 times for each set, and a 1-min rest was given between sets to complete six sets. The participants received strong verbal encouragement during each eccentric contraction to generate maximum force. The torque and angle signals from the isokinetic dynamometer during eccentric exercise were A/D converted (PowerLab 4/20T, ADInstruments, NSW, Australia) and stored in a PC (Dynabook Satellite J50, Toshiba, Tokyo, Japan).
Static stretching
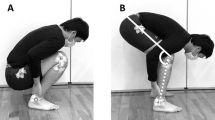
A 300-s continuous static stretching was performed at 2 and 4 days after eccentric exercise for both legs. These time points were chosen, since they were considered to represent the time point when symptoms of muscle damage (e.g. muscle soreness) are peaked (2 days) and the symptoms are subsided but still remained (4 days). This allowed us to examine the effect of stretching on two different conditions of eccentrically damaged muscles. The present study used a sitting position in which the hip joint was flexed (Fig. 1a) that has been shown to efficiently stretch the hamstrings (Matsuo et al. 2013). The subjects were seated on a chair of the isokinetic dynamometer with the seat tilted maximally (35° from horizontal position) and a cushion was inserted between the trunk and the backrest, which set the angle between the seat and the back at approximately 60°. The subject’s chest, pelvis and thigh of the stretched leg were stabilised with Velcro straps. The knee joint was aligned with the axis of rotation of the isokinetic dynamometer, and the lever arm attachment was placed at proximal to the malleolus medialis and stabilised with Velcro straps. In this position, the average angles of hip and knee flexion were 110.0° ± 2.0° and 113.4° ± 1.7°, respectively. The hamstrings of both legs were stretched at this position by the isokinetic dynamometer separately, and the order of stretching for the exercised and non-exercised limbs was randomised among the participants, but the same order was maintained between days for each participant. The range of motion of the dynamometer was set at maximum tolerable intensity without stretching pain (Fig. 1b), and this position was kept for 300 s based on a previous study (Matsuo et al. 2013). Muscle activities of medial and lateral hamstrings during the stretching were monitored and recorded by a Biomonitor ME6000 (Mega Electronics, Kuopio, Finland) with a sampling frequency of 1 kHz. The details are shown below in the “Electromyogram” section below.
Dependent variables
All of the dependent variables except SPT were used to assess muscle damage, and all variables were also used to assess the acute effect of the static stretching. The measurements were taken from both limbs in the same order of the stretching such that the order of the leg was randomised among the subjects, but the same order was maintained for each subject. The sequence of the measurements was fixed as follows: muscle soreness, torque–angle curve (ROM, PT at onset of pain, and passive stiffness), MVC-ISO torque and MVC-CON torque for all time points. The details of each measure are provided below.
Static passive torque (SPT)
Passive torque produced by the hamstrings during static stretching was measured continuously by the isokinetic dynamometer, and the torque signal was transferred to a PowerLab (ADInstruments, NSW, Australia) and stored in a PC (Dynabook Satellite J50, Toshiba, Tokyo, Japan) for analyses performed later. To assess the magnitude of “stress relaxation” (Magnusson 1998; Magnusson et al. 1997) during the stretching, the changes in SPT from the onset of stretching (0 s) to the end of stretching (300 s) were calculated using a LabChart software (LabChart 4, ADInstruments, NSW, Australia) based on a previous study (Matsuo et al. 2013). Moreover, the values of SPT at 0 s and 300 s were compared between the exercised and control limbs to examine the difference in the magnitude of stretching.
ROM, PT at onset of pain, and passive stiffness
ROM, PT at onset of pain, and passive stiffness were calculated from the torque–angle curve obtained using the isokinetic dynamometer, and the torque and angle signals were A/D converted and stored in a PC (Dynabook Satellite J50, Toshiba, Tokyo, Japan) for analyses. While each subject was sitting in the chair (Fig. 1a), the knee was extended passively at 5°/s to a point of the onset of pain, and during which the torque was recorded to obtain torque–angle curve (Matsuo et al. 2013). ROM was defined as the maximum knee extension angle from the initial position (0°), and PT at onset of pain was defined as the torque at the onset of pain (Halbertsma et al. 1996; Mizuno et al. 2013). As shown in Fig. 2, passive stiffness was defined as the slope of the regression line of the torque–angle curve using a least-squares method (Matsuo et al. 2013). Passive stiffness was calculated from the torque corresponding to 50 % to maximum knee extension angles of each subject when the minimum ROM was recorded (Fig. 2a), and the same knee joint angles were used for all time points, and the same range of motion was used for the exercised and non-exercised limbs within a subject.
Typical torque–angle curves (a) and typical row EMG traces from medial hamstrings (b) of a subject before (pre-ECC) and 2 days after eccentric exercise (post-ECC). Stiffness is determined by a regression line between 50 and 100 % of post-exercise ROM–passive torque relationship (a). Stiffness changed from a (0.29) to b (0.33) for this example. On the other hand, no EMG activities from medial hamstrings during measurement of torque–angle curve were observed before and after eccentric exercise (b), and as well as lateral hamstrings
MVC-ISO torque and RFD
MVC-ISO torque was measured at the position that was the same as the static stretching (Fig. 1a) as well as a position where the seat was adjusted parallel to the floor (Fig. 1c), while the participant seated on the chair with the angles of hip and knee flexion were 85° and 90°, respectively. The horizontal position was included, because a previous study (McHugh and Nesse 2008) reported that stretch-induced strength loss was dependent on muscle length, and the horizontal position was normally used to measure MVC-ISO torque in previous studies (e.g. Ford-Smith et al. 2001; Kollock et al. 2010). MVC-ISO torque at the stretching position (Fig. 1a) was measured prior to that at the horizontal position (Fig. 1c). The participants were instructed to generate maximum knee flexion force for 3 s at each position for 3 times with a 45-s rest between trials (Chen et al. 2011b). Verbal encouragement was provided during all measurements. Peak torque was obtained from each contraction, and the average of the three trials was used for further analysis.
RFD was determined from the MVC-ISO torque data for both sitting positions. Based on the previous studies (Andersen and Aagaard 2006; Morais de Oliveira et al. 2012), the RFD was defined as the slope of the regression line that was calculated from the torque–time curve using a least-squares method. RFD was calculated from the onset of contraction to 100 ms (RFD100) and 150 ms (RFD150), because Morais de Oliveira et al. (2012) reported that RFD decreased after static stretching at early phase (<100 ms) but not at late phase (>100 ms). Onset of muscle contraction was defined when the knee flexor torque exceeded the baseline by 2.5 % of the baseline-to-peak amplitude (Andersen et al. 2010; Blazevich et al. 2008). The average of the three trials was used for subsequent analysis.
MVC-CON torque and optimum angle
MVC-CON torque and optimum angle were measured at the horizontal position described above on the isokinetic dynamometer after measuring MVC-ISO torque at the horizontal position. Three maximum voluntary concentric knee flexions were performed continuously at the angular velocity of 60°/s for the range of 85° from a knee-extended position (5°) to a knee-flexed position (90°). Verbal encouragement was provided in a constant manner during measurements. Peak torque (highest torque of the trial) and the joint angle at the peak torque (optimum angle) of each trial were obtained, and the average of the three trials was used for subsequent analysis (Chen et al. 2011a, b).
Electromyogram (EMG)
EMG activities during the maximal isometric and concentric contractions were recorded from medial and lateral hamstrings by a Biomonitor ME6000 (Mega Electronics, Kuopio, Finland) with a sampling frequency of 1 kHz. The skin under the electrodes was shaved, abraded and cleaned with alcohol before putting the electrodes. A pair of Ag/AgCl sensors (Blue Sensor M-00-s, Ambu, Ballerup, Denmark) with a 35-mm inter-electrode distance were placed at 50 % on the line between the ischial tuberosity and the medial epicondyle of the tibia for medial hamstrings, and placed at 50 % on the line between the ischial tuberosity and the lateral epicondyle of the tibia for lateral hamstrings. The raw EMG signals were stored in the PC and root mean square (RMS) amplitude values were calculated using a software (MegaWin, Mega Electronics, Kuopio, Finland). The average of the three trials at each contraction was used for subsequent analysis. EMG activities were also monitored during the static stretching and measurements of SPT, ROM, PT at onset of pain, and passive stiffness.
Muscle soreness
The level of muscle soreness was quantified by a visual analogue scale (VAS) consisting of a 100-mm continuous line anchoring “no pain” at one end (0 mm) and “very, very painful” at the other (100 mm) (Chen et al. 2011a). Each participant was asked to indicate the pain level of both hamstrings on the line when the knee joint was passively extended from the position to 90 % of the ROM that was determined at the pre-exercise measures in the stretching position (Fig. 1a).
Reliability
The test–retest reliability for all dependent variables except muscle soreness was determined before the data collection of the present study using eight men. The two tests were separated by 1–7 days, and the two tests were performed at the same time of the day (±1 h). Based on intra-class correlation coefficients (ICC1,1), the reliability was found to be high for all measures (ROM: 0.95, PT at onset of pain: 0.97, passive stiffness: 0.97, MVC-ISO torque: 0.90, RFD: 0.83, MVC-CON torque: 0.85, optimum angle: 0.82, RMS: 0.82, SPT: 0.94). Coefficient of variation (CV) was also checked for the measures: ROM: 1.1 %, PT at onset of pain: 3.7 %, passive stiffness: 3.9 %, MVC-ISO torque: 5.4 %, RFD: 8.9 %, MVC-CON torque: 5.0 %, optimum angle: 6.3 %, RMS: 9.1 % and SPT: 4.5 %, showing acceptable reliability.
Statistical analysis
Normality of the data was assessed by a Shapiro–Wilk test. This test showed that the data of ROM, stiffness, MVC-CON torque, and SPT were normally disturbed, but the others were not. Parametric tests were applied to the data that were normally disturbed, but non-parametric tests were applied to the other variables and the relative change for all variables. Changes in each dependent variable over time were compared between limbs by a two-way repeated measures analysis of variance (ANOVA) or Friedman test. When a significant interaction effect or time effect was found, a Bonferroni post hoc test was performed to locate a significant difference between limbs for each time point or the difference from the baseline value. The analyses were performed using a SPSS (IBM SPSS statistics, version 21.0, IBM Corp., Armonk, NY, USA), and significance was set at p < 0.05. All results are expressed as mean ± standard deviation (SD).
Results
Changes in dependent variables after eccentric exercise
Significant changes in all dependent variables except for the optimum angle were found only for the exercised limb, and significant (p < 0.05) differences in the changes in all variables were evident between the exercised and control limbs (Fig. 3). The changes in MVC-ISO torque were similar between the two sitting positions (Fig. 1a, c), thus only the results from the horizontal position are reported. MVC-ISO torque decreased by 34 % at immediately after exercise, and still 20 % lower than the baseline at 96 h post-exercise (Fig. 3a). MVC-CON torque also decreased from the baseline by 17 % at immediately after and by 19 % at 48 h after eccentric exercise, but returned to the baseline by 96 h after exercise (Fig. 3b). RFD100 decreased from baseline (0.49 ± 0.14 Nm/ms) at immediately (0.28 ± 0.09 Nm/ms, 43 %) to 96 h (0.33 ± 0.10 Nm/ms, 29 %) after exercise, and RFD150 decreased significantly from the baseline (0.36 ± 0.10 Nm/ms) at immediately (0.22 ± 0.09 Nm/ms, 39 %) and 48 h (0.18 ± 0.07 Nm/ms, 49 %) post-exercise. RMS of EMG from medial and lateral hamstrings during the maximum isometric and concentric contractions did not change for both limbs after exercise, and there was no significant difference between limbs.
Changes in maximal voluntary isometric (a) and concentric contraction torque (b), range of motion (c), passive torque at onset of pain (d), passive stiffness (e) and muscle soreness assessed by a visual analogue scale (f) before (pre), immediately after (post) and 1, 48, 96 h after eccentric exercise for the exercised limb (exercise) and non-exercised limb (control). *Significantly (p < 0.05) different from the pre-exercise value, †significantly (p < 0.05) different from control
ROM decreased more than 20° at 48 and 96 h after exercise for the exercised limb (Fig. 3c). PT at onset of pain increased significantly at immediately after and 1 h after exercise by approximately 13 %, and decreased significantly at 48 and 96 h after exercise by approximately 30 % (Fig. 3d). As shown in Fig. 2, passive stiffness increased after exercise while ROM and PT at onset of pain decreased. Passive stiffness of the exercised limb increased significantly by 15 % at immediately after exercise, and remained for 96 h after exercise (Fig. 3e). During the passive stiffness measurements, no EMG activities were observed. Muscle soreness developed only for the exercised limb at 48 and 96 h after exercise (Fig. 3f).
Changes in the dependent variables after static stretching
Before stretching performed on 2 and 4 days after eccentric exercise, static passive torque (SPT) was significantly smaller for the exercised limb compared with the non-exercised (control) limb, but changes in SPT during stretching were similar between limbs for both days (Fig. 4). EMG activities were not observed during the static stretching and the SPT measurements.
Changes in static passive torque from onset to the end of static stretching performed at 2 and 4 days after eccentric exercise for the exercised limb (exercise) and non-exercised limb (control). *Significantly (p < 0.05) different from the pre-stretching value, †significantly (p < 0.05) different from control
Significant differences in the pre-stretching values between exercised and non-exercised (control) limbs were evident for MVC-ISO torque, RFD100, RFD150, MVC-CON torque, ROM, PT at onset of pain and stiffness on 2 days, and for MVC-ISO, ROM, PT at onset of pain and stiffness on 4 days after eccentric exercise. Since the pre-stretching values were different between limbs, and between days (2 days post- and 4 days post-exercise), changes in the dependent variables after stretching were normalised to the pre-stretching values, and compared between limbs.
Changes in MVC-ISO torque at the horizontal position (Fig. 1c) after stretching are shown in Fig. 5a. MVC-ISO torque decreased significantly after stretching for the non-exercised limb only from 78.3 ± 11.9 Nm to 69.7 ± 14.2 Nm (11 %) at immediately post-stretching performed at 2 days after eccentric exercise, but returned to the baseline by 20 min post-stretching. After the stretching performed at 4 days after eccentric exercise, MVC-ISO torque decreased from 82.6 ± 11.7 Nm to 77.2 ± 13.9 Nm (7 %) at immediately post-stretching, and the decrease lasted for 60 min (20 min post-stretching: 76.6 ± 12.6 Nm, 60 min post-stretching: 76.4 ± 12.3 Nm). This was also the case for the MVC-ISO torque measured at the stretching position. Significant decreases in RFD100 and RFD150 were found only for the non-exercised limb, and the magnitude of decrease (12–16 %) was similar to that of MVC-ISO torque. MVC-CON torque (Fig. 5b) and optimum angle did not change for both limbs after stretching. No significant changes in EMG activities of medial and lateral hamstrings were evident during the maximum isometric contractions before and after stretching.
Normalised changes in maximal voluntary isometric (a) and concentric contraction torque (b), range of motion (c), passive torque at onset of pain (d) and passive stiffness (e) from the pre-stretching values (100 %), and absolute changes in muscle soreness assessed by a visual analogue scale (f), immediately before (pre), immediately after (0), and 20 and 60 min after static stretching performed 2 and 4 days after eccentric exercise for the exercised limb (exercise) and non-exercised limb (control). *Significantly (p < 0.05) different from the pre-stretching values (absolute value), †significantly (p < 0.05) different from control (relative value)
ROM increased significantly for both limbs for 60 min after stretching (Fig. 5c), but the magnitude of the increase was significantly greater for the exercised limb (9°–13°) than non-exercised limb (4°–8°). PT at onset of pain increased significantly after stretching for both limbs (Fig. 5d), but the magnitude of the increase was significantly greater for the exercised limb (3–6 Nm) than non-exercised limb (2–3 Nm). Significant decreases in passive stiffness were evident after stretching for both limbs, and the magnitude of the decrease (4–7 %) was not significantly between limbs (Fig. 5e). VAS of muscle soreness decreased significantly after stretching by 37 % at 2 days and 41 % at 4 days post-exercise for the exercised limb (Fig. 5f). Moreover, no EMG activities of medial and lateral hamstrings were observed during these measurements.
Discussion
The present study tested the hypothesis that the decreases in maximum voluntary contraction torque and passive stiffness, and the increase in ROM after 300-s continuous static stretching would be smaller for damaged than non-damaged muscles. The results showed that the magnitude of increase in ROM and PT at onset of pain after stretching was significantly greater for the exercised limb (damaged muscle) than non-exercised (control) limb, and MVC-ISO torque decreased only for the non-exercised limb (non-damaged muscle), but MVC-CON torque did not change after stretching, and changes in passive stiffness after stretching were not different between limbs (Fig. 5). These results partially supported the hypothesis and suggested that the static stretching could produce greater positive effects without stretch-induced strength loss on damaged muscle than non-damaged muscle.
The baseline value of MVC-ISO torque in the present study was similar to that reported in our previous study (Matsuo et al. 2013), but the baseline values of ROM and PT at onset of pain appear to be smaller than those reported in a previous study (Magnusson et al. 1997). It seems likely that the differences in the baseline values between the studies were due to the difference in the participants used in the studies such that the previous study (Magnusson et al. 1997) used male elite orienteers, but the present study used non-athletes. It should be noted that the baseline values of all criterion measures were not different between limbs, and ICC and CV showed good test–retest reliability of the measures. Therefore, it seems reasonable to assume that the present study design was appropriate to compare the responses to the static stretching between damaged and non-damaged muscles.
As shown in Fig. 3, all dependent variables changed significantly only for the exercised limb. The changes in the dependent variables after the eccentric exercise in the present study were comparable to those reported in previous studies in which a similar eccentric exercise of the knee flexors was performed (Chen et al. 2011a, b). These changes appear to indicate that muscle damage was induced to the knee flexors by the eccentric exercise. It seems unlikely that the eccentric exercise of one limb affected the other limb (control limb), at least for the muscle function and flexibility.
As shown in Figs. 4 and 5, the 300-s static stretching of hamstrings decreased SPT, passive stiffness and MVC-ISO torque, and increased ROM and PT at onset of pain for the non-exercised limb immediately after stretching. These changes were similar to those reported in previous studies in which a long (>180 s) static stretching of hamstrings was performed (Halbertsma et al. 1996; Matsuo et al. 2013). It appears that the static stretching for the control limb worked as expected, thus the differences between the exercised and non-exercised (control) limbs were due to the muscle damage that was induced to the exercised limb, or a different absolute magnitude of the stretching applied to each limb such that the intensity was lower for the exercised limb, because of lower pain threshold of the exercised than non-exercise limb.
The most striking finding of the present study was that MVC-ISO torque did not decrease after the 300-s static stretching for the exercised limb (Fig. 5a). Several studies have shown significant decreases in MVC-ISO torque after a static stretching that is longer than 60 s (Kay and Blazevich 2012; Matsuo et al. 2013; Morais de Oliveira et al. 2012; Siatras et al. 2008). For example, Trajano et al. (2013) reported 16 % decrease in MVC-ISO torque immediately after a 300-s static stretching of triceps surae, but it recovered within 15 min, and stated that the decrease in muscle force was caused by reduction in central drive. Previous systematic reviews reported that MVC-ISO force decreased by 7–8 % after static stretching (Simic et al. 2013; Kay and Blazevich 2012). The magnitude of the decrease in MVC-ISO torque after stretching for the non-exercised (control) limb was 7–11 % in the present study, which appears to be comparable to that reported in previous studies. However, no such decreases were evident for the exercised limb (eccentrically damaged muscle). It has been documented that the stretch-induced force loss is caused by a reduced neural drive and/or reduced peripheral force-generating capacity due to a decrease in musculotendinous stiffness and/or change in muscle’s length–tension relationship (Ryan et al. 2008; Fowles et al. 2000). The total force exerted by a muscle is the sum of active force generated and passive force provided (MacIntosh and MacNaughton 2005). In the present study, the decrease in passive stiffness after stretching was not different between limbs (Fig. 5e). Thus, it is likely that the decreases in MVC-ISO torque after stretching were manly associated with the decreases in active force. It should be noted that VAS of muscle soreness for the exercised limb decreased significantly after the static stretching (Fig. 5f). It is possible that the decrease in pain sensation by approximately 40 % counteracted the force loss. It may be that a decrease in MVC-ISO torque after stretching would have been observed, if the static stretching had been performed at the same intensity and at the same angle as that of the non-exercised limb, because this would induce greater pain during stretching. Further studies are necessary to investigate changes in voluntary activation of the damaged muscle with muscle soreness after static stretching, and if attenuation of pain is associated with the no changes in the MVC-ISO torque after stretching.
It is interesting that MVC-CON torque did not change after the static stretching for both limbs (Fig. 5b). Previous studies reported that knee extension concentric muscle force decreased (3–12 %) after a static stretching of quadriceps (Cramer et al. 2005, 2007; Siatras et al. 2008). Kay and Blazevich (2012) stated that the magnitude of the reduction was greater for isometric (9 %) than concentric (5 %) strength. McHugh and Nesse (2008) reported that stretch-induced strength loss was dependent on muscle length, such that strength was decreased with a short muscle length, but not with a long muscle length for the knee flexors. In the present study, the optimum angle of the knee flexors was approximately 30° knee flexion, thus MVC-ISO torque was measured at a short muscle length (approximately 110° knee flexion). It is possible that the MVC-CON torque was not reduced by static stretching, because its maximum torque was produced at a long muscle length (e.g. 30°), where the effect of stretching was minimum.
Contrary to the hypothesis, the magnitude of the increase in ROM and PT at onset of pain after stretching was greater for the exercised limb than the non-exercised limb, despite the intensity of stretching was lower for the exercised limb than exercised limb (Figs. 4, 5c, d). A previous study (Reisman et al. 2009) reported that the magnitude of improvement in flexibility assessed by passive torque after a 30-s stretching was not different between damaged and non-damaged muscle. However, it should be noted that the same absolute intensity of stretching was applied for both damaged and non-damaged muscle, and the stretching duration was only 30 s in the previous study. In contrast, the present study applied longer (300-s) continuous passive static stretching at tolerable intensity without pain. Thus, because of DOMS of the exercised muscle, the absolute stretching intensity was lower for the damaged muscle. It is important to note that the reduced stretch tolerance in the damaged muscle after exercise was not directly linked to the higher passive tension after damage. Mizuno et al. (2013) reported that an increase in ROM after static stretching was attributable to an increase in PT at onset of pain and a decrease in stiffness. It has been shown that “stretch tolerance” increases after static stretching (Blazevich et al. 2014; Magnusson et al. 1996). It is therefore possible that the greater increase in PT at onset of pain for the exercised limb was associated with the analgesic effect of stretching. In fact, VAS of muscle soreness for the exercised limb decreased significantly for the exercised limb after static stretching (Fig. 5f).
The magnitude of the decrease in passive stiffness was not different between limbs (Fig. 5e), although the intensity of stretching for damaged muscle was lower than non-damaged muscle. Nakamura et al. (2011) documented that a decrease in muscle–tendon unit stiffness after 5-min static stretching was due to a decrease in muscle stiffness not tendon stiffness, and speculated that a decrease in passive muscle–tendon unit stiffness after 5-min static stretching was likely due to increased flexibility and movement of the aponeurosis and the connective tissue such as the endomysium, perimysium, and epimysium, instead of lengthening muscle fibre. In the present study, passive stiffness was calculated from the same range of motion for the exercised and non-exercised limbs within a subject for all time points. Moreover, no EMG activities were found during the passive stiffness measures for both limbs (Fig. 2b). Therefore, it seems likely that the decrease in passive stiffness was mainly associated with changes in mechanical factors rather than neurological factors.
In summary, the present study showed that a long-duration (300 s) static passive stretching produced greater positive effects on flexibility without stretch-induced force loss for damaged muscle than non-damaged muscle. It is possible that analgesic effect of the static stretching for the damaged muscle eliminated the post-stretch force loss, and induced the greater increases in the flexibility for the damaged muscle than non-damaged muscle. It is concluded that a static stretching is effective for improving decreased flexibility of eccentrically damaged muscle without stretch-induced force loss, when the intensity of stretching does not exceed the pain threshold.
Abbreviations
- ANOVA:
-
Analysis of variance
- CK:
-
Creatine kinase
- CV:
-
Coefficient of variation
- DOMS:
-
Delayed onset muscle soreness
- EMG:
-
Electromyogram
- ICC:
-
Intra-class correlation coefficients
- MVC-CON torque:
-
Maximum voluntary concentric contraction torque
- MVC-ISO torque:
-
Maximum voluntary isometric contraction torque
- PT:
-
Passive torque
- RFD:
-
Rate of force development
- RMS:
-
Root mean square
- ROM:
-
Range of motion
- SD:
-
Standard deviation
- SPT:
-
Static passive torque
- VAS:
-
Visual analogue scale
References
Andersen LL, Aagaard P (2006) Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol 96(1):46–52. doi:10.1007/s00421-005-0070-z
Andersen LL, Andersen JL, Zebis MK, Aagaard P (2010) Early and late rate of force development: differential adaptive responses to resistance training? Scand J Med Sci Sports 20(1):e162–e169. doi:10.1111/j.1600-0838.2009.00933.x
Blazevich AJ, Horne S, Cannavan D, Coleman DR, Aagaard P (2008) Effect of contraction mode of slow-speed resistance training on the maximum rate of force development in the human quadriceps. Muscle Nerve 38(3):1133–1146. doi:10.1002/mus.21021
Blazevich AJ, Cannavan D, Waugh CM, Miller SC, Thorlund JB, Aagaard P, Kay AD (2014) Range of motion, neuromechanical, and architectural adaptations to plantar flexor stretch training in humans. J Appl Physiol 117(5):452–462. doi:10.1152/japplphysiol.00204.2014
Chen CH, Nosaka K, Chen HL, Lin MJ, Tseng KW, Chen TC (2011a) Effects of flexibility training on eccentric exercise-induced muscle damage. Med Sci Sports Exerc 43(3):491–500. doi:10.1249/MSS.0b013e3181f315ad
Chen TC, Lin KY, Chen HL, Lin MJ, Nosaka K (2011b) Comparison in eccentric exercise-induced muscle damage among four limb muscles. Eur J Appl Physiol 111(2):211–223. doi:10.1007/s00421-010-1648-7
Cramer JT, Housh TJ, Weir JP, Johnson GO, Coburn JW, Beck TW (2005) The acute effects of static stretching on peak torque, mean power output, electromyography, and mechanomyography. Eur J Appl Physiol 93(5–6):530–539. doi:10.1007/s00421-004-1199-x
Cramer JT, Beck TW, Housh TJ, Massey LL, Marek SM, Danglemeier S, Purkayastha S, Culbertson JY, Fitz KA, Egan AD (2007) Acute effects of static stretching on characteristics of the isokinetic angle–torque relationship, surface electromyography, and mechanomyography. J Sports Sci 25(6):687–698. doi:10.1080/02640410600818416
Ford-Smith CD, Wyman JF, Elswick RK Jr, Fernandez T (2001) Reliability of stationary dynamometer muscle strength testing in community-dwelling older adults. Arch Phys Med Rehabil 82(8):1128–1132. doi:10.1053/apmr.2001.24291
Fowles JR, Sale DG, MacDougall JD (2000) Reduced strength after passive stretch of the human plantarflexors. J Appl Physiol 89(3):1179–1188
Halbertsma JP, van Bolhuis AI, Goeken LN (1996) Sport stretching: effect on passive muscle stiffness of short hamstrings. Arch Phys Med Rehabil 77(7):688–692. doi:10.1016/S0003-9993(96)90009-X
Herbert RD, de Noronha M, Kamper SJ (2011) Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database Syst Rev 7:CD004577. doi:10.1002/14651858.CD004577.pub3
Janecki D, Jarocka E, Jaskolska A, Marusiak J, Jaskolski A (2011) Muscle passive stiffness increases less after the second bout of eccentric exercise compared to the first bout. J Sci Med Sport 14(4):338–343. doi:10.1016/j.jsams.2011.02.005
Johansson PH, Lindstrom L, Sundelin G, Lindstrom B (1999) The effects of preexercise stretching on muscular soreness, tenderness and force loss following heavy eccentric exercise. Scand J Med Sci Sports 9(4):219–225. doi:10.1111/j.1600-0838.1999.tb00237.x
Kay AD, Blazevich AJ (2012) Effect of acute static stretch on maximal muscle performance: a systematic review. Med Sci Sports Exerc 44(1):154–164. doi:10.1249/MSS.0b013e318225cb27
Kollock RO Jr, Onate JA, Van Lunen B (2010) The reliability of portable fixed dynamometry during hip and knee strength assessments. J Athl Train 45(4):349–356. doi:10.4085/1062-6050-45.4.349
MacIntosh BR, MacNaughton MB (2005) The length dependence of muscle active force: considerations for parallel elastic properties. J Appl Physiol 98(5):1666–1673. doi:10.1152/japplphysiol.01045.2004
Magnusson SP (1998) Passive properties of human skeletal muscle during stretch maneuvers. A review. Scand J Med Sci Sports 8(2):65–77. doi:10.1111/j.1600-0838.1998.tb00171.x
Magnusson SP, Simonsen EB, Aagaard P, Sorensen H, Kjaer M (1996) A mechanism for altered flexibility in human skeletal muscle. J Physiol 497(Pt 1):291–298
Magnusson SP, Simonsen EB, Aagaard P, Boesen J, Johannsen F, Kjaer M (1997) Determinants of musculoskeletal flexibility: viscoelastic properties, cross-sectional area, EMG and stretch tolerance. Scand J Med Sci Sports 7(4):195–202. doi:10.1111/j.1600-0838.1997.tb00139.x
Matsuo S, Suzuki S, Iwata M, Banno Y, Asai Y, Tsuchida W, Inoue T (2013) Acute effects of different stretching durations on passive torque, mobility, and isometric muscle force. J Strength Cond Res 27(12):3367–3376. doi:10.1519/JSC.0b013e318290c26f
McHugh MP, Cosgrave CH (2010) To stretch or not to stretch: the role of stretching in injury prevention and performance. Scand J Med Sci Sports 20(2):169–181. doi:10.1111/j.1600-0838.2009.01058.x
McHugh MP, Nesse M (2008) Effect of stretching on strength loss and pain after eccentric exercise. Med Sci Sports Exerc 40(3):566–573. doi:10.1249/MSS.0b013e31815d2f8c
Mizuno T, Matsumoto M, Umemura Y (2013) Viscoelasticity of the muscle-tendon unit is returned more rapidly than range of motion after stretching. Scand J Med Sci Sports 23(1):23–30. doi:10.1111/j.1600-0838.2011.01329.x
Morais de Oliveira AL, Greco CC, Molina R, Denadai BS (2012) The rate of force development obtained at early contraction phase is not influenced by active static stretching. J Strength Cond Res 26(8):2174–2179. doi:10.1519/JSC.0b013e31823b0546
Nakamura M, Ikezoe T, Takeno Y, Ichihashi N (2011) Acute and prolonged effect of static stretching on the passive stiffness of the human gastrocnemius muscle tendon unit in vivo. J Orthop Res 29(11):1759–1763. doi:10.1002/jor.21445
Nosaka K, Sakamoto K (2001) Effect of elbow joint angle on the magnitude of muscle damage to the elbow flexors. Med Sci Sports Exerc 33(1):22–29. doi:10.1097/00005768-200101000-00005
Nosaka K, Newton M, Sacco P (2002) Delayed-onset muscle soreness does not reflect the magnitude of eccentric exercise-induced muscle damage. Scand J Med Sci Sports 12(6):337–346. doi:10.1034/j.1600-0838.2002.10178.x
Reisman S, Allen TJ, Proske U (2009) Changes in passive tension after stretch of unexercised and eccentrically exercised human plantarflexor muscles. Exp Brain Res 193(4):545–554. doi:10.1007/s00221-008-1657-5
Ryan ED, Beck TW, Herda TJ, Hull HR, Hartman MJ, Stout JR, Cramer JT (2008) Do practical durations of stretching alter muscle strength? A dose–response study. Med Sci Sports Exerc 40(8):1529–1537. doi:10.1249/MSS.0b013e31817242eb
Shrier I (2005) When and whom to stretch? Gauging the benefits and drawbacks for individual patients. Physician Sportsmed 33(3):22–26. doi:10.3810/psm.2005.03.61
Siatras TA, Mittas VP, Mameletzi DN, Vamvakoudis EA (2008) The duration of the inhibitory effects with static stretching on quadriceps peak torque production. J Strength Cond Res 22(1):40–46. doi:10.1519/JSC.0b013e31815f970c
Simic L, Sarabon N, Markovic G (2013) Does pre-exercise static stretching inhibit maximal muscular performance? A meta-analytical review. Scand J Med Sci Sports 23(2):131–148. doi:10.1111/j.1600-0838.2012.01444.x
Small K, Mc Naughton L, Matthews M (2008) A systematic review into the efficacy of static stretching as part of a warm-up for the prevention of exercise-related injury. Res Sports Med 16(3):213–231. doi:10.1080/15438620802310784
Smith CA (1994) The warm-up procedure: to stretch or not to stretch. A brief review. J Orthop Sports Phys Ther 19(1):12–17. doi:10.2519/jospt.1994.19.1.12
Torres R, Appell H-J, Duarte JA (2007) Acute effects of stretching on muscle stiffness after a bout of exhaustive eccentric exercise. Int J Sports Med 28(7):590–594. doi:10.1055/s-2007-964865
Trajano GS, Seitz L, Nosaka K, Blazevich AJ (2013) Contribution of central vs. peripheral factors to the force loss induced by passive stretch of the human plantar flexors. J Appl Physiol 115(2):212–218. doi:10.1152/japplphysiol.00333.2013
Woods K, Bishop P, Jones E (2007) Warm-up and stretching in the prevention of muscular injury. Sports Med 37(12):1089–1099. doi:10.2165/00007256-200737120-00006
Acknowledgments
This work was supported in part by a grant from A-kit Co., Ltd. and the Public Advertisement Research Project of Nihon Fukushi University.
Conflict of interest
All the authors declare that there is no conflict of interest regarding this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Olivier Seynnes.
Rights and permissions
About this article
Cite this article
Matsuo, S., Suzuki, S., Iwata, M. et al. Changes in force and stiffness after static stretching of eccentrically-damaged hamstrings. Eur J Appl Physiol 115, 981–991 (2015). https://doi.org/10.1007/s00421-014-3079-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-3079-3