Abstract
Purpose
Eccentric muscle actions are important to the development of muscle mass and strength and may affect bone mineral density (BMD). This study’s purpose was to determine the relative effectiveness of five different eccentric:concentric load ratios to increase musculoskeletal parameters during early adaptations to resistance training.
Methods
Forty male subjects performed a supine leg press and calf press training program 3 days week−1 for 8 weeks. Subjects were matched for pre-training leg press 1-repetition maximum strength (1-RM) and randomly assigned to one of five training groups. Concentric training load (% 1-RM) was constant across groups, but within groups, eccentric load was 0, 33, 66, 100, or 138 % of concentric load. Muscle mass (dual energy X-ray absorptiometry; DXA), strength (1-RM), and BMD (DXA) were measured pre- and post-training. Markers of bone metabolism were assessed pre-, mid- and post-training.
Results
The increase in leg press 1-RM in the 138 % group (20 ± 4 %) was significantly greater (P < 0.05) than the 0 % (8 ± 3 %), 33 % (8 ± 5 %) and 66 % (8 ± 4 %) groups, but not the 100 % group (13 ± 6 %; P = 0.15). All groups, except the 0 % group, increased calf press 1-RM (P < 0.05). Leg lean mass and greater trochanter BMD were increased only in the 138 % group (P < 0.05).
Conclusions
Early-phase adaptations to eccentric overload training include increases in muscle mass and site-specific increases in BMD and muscle strength which are not present or are less with traditional and eccentric underload training. Eccentric overload provides a robust musculoskeletal stimulus that may benefit bedridden patients, individuals recovering from injury or illness, and astronauts during spaceflight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle strength and adequate bone mineral density (BMD) are indispensable components of independent human function. Resistance exercise is a well-documented training modality that effectively increases muscle strength in a wide range of populations: young and old (Campos et al. 2002; Kryger and Andersen 2007; Menkes et al. 1993), male and female (Hawkins et al. 1999; Josse et al. 2010; Schroeder et al. 2004; Staron et al. 1990; Vuori et al. 1994), athletically trained and untrained (Brandenburg and Docherty 2002; Campos et al. 2002; Friedmann-Bette et al. 2010), healthy and rehabilitative (Alfredson et al. 1999). Bone remodeling is tightly regulated by mechanical stimulation, the chief source of which is skeletal muscle contractions. Beginning with the pioneering work of Harold Frost (1987a, b, 1990a, b, 2001), numerous studies over the last 20 years have demonstrated that high force muscle contractions (e.g., chronic resistance exercise) can increase BMD (Bassey and Ramsdale 1994; Bassey et al. 1998; Hawkins et al. 1999; Kohrt et al. 2004; Lohman et al. 1995; Menkes et al. 1993; Nickols-Richardson et al. 2007; Ryan et al. 2004; Schroeder et al. 2004).
Early-phase (<8–10 weeks) skeletal muscle adaptations to resistance exercise in untrained subjects follow a well-characterized progression with neural changes observed in the first several weeks (Moritani and deVries 1979) and hypertrophic responses in muscle fiber cross-sectional area (CSA) seen after 4–8 weeks (Schuenke et al. 2012; Staron et al. 1994); increases in strength are a product of both neuromuscular and hypertrophic adaptations and are thus also observed within the first 4 weeks of training (Schuenke et al. 2012; Staron et al. 1994). Conversely, positive adaptations in bone mineral density or content are rarely detected by DXA (or even measured) (Alcaraz et al. 2011) in the first 8 weeks of resistance exercise training, although one study recently has documented an increase in greater trochanter BMD after 8 weeks of training (Loehr et al. 2011). Numerous studies have reported increases in bone mineral density or content after 16 weeks of training (Hawkins et al. 1999; Menkes et al. 1993; Nickols-Richardson et al. 2007; Schroeder et al. 2004).
Biomechanically, force is generated both as a muscle shortens (concentric action) and lengthens (eccentric action) with both types of muscle action integral to activities of daily living as well as athletic performance. Due to the mechanical properties of skeletal muscle, muscle fibers can generate greater force during an eccentric action than during a concentric action (Crenshaw et al. 1995; Westing et al. 1991; Westing and Seger 1989). Thus, during conventional isoinertial resistance exercise, the prime mover or agonist muscle is trained maximally for concentric actions, while the eccentric actions for that muscle are performed at a lower relative intensity. Because muscle tension is a key factor that stimulates positive musculoskeletal adaptations, numerous studies have investigated both the discrete and cumulative effects of concentric and eccentric muscle actions on various training adaptations (e.g., increases in muscle mass, muscle strength, and BMD).
The effects of traditional (eccentric loads equal to concentric loads), concentric-only, eccentric-only, and eccentric overload (eccentric loads greater than concentric loads) training on muscle strength and mass are well studied. Both eccentric-only and concentric-only training increase muscle mass and strength (Colliander and Tesch 1990; Higbie et al. 1996; Jones and Rutherford 1987; Seger et al. 1998), but the combination of eccentric and concentric contractions using the same load, as in traditional training paradigms, appears to be more effective at increasing muscle mass and strength than either eccentric-only or concentric-only training (Dudley et al. 1991b; Hather et al. 1991; Lacerte et al. 1992; Tesch et al. 1990). Further, eccentric overload elicits greater strength gains than traditional isoinertial training (Brandenburg and Docherty 2002; Doan et al. 2002; Hortobagyi et al. 2001). Maximal eccentric training also protects against acute decreases in maximum strength after high intensity running exercise (Oliveira et al. 2013). However, to our knowledge, no previous work has evaluated specifically the effects of eccentric underload training (eccentric loads less than concentric loads).
Much less information exists on the relative importance of eccentric and concentric muscle actions on BMD. Most studies reporting bone outcomes have employed resistance exercise protocols with equal eccentric and concentric loads such that information regarding the discrete effects of the different muscle actions is limited. We might expect that higher levels of eccentric loading would promote greater increases in BMD due to the more forceful muscle contractions that they elicit; some evidence is available to support this concept. Hawkins et al. reported an increase in mid-femur BMD following 18 weeks of eccentric-only exercise compared with no change in a concentric-only exercise group (Hawkins et al. 1999). Also, Alfredson et al. demonstrated that high load eccentric-only muscle contractions effectively prevented losses in calcaneus bone mass in individuals with Achilles tendonitis (Alfredson et al. 1999). However, similar to the knowledge base regarding muscle, the skeletal responses to eccentric underload training have not been investigated.
Intuitively, it appears that an exercise training program designed to maximize gains in muscle strength and BMD would include eccentric loading equal to or greater than the concentric load. Conversely, it is not uncommon to minimize or reduce eccentric muscle loading in some circumstances, such as in unfit individuals embarking on a new exercise training program or in the elderly, when muscle soreness (Crameri et al. 2007) or the potential for increased injury risk due to high eccentric loads might be deleterious and counterproductive. For example, concentric-only and eccentric underload exercises have been employed to rehabilitate from an injury, immobilization, and bed rest when the potential for muscle injury may be elevated (Gabriel et al. 2006; Narici et al. 2003; Riley et al. 2000). In a more unique population, astronauts performing resistance exercise during spaceflight historically have not had access to an exercise device that provided eccentric loads that were equal to the concentric loads, which may have contributed to spaceflight-induced reductions in muscle mass, strength, and BMD in the lower body despite regular performance of exercise countermeasures (Gopalakrishnan et al. 2010; LeBlanc et al. 2000a, b; Smith et al. 2005a; Tesch et al. 2005; Trappe et al. 2009).
Thus, the purpose of this investigation was to determine the relative effectiveness of training with different ratios of eccentric to concentric resistance to induce early-phase changes in lean tissue mass, muscle strength, BMD, and biomarkers of bone metabolism. We hypothesized that subjects who performed 8 weeks of resistance exercise with training loads comprised of proportionally greater eccentric to concentric loads would experience greater increases in lean mass and muscle strength compared to subjects that exercised with lower eccentric to concentric loading. Additionally, given the paucity of data regarding BMD adaptations with eccentric overload training, we sought to determine the effectiveness of different eccentric loads on site-specific BMD and whole body measures of bone metabolism. While few previous studies have measured changes in BMD during early-phase adaptations, we suspected that the high muscle loading associated with eccentric overload training might be effective.
This study is unique in that a range of eccentric to concentric loading was evaluated during resistance training and that both muscle and bone outcomes were assessed. The results can be used to inform the development of appropriate exercise prescriptions for a range of populations and to guide resistance exercise hardware requirements for exploration spaceflight.
Methods
Subjects
Forty healthy males (34.9 ± 7 years, 80.9 ± 9.8 kg, 178.2 ± 7.1 cm; mean ± SD) volunteered to participate in this training study. Subjects were screened for participation by the Human Test Subject Facility at NASA Johnson Space Center (JSC) using an Air Force Class III-equivalent physical examination. Subjects were free from any orthopedic or other medical conditions that would have precluded safe participation and normal adaptations to strength training. Testing protocols were reviewed and approved by the NASA JSC Institutional Review Board. Subjects received written and verbal explanations of the study and provided written informed consent before participation.
Subjects had not participated in a strength training program for at least 6 months prior to entering the study; only five subjects had any history of strength training in the previous 5 years. Subjects were instructed not to initiate any new exercise programs while participating in the study. No upper body resistance exercise was allowed while enrolled in the study. Eight subjects already participating in moderate volume/intensity recreational aerobic exercise (e.g., jogging or cycling 2-3 days week-1) were instructed to maintain the intensity, frequency, and duration of their aerobic exercise training during the study; these subjects were distributed among all five training groups. Although diet was not specifically controlled, subjects were instructed to maintain their typical dietary habits for the duration of the study. Subjects were prohibited to take any nutritional supplements or similar products that might affect muscle performance, lean tissue mass, or BMD. Each subject also completed a pre-study dual energy X-ray absorptiometry (DXA) scan of the lumbar spine to ensure that BMD was not more than 2 SD from the population mean prior to study enrollment. We have employed these same screening criteria in previous resistance exercise training studies (Loehr et al. 2011; Schneider et al. 2003).
Experimental design and procedures
This 12-week study comprised 3 weeks of pre-testing, 8 weeks of training, and 1 week of post-testing (Fig. 1). Exercise testing and training were conducted using supine leg press and supine calf press exercises. All exercise testing and training were supervised by experienced strength training coaches.
During each of the 3 weeks that preceded the start of exercise training, each subject performed one-repetition maximum (1-RM) strength tests for both exercises. Each 1-RM testing session was separated by 5–9 days. During the final week before training was initiated, BMD and lean tissue mass were measured using DXA, and urine and blood samples were collected to measure baseline markers of bone metabolism. The last pre-training 1-RM was conducted after these tests to avoid any potential confounding effect of the 1-RM session on these measures.
Subjects were matched according to their highest pre-training 1-RM for leg press and assigned to one of five training groups in a counterbalanced fashion (n = 8 per group). All subjects trained with the same prescribed concentric load (% pre-training 1-RM; Table 1) but eccentric loads were prescribed according to group assignment: 0 % (concentric-only training), 33, 66, 100, or 138 % of the concentric load.
Mid- and post-training urine and blood samples were collected during week 4 of training and in the week after the last training session. Blood draws were performed on the first day of the week in the morning after an overnight fast and at least 60 h after the most recent resistance exercise session. Post-training BMD, lean tissue mass, and 1-RM for each exercise also were measured. As with pre-training tests, 1-RM testing was conducted after urine and blood sampling and DXA scans.
Exercise training
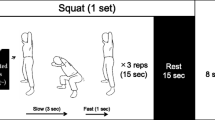
Each group performed the supine leg press and calf press exercises 3 days week−1. These exercises were chosen to train the locomotor and postural muscles of the lower body which are most affected by the unloading of bed rest disuse or spaceflight. The concentric resistance varied in a periodized fashion within each week and incrementally increased every subsequent week (Table 1). Concentric resistance was prescribed as a percentage of pre-training 1-RM and was the same for each group. Within each week, the first exercise day (day 1) was prescribed as the heavy day, day 2 was the light day (~10 % of 1-RM less than day 1), and day 3 was a medium day (~5 % of 1-RM less than day 1). Eccentric loads, prescribed as a percentage of the concentric load for each day, therefore varied in an identical fashion within and across weeks as the concentric loads. Subjects performed the same number of warm-up and training sets and repetitions in each session within a given week. Subjects performed two to four lighter warm-up sets which began at 50 % 1-RM and progressed in intensity before performing their training sets (highest load) during each session (Table 1).
Exercise device
Leg press and calf press 1-RM testing and exercise training was performed using an Agaton Fitness System (Agaton Fitness AB, Boden, Sweden; Fig. 2). This commercial system was designed to perform traditional resistance exercise training (1:1 eccentric:concentric load ratio) as well as eccentric overload training. In the traditional training mode (corresponding to the 100 % group), subjects raised and lowered the weight on the left side only. When the machine was placed in the eccentric overload mode (138 % group), subjects raised the weight on the left side, and in the eccentric phase lowered weight placed on both the left and right sides. A motor located inside the tower raised the load on the right side to the uppermost position during the concentric phase (right side not lifted by the subject), and as the subject began the eccentric phase, a clutch in the tower engaged the right side in the load path such that the subject received loading from the left and right sides concurrently. For the purposes of this study, the manufacturer modified the device to provide an eccentric underload capability. In the eccentric underload mode (0, 33, and 66 % groups), the clutch was engaged so that the subject lifted the loads on both the right and left sides, but lowered the weight on only the left side. To achieve this, when the subject reached the top of the range of motion during the concentric phase, the clutch disengaged the right side from the load path, and the motor lowered the weight on the right side to the bottom position.
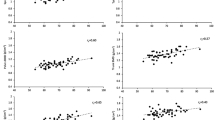
Loads were verified prior to the beginning of the study by fixing an inline load cell between the subject carriage and the loaded weight; data were collected statically during coupled eccentric:concentric leg press and calf press in each of the loading configurations (eccentric underload, traditional resistance, and eccentric overload) over a range of concentric loads (leg press: 45.5–136.4 kg, 22.7 kg increments; calf press: 136.4–227.3 kg, 45.5 kg increments) that corresponded to the loads that we anticipated would be used by the subjects during training (Fig. 3). R 2 values (actual vs. expected) were >0.99 for both eccentric and concentric loading across all loading configurations.
Actual vs. expected static loads measured during eccentric phase of exercise during leg press (circle) and calf press (triangle) exercises to verify loading in underload (solid black), traditional (open), and overload (gray) operating configurations; concentric data not shown. Solid line represents line of identity (100 % agreement)
One-repetition maximum testing
Each subject completed three 1-RM testing sessions using traditional 1:1 eccentric:concentric loading prior to the start of the training period according to the schedule previously described. The first session was considered a familiarization session and was used to instruct the subjects in proper exercise technique and acquaint them with the testing procedures. In this session, subjects first performed two sets of eight repetitions at a moderate load followed by a set of five repetitions and a set of three repetitions of increased intensity. Thereafter, subjects performed sets of one repetition until the subject could not lift the load through the desired range of motion or with proper technique. The load increased incrementally for each set based on the subjects’ perceived level of effort.
The second and third pre-training 1-RM sessions followed the same protocol as the familiarization session, except that the loads for each set were based on percentages of the 1-RM determined in the previous test session. The first two sets consisted of eight reps at 50 and 60 %, followed by five reps at 70 %, and three reps at 80 % of the previous 1-RM. Following the warm-up, subjects attempted to complete one repetition at 90 % and another repetition at 100 %. The subjects then attempted to complete one repetition with additional load until a 1-RM was reached.
For each 1-RM session, testing was terminated if the subject failed on two consecutive attempts to complete a repetition with additional load or the technique and form were poor (Loehr et al. 2011). Approximately 2–3 min of rest were given between each set. Pre-training 1-RM for each exercise was considered to be the highest load lifted in the second and third 1-RM session. The post-training 1-RM testing was performed in the same manner as the pre-training tests, and used the same progression of loads, prescribed based upon the highest pre-training 1-RM. Intraclass correlation coefficients for the second and third pre-training 1-RM sessions were 0.95 and 0.96 for leg press and calf press, respectively. This protocol has previously been shown to elicit reliable 1-RM values in untrained subjects (English et al. 2008; Loehr et al. 2011).
DXA measurements
Pre- and post-training whole body, lumbar spine, and hip BMD (g cm−2) and whole body lean tissue mass were measured in triplicate using DXA (4500 W ELITE fan-beam X-ray densitometer, Hologic Inc., Bedford, MA). To improve the reliability of BMD and lean tissue mass measures, all pre- and post-training scans were conducted and analyzed by the same operator. The mean value from the three scans was considered representative of BMD and lean tissue mass. Using this methodology, the precision of measurements for BMD using these procedures is 1.0 % for the whole body, 1.4 % for the lumbar spine, and 1.5 % for the femoral neck (Shackelford et al. 2004). The precision for lean tissue measurements, whole body lean mass (WBLM), and leg lean mass (LLM) calculated from the whole body scans, is 0.9 % for WBLM and 1.3 % for LLM (Shackelford et al. 2004).
Blood and urine sample collections and processing
Blood and urine samples were collected for analysis of bone- and calcium-related factors. This aspect of the study was managed by the NASA Nutritional Biochemistry Laboratory. This team has extensive experience with these types of sample collections, sample handling, and analyses. The laboratory is ISO certified, and participates in a number of external proficiency testing programs, including American Proficiency Institute (API), College of American Pathologists (CAP), and Vitamin D External Quality Assessment Scheme (DEQAS), and National Institute of Standards and Technology (NIST), as detailed per test below. All analyses (detailed below) were run in duplicate, and had to meet assay specifications (e.g., within the assayed standard curve, and with high and low level quality control samples having met laboratory guidelines for run acceptability). Results of these types of tests from this laboratory have been published many times (Morgan et al. 2012; Smith et al. 2003, 2009a, b, 2012b, 1998, 2005a, 2014, 2008; Spector et al. 2009; Zwart et al. 2011a, 2009a, b, 2005, 2007, 2004, 2011b; 2013a, b, 2010).
Fasting (>10 h) blood samples for endocrine and other biochemical measurements were collected at the same time of day to minimize the effect of diurnal changes in endocrine and biochemical markers. Trained medical technologists performed blood collection, using blood drawn from an antecubital vein using butterfly needle collection techniques. Appropriate tubes, anticoagulants, and processing techniques were used to obtain serum, plasma, or whole blood, as required. All samples were stored at −70 °C and assayed simultaneously after completion of the study. Each of the biochemical/endocrine profile determinations required 15 mL of blood. Thus, a total of 45 mL of blood was collected over the 12-week study period per subject.
Urine samples were collected as separate voids into individual bottles over a period of 24 h. The volume of each was determined, and a 24-h pool was prepared using standard techniques. After total volume and pH measurements were made, aliquots were removed, processed, and frozen at −70 °C until batch analysis.
Blood measurements
PTH was assayed for the intact peptide by radioimmunoassay (Active iPTH IRMA Kit, Diagnostic Systems Laboratories, Webster, TX, <4 % within and <6 % between assay CV, API proficiency testing). Serum 25-hydroxy- and 1,25-dihydroxyvitamin D were measured by Diasorin Inc (Minneapolis, MN) radioimmunoassay kits. CVs were 4.78 % (within-assay) and 13.25 % (between-assay) for serum 25-hydroxy-vitamin D; CVs for 1,25 dihydroxyvitamin D were 7.46 % (within-assay) and 15.20 % (between-assay). Proficiency testing was conducted with DEQAS, CAP, API and NIST for 25-hydroxy-vitamin D and with DEQAS and CAP for 1,25-dihydroxyvitamin D as described previously (Smith et al. 2009a, 2012a, 2005a, b, 2008; Zwart et al. 2011b, 2013b). Serum total osteocalcin was determined by radioimmunoassay (Biomedical Technologies/Alfa Aesar, Ward Hill, MA, 5.66 % within and 5.63 % between assay CV) (Smith et al. 2012a, 2005b, 2014, 2008, 2009b; Zwart et al. 2011a, 2009b). BSAP activity was determined by enzyme-linked immunoassay (MicroVue™ BAP Kit, Quidel, San Diego, CA, 4.56 % within and 7.79 % between assay CV, CAP proficiency testing) (Smith et al. 2012a, 2005b, 2014, 2008, 2009b; Zwart et al. 2011a, 2009b). Total alkaline phosphatase was measured by colorimetry on an Olympus AU480® analyzer (Beckman Coulter, Inc., Brea, CA, 0.9 % within and 2.6 % between assay CV, API proficiency testing) (Smith et al. 2012a, 2005b, 2014, 2008, 2009b; Zwart et al. 2011a, 2009b). Serum calcium was measured by flame atomic absorption spectroscopy (PerkinElmer, Inc., Waltham, MA, 1.9 % within and 2.5 % between assay CV, API proficiency testing) (Smith et al. 2012a, b, 2005a, b, 2014, 2008, 2009b; Zwart et al. 2011a, 2009b).
Urine measurements
Urine samples were analyzed for collagen crosslinks using commercially available kits (Pyrilinks™ and Pyrilinks™-D, Quidel, Inc. Santa Clara, CA; Osteomark® ELISA kit, Ostex International, Inc., Seattle, WA). CVs were 5.96 % within and 9.49 % between assay for Pyrilinks™ and 5.23 % within and 11.76 % between assay for Pyrilinks™-D, as previously published (Smith et al. 2003, 2004, 1999, 2005a, b, 2014, 2008, 2009b). CAP proficiency testing is performed for these assays. These widely used markers of bone resorption are extremely reliable when evaluating within-subject interventions (e.g., spaceflight countermeasure use), and among other benefits, these are not affected by dietary gelatin intake (Eastell et al. 1997; Eyre 1992; Smith et al. 2004). In addition to bone resorption markers, 3-methyl histidine (3-MH), a marker of muscle catabolism was evaluated (L-8800 Amino Acid Analyzer, Hitachi Instruments Incorporated, Danbury, CT, 1.53 % within and 12.70 % between assay CV) (Morgan et al. 2012; Zwart et al. 2009b). Urinary calcium was determined by flame atomic absorption spectroscopy (PerkinElmer, Inc., Waltham, MA, 1.86 % within and 2.52 % between assay CV, CAP proficiency testing) (Smith et al. 2012a, b, 2005a, b, 2014, 2008, 2009b; Zwart et al. 2011a, 2009b). Creatinine was determined by photometry on an ACE Alera® analyzer (Alfa Wassermann, Inc., West Caldwell, NJ, 2.99 % within and 3.30 % between assay CV, CAP proficiency testing) (Smith et al. 2012a, 2005a, b, 2014, 2008, 2009b; Zwart et al. 2011a, 2009b). Urine pH was determined by potentiometry (Thermo Electron Corp., Beverly, MA, 0.29 % within and 0.24 % between assay CV, CAP proficiency testing) (Morgan et al. 2012; Smith et al. 2012a, 2005a, 2008; Zwart et al. 2009a).
Statistical analyses
All measures were analyzed using a two-way repeated measures analysis of variance. Tukey’s post hoc analysis was performed when significant differences were revealed. Within group pre- and post-training measures were analyzed using paired t tests with a Bonferroni adjustment to detect a significant effect of training. The average concentric and eccentric loads lifted across groups were analyzed using a one-way analysis of variance. Significance level was set a priori at P ≤ 0.05. All data are presented as mean ± SE unless otherwise noted. Statistical analyses were completed using SPSS version 13.0 (SPSS, Inc., Chicago, Il).
Results
Subject characteristics and exercise training
There were no pre-training differences between groups in age, height, or body weight (Table 2). Body weight did not change in any of the groups from pre- to post-training.
Subject compliance with the training protocol was excellent; no individual subject missed more than two scheduled training sessions. The mean compliance for each group was >95 % (0 %: 97 ± 1 %; 33 %: 97 ± 1 %; 66 %: 99 ± 1 %; 100 %: 96 ± 1 %; 138 %: 97 ± 1 %). Primarily because groups were balanced according to pre-training 1-RM, there were no differences between groups for the average concentric load lifted over the course of the 8-week training period.
Muscle strength and lean tissue mass
There was a main effect of training on leg press 1-RM and a significant group by training interaction (P < 0.05). All groups significantly increased leg press 1-RM with training (P < 0.05), but the 138 % group experienced a greater increase in leg press strength (20.1 ± 3.7 %) than the 0 % (7.9 ± 2.8 %), 33 % (7.7 ± 4.6 %) and 66 % (7.5 ± 4.3 %) groups. However, there was no difference (P = 0.15) between the 100 % (12.8 ± 5.9 %) and 138 % groups (Fig. 4). There was a main effect of training on calf press 1-RM (P < 0.05), but there was no group by training interaction. All groups (33 %: 7.5 ± 6.1 %; 66 %: 6.6 ± 3.7 %; 100 %: 12.2 ± 11.8 %; 138 %: 11.0 ± 6.4 %) significantly increased calf press 1-RM from pre- to post-training, except for the 0 % group (4.9 ± 9.1 %). WBLM did not change from pre- to post-training in any group. LLM increased significantly (P ≤ 0.01) from pre- to post-training in the 138 % group only (Table 3). No main effects or interactions were detected for WBLM or LLM.
Bone mineral density
Total hip, femoral neck, and hip intertrochanter BMD were unchanged from pre- to post-training in all groups (Table 4). Greater trochanter BMD increased after training (P ≤ 0.03) only in the 138 % group. There was a main effect of training on lumbar spine (L1, L2, L3, L4 and total lumbar), but there were no group by training interactions. Total lumbar BMD increased in every group except the 100 % group, but there were no between group differences (Table 5; Fig. 5). For both leg and whole body BMD, no main effects or within-group pre- to post-training differences were observed (Table 6).
Bone metabolism markers
There were no pre- to post-training differences between groups in the bone biochemical markers, but several main effects of training were observed (Tables 7, 8). Parathyroid hormone concentrations were higher at the mid- and post-training measurements (P = 0.01). While bone-specific alkaline phosphatase (P = 0.12) only tended to increase as a result of training, a significant effect was observed for total alkaline phosphatase (P < 0.001). There was no effect of training on blood sodium, potassium, or ionized calcium. Bone resorption markers (i.e., n-telopeptide, pyridinium crosslinks, deoxypyridinoline) were not affected by training.
Discussion
We evaluated the early-phase musculoskeletal effects of 8 weeks of lower body resistance exercise at different levels of eccentric loading in young, untrained males. Our results indicate that eccentric overload training, in which the eccentric load exceeds the concentric load, produces superior increases in leg press strength compared to eccentric underload training, in which the eccentric load is less than the concentric load. Further, only eccentric overload training elicited an increase in LLM and greater trochanter BMD. Thus, eccentric overload training appears to be an efficient resistance exercise training modality, providing positive early-phase adaptations for both muscle and bone. These findings are relevant to a variety of clinical and athletic populations desiring to improve or maintain musculoskeletal health. Further, these findings may influence the design of exercise hardware for long-duration exploration spaceflight. This is the first training study to evaluate a range of eccentric loads and to report both muscle and bone outcomes.
Muscle mass and strength
To our knowledge, no other studies have examined the effects of training with reduced eccentric loads (i.e., <100 % of concentric load), although concentric-only training has been previously evaluated (Colliander and Tesch 1990; Dudley et al. 1991b; Hather et al. 1991; Vikne et al. 2006) and on the whole was shown to be inferior to coupled eccentric-concentric exercise and eccentric-only training. Our observations that eccentric underload training resulted in leg press strength gains which were less than the eccentric overload and were quantitatively less than conventional training agree with these previous reports. It appears that training with eccentric loads <100 % of the concentric load is no more effective than concentric-only training, and decidedly inferior to eccentric overload training. However, at what point there is no benefit to eccentric exercise, perhaps somewhere between 66 and 99 % of the concentric load, cannot be determined at this time from this or other studies.
Our findings regarding eccentric overload training are in broad agreement with previous investigations that also report similar, if not superior, early-phase gains in muscle mass and strength from eccentric overload training compared to conventional 1:1 training. Brandenburg et al. observed greater increases in elbow extensor/flexor concentric 1-RM strength after 9 weeks for an eccentric overload training group compared to traditional 1:1 training in habitually weight-trained young men; however, neither group increased muscle CSA, perhaps due to their weight-trained status (Brandenburg and Docherty 2002). In a study that examined adaptations to low intensity-high repetition training (30 % 1-RM), such training with eccentric overload elicited gains in muscle CSA and strength in contrast to a traditional 1:1 regimen which did not (Friedmann et al. 2004). Friedmann-Bette et al. also evaluated a high intensity lower body protocol (8-RM loads) over 6 weeks in resistance-trained men who used either an eccentric overload or conventional 1:1 training. Both groups increased muscle CSA and maximal concentric strength with no differences between the groups; however, only eccentric overload training increased vertical jump height and Type IIx fiber CSA (Friedmann-Bette et al. 2010). Conversely, in untrained young women, Schroeder et al. reported no differences between eccentric overload (120 % concentric 1-RM) and a conventional 1:1 training group in concentric strength increases for five of six exercises assessed (Schroeder et al. 2004). Both groups also increased WBLM with no differences between them (Schroeder et al. 2004). Although eccentric overload training does not definitively outperform conventional 1:1 resistance exercise training, it is clear from our work and that of others (Roig et al. 2009) that it elicits comparable, if not superior, muscle outcomes for the lower and upper body in both trained (Vogt and Hoppeler 2014) and untrained individuals.
The benefits of increasing eccentric loading during training, however, were not evident in the plantar flexor muscles. In the present study, four of the five groups increased calf press strength, but there were no differences between groups. The calf musculature appears to be more resistant to adaptation with strength training (Alkner and Tesch 2004), and therefore, a longer training period, greater volume (Trappe et al. 2007), or more intense eccentric loading may be necessary to elicit group differences. This may be partly due to the primarily type I fiber composition of the gastrocnemius and soleus muscles (Trappe et al. 2001). Eccentric overload training appears to preferentially affect type II muscle fibers, which are more abundant in the quadriceps (Friedmann-Bette et al. 2010; Hather et al. 1991; Hortobagyi et al. 1996).
While increases in muscle strength were expected, it was unclear whether this relatively short 8-week training program would increase LLM in all the groups studied. It is well accepted that much of the strength increase realized during the early weeks of resistance exercise training is attributable to neural adaptations (Moritani and deVries 1979; Sale 1988), i.e., increases in strength per unit muscle mass (Ploutz et al. 1994). The present study supports this paradigm as the 0–100 % groups increased leg press strength despite non-significant changes in LLM. However, LLM was increased in the 138 % group, which likely contributed to the greater increase in leg press strength compared to the other training groups. Our results indicate that eccentric overload training provides sufficient hypertrophic stimulus for gains to be realized in a shorter time than more traditional 1:1 exercise training. Similar observations, suggesting an accelerated rate of muscle hypertrophy induced by eccentric overload training compared to conventional training over a relatively short training duration (≤9 weeks) are found in some (Friedmann-Bette et al. 2010; Norrbrand et al. 2008), but not all, previous reports (Brandenburg and Docherty 2002).
Bone mineral density and metabolism
Previous investigators have demonstrated that increases in BMD with resistance exercise are slow and that often at least 4 months are required to observe significant changes (Hawkins et al. 1999; Layne and Nelson 1999; Loehr et al. 2011; Schneider et al. 2003). To our knowledge, only two studies have even reported bone outcomes measured by DXA after only 8 weeks of resistance exercise training; one found no changes (Alcaraz et al. 2011) while we reported an increase in greater trochanter BMD in a traditional free weight training group at the mid-point of a 16-week study (Loehr et al. 2011). However, a recent study used a more sensitive measure of bone density and architecture, peripheral quantitative computed tomography (pQCT), along with custom analysis software to detect increases in tibial trabecular BMD after 8 weeks of exercise training in young women (Evans et al. 2012). The unique contributions of the present study are that no other work has examined the changes in BMD to decreased levels of eccentric loading and no one has previously reported the effects of eccentric overload training on bone after only 8 weeks of resistance exercise. Although there were no between group differences for the BMD measurements, the leg press and calf press exercise training protocol did elicit improvements in BMD. In our experiences (Loehr et al. 2011; Schneider et al. 2003) and those of others (Martyn-St James and Carroll 2006; Schroeder et al. 2004) using traditional resistance exercise consisting of a 1:1 eccentric:concentric ratio emphasizing axial loading of the spine and lower body, the most consistent observation has been the increase in lumbar BMD. In the present study, after only 8 weeks of training, subjects also experienced an increase in lumbar BMD, across all groups independent of eccentric loading (main effect). Paradoxically, within the training group that performed exercises with loading most similar to previous reports, the 100 % group, lumbar BMD was not increased; the reason for this finding is unclear. However, as previously discussed, only a few studies have reported BMD responses after just 8 weeks of training; thus, our understanding of early-phase bone adaptations to resistance exercise training and individual response variability is limited. Although it did not examine a response to exercise, a fascinating cross-sectional study offers some insight into this largely unexplored area of individual variation (Jepsen et al. 2011). The investigators compared the functional traits (e.g., bending stiffness) of large, robust tibias with those of slender tibias and found that, to compensate for their inferior morphology, the slender tibias modulated mineralization and cortical porosity although the compensatory changes were insufficient to result in equivalent bending stiffness (Jepsen et al. 2011). Baseline differences such as these may affect exercise-induced adaptations in bone, but to our knowledge, this has not been evaluated.
Increased BMD has been associated with increased mechanical loading and muscle strength following resistance exercise (Hawkins et al. 1999; Nickols-Richardson et al. 2007; Snow-Harter et al. 1992). Supporting this concept, we observed an increase in BMD of the greater trochanter only in the 138 % group. The eccentric overload group not only experienced a greater mechanical load during training, but also demonstrated the greatest increase in muscle strength. Because no other training groups experienced this increase in BMD, there may be some minimal threshold of musculoskeletal loading that must be achieved before the change in BMD can be detected in this length of study; the work of Frost and others supports this notion (Burr and Martin 1992; Frost 1987a, b, 1990a, b; Jee and Frost 1992; Turner 1998). If maximal eccentric muscle actions are capable of producing site-specific responses in BMD (Hawkins et al. 1999), then performing exercises with an eccentric overload may provide the necessary stimulus to increase BMD.
Varying levels of eccentric training did not have a differential effect on bone biochemistry as assessed with blood and urine biochemical markers. Across all groups, parathyroid hormone concentrations were increased as a result of exercise, and there was a trend for increased markers of bone formation and turnover (bone-specific alkaline phosphatase, total alkaline phosphatase, osteocalin). We (Loehr et al. 2011) and others (Schroeder et al. 2004) have reported similar responses in bone formation markers with resistance training and in bed rest subjects performing heavy resistance exercise (Shackelford et al. 2004). The combination of these results suggests that bone formation is elevated relatively early in a resistance training program and remains elevated without further increases through 16 weeks of training. The elevated markers of bone formation from these studies are congruent with the increase in BMD observed in the lower body, particularly the spine. Bone resorption markers were unaffected by exercise in this study, and in other training studies (Schneider et al. 2003), and the same has been seen in bed rest (Shackelford et al. 2004), and on the ISS (Smith et al. 2012a). There are some inconsistencies in this literature, however, as there was an increase in N-telopeptide and helical peptide in a 16-week training study with a more aggressive training protocol (Loehr et al. 2011). Further, Schroeder et al. observed a decrease in deoxypyridinoline after 16 weeks of intense eccentric training (Schroeder et al. 2004).
Spaceflight implications
Inclusion of high load eccentric and concentric muscle actions has been suggested as an important component of exercise countermeasures during spaceflight (Baldwin et al. 1996; Hargens et al. 1989), particularly during long-duration International Space Station (ISS) missions and extended exploration missions to the moon, Mars or an asteroid. Muscle atrophy, reduced muscle strength, increased calcium turnover, and decreased BMD have been consistently observed following spaceflight (Gopalakrishnan et al. 2010; LeBlanc et al. 2000a, b; Smith et al. 2005a; Tesch et al. 2005; Trappe et al. 2009), despite the regular performance of inflight countermeasures. However, the inability to maintain musculoskeletal health during spaceflight may be related to the forms of exercise that have been available historically to the astronauts and/or their nutritional practices. Aerobic-style cycle and treadmill exercise have predominated the inflight exercise countermeasure program since the early spaceflight program (Moore et al. 2010), with only very recent inclusion of high load resistance exercise. The resistance exercise device used on early ISS missions, the interim Resistive Exercise Device (iRED), failed to prevent losses in muscle mass, strength (Trappe et al. 2009), and BMD (Lang et al. 2004); losses were no different than on the Russian Mir Space Station when resistance exercise was limited to high tension bungee cords (Lee et al. 2004a). This is perhaps not surprising given that iRED provided only a 136 kg peak load and eccentric loading was only ~70 % of the correspondent concentric resistance (Amonette et al. 2004; Lee et al. 2004b). More recently, a resistance exercise device was deployed on the ISS with enhanced capabilities compared to iRED, including peak loads of 272 kg and eccentric loads >90 % of the concentric load (Loehr et al. 2011). The Advanced Resistive Exercise Device (ARED) has served as the primary resistance exercise device on the ISS since 2009 and, in combination with adequate energy intake, has shown promise to better protect muscle mass, strength, and BMD than its predecessor (Smith et al. 2012a, 2014). These observations in spaceflight are in agreement with reports from bed rest using conventional resistance exercise training with weight stack machines to protect muscle and bone (Akima et al. 2003; Bamman et al. 1998, 1997; Ferrando et al. 1997; Shackelford et al. 2004; Trappe et al. 2007).
Current plans for exploration missions to the moon, Mars, or other distant surfaces will entail long transit periods in microgravity in a small capsule and stays on extraterrestrial surfaces with <1-Earth gravity. Spaceflight hardware for exploration missions will be severely constrained in mass, volume, and the availability of electric power, which likely precludes a large exercise device like ARED. Thus, there is a need to optimize the resistance exercise device to achieve the greatest musculoskeletal benefits possible with minimal volume and power requirements. A gravity-independent resistance exercise device utilizing an inertial flywheel has been developed to provide eccentric overload with minimal power demand. Training with this device has been shown to protect muscle volume and strength (Alkner and Tesch 2004) and attenuate bone losses (Rittweger et al. 2005) during prolonged bed rest. Thus, eccentric overload training may be effective in spaceflight to prevent losses in muscle mass, strength, and BMD in a more time- (i.e., more mechanical work performed per unit time) and energy-efficient manner (Dudley et al. 1991a). Further, eccentric overload training may minimize muscle damage resulting from eccentric contractions after reloading upon return to a gravitational environment (Prisby et al. 2004). For these reasons, eccentric overload capabilities merit strong consideration for incorporation into future exploration exercise hardware.
Conclusions
Eccentric overload training was superior to eccentric underload training in its ability to stimulate increases in leg press strength and was the only training regimen to increase leg lean tissue mass and greater trochanter BMD after only 8 weeks of training. While we demonstrated this effect in young, healthy subjects, we suspect that a similar training protocol would benefit aging and rehabilitative populations when employed with appropriate professional oversight. Further, a similar training protocol may improve the efficacy of exercise countermeasures employed to prevent unloading-induced muscle atrophy, decreased muscle strength, and reduced BMD in bedridden patients and in astronauts during prolonged spaceflight.
Abbreviations
- 1-RM:
-
One-repetition maximum
- ARED:
-
Advanced Resistive Exercise Device
- BMD:
-
Bone mineral density
- CSA:
-
Cross-sectional area
- DXA:
-
Dual energy X-ray absorptiometry
- iRED:
-
interim Resistive Exercise Device
- ISS:
-
International Space Station
- JSC:
-
Johnson Space Center
- LLM:
-
Leg lean mass
- NASA:
-
National Aeronautics and Space Administration
- pQCT:
-
Peripheral quantitative computed tomography
- WBLM:
-
Whole body lean mass
References
Akima H, Ushiyama J, Kubo J, Tonosaki S, Itoh M, Kawakami Y, Fukuoka H, Kanehisa H, Fukunaga T (2003) Resistance training during unweighting maintains muscle size and function in human calf. Med Sci Sports Exerc 35:655–662
Alcaraz PE, Perez-Gomez J, Chavarrias M, Blazevich AJ (2011) Similarity in adaptations to high-resistance circuit vs. traditional strength training in resistance-trained men. J Strength Cond Res 25:2519–2527
Alfredson H, Nordstrom P, Pietila T, Lorentzon R (1999) Bone mass in the calcaneus after heavy loaded eccentric calf-muscle training in recreational athletes with chronic achilles tendinosis. Calcif Tissue Int 64:450–455
Alkner BA, Tesch PA (2004) Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 93:294–305
Amonette WE, Bentley JR, Lee SMC, Loehr JA, Schneider SM (2004) Ground reaction force and mechanical differences between the interim resistive exercise device (ired) and smith machine while performing a squat. NASA Technical Paper, TP-212063, Washington, D.C
Baldwin KM, White TP, Arnaud SB, Edgerton VR, Kraemer WJ, Kram R, Raab-Cullen D, Snow CM (1996) Musculoskeletal adaptations to weightlessness and development of effective countermeasures. Med Sci Sports Exerc 28:1247–1253
Bamman MM, Hunter GR, Stevens BR, Guilliams ME, Greenisen MC (1997) Resistance exercise prevents plantar flexor deconditioning during bed rest. Med Sci Sports Exerc 29:1462–1468
Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC (1998) Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 84:157–163
Bassey EJ, Ramsdale SJ (1994) Increase in femoral bone density in young women following high-impact exercise. Osteoporos Int 4:72–75
Bassey EJ, Rothwell MC, Littlewood JJ, Pye DW (1998) Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res 13:1805–1813
Brandenburg JP, Docherty D (2002) The effects of accentuated eccentric loading on strength, muscle hypertrophy, and neural adaptations in trained individuals. J Strength Cond Res 16:25–32
Burr DB, Martin RB (1992) Mechanisms of bone adaptation to the mechanical environment. Triangle (Sandoz) 31:59–76
Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS (2002) Muscular adaptations in response to three different resistance-training regimens: Specificity of repetition maximum training zones. Eur J Appl Physiol 88:50–60
Colliander EB, Tesch PA (1990) Effects of eccentric and concentric muscle actions in resistance training. Acta Physiol Scand 140:31–39
Crameri RM, Aagaard P, Qvortrup K, Langberg H, Olesen J, Kjaer M (2007) Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol 583:365–380
Crenshaw AG, Karlsson S, Styf J, Backlund T, Friden J (1995) Knee extension torque and intramuscular pressure of the vastus lateralis muscle during eccentric and concentric activities. Eur J Appl Physiol Occup Physiol 70:13–19
Doan BK, Newton RU, Marsit JL, Triplett-McBride NT, Koziris LP, Fry AC, Kraemer WJ (2002) Effects of increased eccentric loading on bench press 1rm. J Strength Cond Res 16:9–13
Dudley GA, Tesch PA, Harris RT, Golden CL, Buchanan P (1991a) Influence of eccentric actions on the metabolic cost of resistance exercise. Aviat Space Environ Med 62:678–682
Dudley GA, Tesch PA, Miller BJ, Buchanan P (1991b) Importance of eccentric actions in performance adaptations to resistance training. Aviat Space Environ Med 62:543–550
Eastell R, Colwell A, Hampton L, Reeve J (1997) Biochemical markers of bone resorption compared with estimates of bone resorption from radiotracer kinetic studies in osteoporosis. J Bone Miner Res 12:59–65
English KL, Loehr JA, Laughlin MA, Lee SMC, Hagan RD (2008) Reliability of strength testing using the advanced resistive exercise device and free weights. NASA Technical Paper, TP-214728, Washington, D.C
Evans RK, Negus CH, Centi AJ, Spiering BA, Kraemer WJ, Nindl BC (2012) Peripheral qct sector analysis reveals early exercise-induced increases in tibial bone mineral density. J Musculoskelet Neuronal Interact 12:155–164
Eyre DR (1992) New biomarkers of bone resorption. J Clin Endocrinol Metabol 74:470A–470C
Ferrando AA, Tipton KD, Bamman MM, Wolfe RR (1997) Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol 82:807–810
Friedmann B, Kinscherf R, Vorwald S, Muller H, Kucera K, Borisch S, Richter G, Bartsch P, Billeter R (2004) Muscular adaptations to computer-guided strength training with eccentric overload. Acta Physiol Scand 182:77–88
Friedmann-Bette B, Bauer T, Kinscherf R, Vorwald S, Klute K, Bischoff D, Muller H, Weber MA, Metz J, Kauczor HU, Bartsch P, Billeter R (2010) Effects of strength training with eccentric overload on muscle adaptation in male athletes. Eur J Appl Physiol 108:821–836
Frost HM (1987a) Bone “mass” and the “mechanostat”: a proposal. Anat Rec 219:1–9
Frost HM (1987b) The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner 2:73–85
Frost HM (1990a) Skeletal structural adaptations to mechanical usage (satmu): 1. Redefining wolff’s law: the bone modeling problem. Anat Rec 226:403–413
Frost HM (1990b) Skeletal structural adaptations to mechanical usage (satmu): 2. Redefining wolff’s law: the remodeling problem. Anat Rec 226:414–422
Frost HM (2001) From wolff’s law to the utah paradigm: insights about bone physiology and its clinical applications. Anat Rec 262:398–419
Gabriel DA, Kamen G, Frost G (2006) Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med 36:133–149
Gopalakrishnan R, Genc KO, Rice AJ, Lee SM, Evans HJ, Maender CC, Ilaslan H, Cavanagh PR (2010) Muscle volume, strength, endurance, and exercise loads during 6-month missions in space. Aviat Space Environ Med 81:91–102
Hargens AR, Parazynski S, Aratow M, Friden J (1989) Muscle changes with eccentric exercise: implications on earth and in space. Adv Myochem 2:299–312
Hather BM, Tesch PA, Buchanan P, Dudley GA (1991) Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand 143:177–185
Hawkins SA, Schroeder ET, Wiswell RA, Jaque SV, Marcell TJ, Costa K (1999) Eccentric muscle action increases site-specific osteogenic response. Med Sci Sports Exerc 31:1287–1292
Higbie EJ, Cureton KJ, Warren GL 3rd, Prior BM (1996) Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J Appl Physiol 81:2173–2181
Hortobagyi T, Hill JP, Houmard JA, Fraser DD, Lambert NJ, Israel RG (1996) Adaptive responses to muscle lengthening and shortening in humans. J Appl Physiol 80:765–772
Hortobagyi T, Devita P, Money J, Barrier J (2001) Effects of standard and eccentric overload strength training in young women. Med Sci Sports Exerc 33:1206–1212
Jee WSS, Frost HM (1992) Skeletal adaptations during growth. Triangle (Sandoz) 31:77–88
Jepsen KJ, Centi A, Duarte GF, Galloway K, Goldman H, Hampson N, Lappe JM, Cullen DM, Greeves J, Izard R, Nindl BC, Kraemer WJ, Negus CH, Evans RK (2011) Biological constraints that limit compensation of a common skeletal trait variant lead to inequivalence of tibial function among healthy young adults. J Bone Miner Res 26:2872–2885
Jones DA, Rutherford OM (1987) Human muscle strength training: the effects of three different regimens and the nature of the resultant changes. J Physiol 391:1–11
Josse AR, Tang JE, Tarnopolsky MA, Phillips SM (2010) Body composition and strength changes in women with milk and resistance exercise. Med Sci Sports Exerc 42:1122–1130
Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR (2004) American college of sports medicine position stand: physical activity and bone health. Med Sci Sports Exerc 36:1985–1996
Kryger AI, Andersen JL (2007) Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and mhc isoforms. Scand J Med Sci Sports 17:422–430
Lacerte M, de Lateur BJ, Alquist AD, Questad KA (1992) Concentric versus combined concentric-eccentric isokinetic training programs: effect on peak torque of human quadriceps femoris muscle. Arch Phys Med Rehabil 73:1059–1062
Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A (2004) Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 19:1006–1012
Layne JE, Nelson ME (1999) The effects of progressive resistance training on bone density: a review. Med Sci Sports Exerc 31:25–30
LeBlanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, Schenkman B, Kozlovskaya I, Oganov V, Bakulin A, Hedrick T, Feeback D (2000a) Muscle volume, mri relaxation times (t2), and body composition after spaceflight. J Appl Physiol 89:2158–2164
LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, Voronin L (2000b) Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact 1:157–160
Lee S, Shackelford L, Smith S, Guilliams M, Shepherd B, Loehr JA, Laughlin MA, Chauvin J, Hagan RD (2004a) Lean tissue mass and muscle strength: does resistive exercise during space flight prevent deconditioning? Med Sci Sports Exerc 36:S272
Lee SM, Cobb K, Loehr JA, Nguyen D, Schneider SM (2004b) Foot-ground reaction force during resistive exercise in parabolic flight. Aviat Space Environ Med 75:405–412
Loehr JA, Lee SM, English KL, Sibonga J, Smith SM, Spiering BA, Hagan RD (2011) Musculoskeletal adaptations to training with the advanced resistive exercise device. Med Sci Sports Exerc 43:146–156
Lohman T, Going S, Pamenter R, Hall M, Boyden T, Houtkooper L, Ritenbaugh C, Bare L, Hill A, Aickin M (1995) Effects of resistance training on regional and total bone mineral density in premenopausal women: A randomized prospective study. J Bone Miner Res 10:1015–1024
Martyn-St James M, Carroll S (2006) Progressive high-intensity resistance training and bone mineral density changes among premenopausal women: evidence of discordant site-specific skeletal effects. Sports Med 36:683–704
Menkes A, Mazel S, Redmond RA, Koffler K, Libanati CR, Gundberg CM, Zizic TM, Hagberg JM, Pratley RE, Hurley BF (1993) Strength training increases regional bone mineral density and bone remodeling in middle-aged and older men. J Appl Physiol 74:2478–2484
Moore AD, Lee SMC, Stenger MB, Platts SH (2010) Cardiovascular exercise in the U.S. Space program: past, present and future. Acta Astronaut 66:974–988
Morgan JL, Zwart SR, Heer M, Ploutz-Snyder R, Ericson K, Smith SM (2012) Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J Appl Physiol 113:1519–1529
Moritani T, de Vries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58:115–130
Narici M, Kayser B, Barattini P, Cerretelli P (2003) Effects of 17-day spaceflight on electrically evoked torque and cross-sectional area of the human triceps surae. Eur J Appl Physiol 90:275–282
Nickols-Richardson SM, Miller LE, Wootten DF, Ramp WK, Herbert WG (2007) Concentric and eccentric isokinetic resistance training similarly increases muscular strength, fat-free soft tissue mass, and specific bone mineral measurements in young women. Osteoporos Int 18:789–796
Norrbrand L, Fluckey JD, Pozzo M, Tesch PA (2008) Resistance training using eccentric overload induces early adaptations in skeletal muscle size. Eur J Appl Physiol 102:271–281
Oliveira AS, Caputo F, Aagaard P, Corvino RB, Goncalves M, Denadai BS (2013) Isokinetic eccentric resistance training prevents loss in mechanical muscle function after running. Eur J Appl Physiol 113:2301–2311
Ploutz LL, Tesch PA, Biro RL, Dudley GA (1994) Effect of resistance training on muscle use during exercise. J Appl Physiol 76:1675–1681
Prisby RD, Nelson AG, Latsch E (2004) Eccentric exercise prior to hindlimb unloading attenuated reloading muscle damage in rats. Aviat Space Environ Med 75:941–946
Riley DA, Bain JL, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL (2000) Decreased thin filament density and length in human atrophic soleus muscle fibers after spaceflight. J Appl Physiol 88:567–572
Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, Felsenberg D (2005) Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: results from the ltbr study. Bone 36:1019–1029
Roig M, O’Brien K, Kirk G, Murray R, McKinnon P, Shadgan B, Reid WD (2009) The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: A systematic review with meta-analysis. Br J Sports Med 43:556–568
Ryan AS, Ivey FM, Hurlbut DE, Martel GF, Lemmer JT, Sorkin JD, Metter EJ, Fleg JL, Hurley BF (2004) Regional bone mineral density after resistive training in young and older men and women. Scand J Med Sci Sports 14:16–23
Sale DG (1988) Neural adaptation to resistance training. Med Sci Sports Exerc 20:S135–S145
Schneider SM, Amonette WE, Blazine K, Bentley J, Lee SMC, Loehr JA, Moore AD Jr, Rapley M, Mulder ER, Smith SM (2003) Training with the international space station interim resistive exercise device. Med Sci Sports Exerc 35:1935–1945
Schroeder ET, Hawkins SA, Jaque SV (2004) Musculoskeletal adaptations to 16 weeks of eccentric progressive resistance training in young women. J Strength Cond Res 18:227–235
Schuenke MD, Herman JR, Gliders RM, Hagerman FC, Hikida RS, Rana SR, Ragg KE, Staron RS (2012) Early-phase muscular adaptations in response to slow-speed versus traditional resistance-training regimens. Eur J Appl Physiol 112:3585–3595
Seger JY, Arvidsson B, Thorstensson A (1998) Specific effects of eccentric and concentric training on muscle strength and morphology in humans. Eur J Appl Physiol Occup Physiol 79:49–57
Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D (2004) Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 97:119–129
Smith SM, Nillen JL, Leblanc A, Lipton A, Demers LM, Lane HW, Leach CS (1998) Collagen cross-link excretion during space flight and bed rest. J Clin Endocrinol Metab 83:3584–3591
Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE, Rice BL, Lane HW (1999) Calcium metabolism before, during, and after a 3-mo spaceflight: Kinetic and biochemical changes. Am J Physiol 277:R1–R10
Smith SM, Davis-Street JE, Fesperman JV, Calkins DS, Bawa M, Macias BR, Meyer RS, Hargens AR (2003) Evaluation of treadmill exercise in a lower body negative pressure chamber as a countermeasure for weightlessness-induced bone loss: a bed rest study with identical twins. J Bone Miner Res 18:2223–2230
Smith SM, Dillon EL, DeKerlegand DE, Davis-Street JE (2004) Variability of collagen crosslinks: impact of sample collection period. Calcif Tissue Int 74:336–341
Smith SM, Wastney ME, O’Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V, Shackelford LC (2005a) Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J Bone Miner Res 20:208–218
Smith SM, Zwart SR, Block G, Rice BL, Davis-Street JE (2005b) The nutritional status of astronauts is altered after long-term space flight aboard the international space station. J Nutr 135:437–443
Smith SM, Zwart SR, Heer M, Lee SMC, Baecker N, Meuche S, Macias BR, Shackelford LC, Schneider S, Hargens AR (2008) Wise-2005: Supine treadmill exercise within lower body negative pressure and flywheel resistive exercise as a countermeasure to bed rest-induced bone loss in women during 60-day simulated microgravity. Bone 42:572–581
Smith SM, Gardner KK, Locke J, Zwart SR (2009a) Vitamin d supplementation during antarctic winter. Am J Clin Nutr 89:1092–1098
Smith SM, Zwart SR, Heer MA, Baecker N, Evans HJ, Feiveson AH, Shackelford LC, Leblanc AD (2009b) Effects of artificial gravity during bed rest on bone metabolism in humans. J Appl Physiol 107:47–53
Smith SM, Heer MA, Shackelford L, Sibonga JD, Ploutz-Snyder L, Zwart SR (2012a) Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J Bone Miner Res 27:1896–1906
Smith SM, McCoy T, Gazda D, Morgan JL, Heer M, Zwart SR (2012b) Space flight calcium: implications for astronaut health, spacecraft operations, and earth. Nutrients 4:2047–2068
Smith SM, Zwart SR, Heer M, Hudson EK, Shackelford L, Morgan JL (2014) Men and women in space: bone loss and kidney stone risk after long-duration spaceflight. J Bone Miner Res 29(7):1639–1645
Snow-Harter C, Whalen R, Myburgh K, Arnaud S, Marcus R (1992) Bone mineral density, muscle strength, and recreational exercise in men. J Bone Miner Res 7:1291–1296
Spector ER, Smith SM, Sibonga JD (2009) Skeletal effects of long-duration head-down bed rest. Aviat Space Environ Med 80:A23–A28
Staron RS, Malicky ES, Leonardi MJ, Falkel JE, Hagerman FC, Dudley GA (1990) Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. Eur J Appl Physiol Occup Physiol 60:71–79
Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, Hagerman FC, Hikida RS (1994) Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol 76:1247–1255
Tesch PA, Thorsson A, Colliander EB (1990) Effects of eccentric and concentric resistance training on skeletal muscle substrates, enzyme activities and capillary supply. Acta Physiol Scand 140:575–580
Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD (2005) Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol 93:463–468
Trappe SW, Trappe TA, Lee GA, Costill DL (2001) Calf muscle strength in humans. Int J Sports Med 22:186–191
Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW (2007) Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 191:147–159
Trappe S, Costill D, Gallagher P, Creer A, Peters JR, Evans H, Riley DA, Fitts RH (2009) Exercise in space: Human skeletal muscle after 6 months aboard the international space station. J Appl Physiol 106:1159–1168
Turner CH (1998) Three rules for bone adaptation to mechanical stimuli. Bone 23:399–407
Vikne H, Refsnes PE, Ekmark M, Medbo JI, Gundersen V, Gundersen K (2006) Muscular performance after concentric and eccentric exercise in trained men. Med Sci Sports Exerc 38:1770–1781
Vogt M, Hoppeler HH (2014) Eccentric exercise: mechanisms and effects when used as training regime or training adjunct. J Appl Physiol 116(11):1446–1454
Vuori I, Heinonen A, Sievanen H, Kannus P, Pasanen M, Oja P (1994) Effects of unilateral strength training and detraining on bone mineral density and content in young women: A study of mechanical loading and deloading on human bones. Calcif Tissue Int 55:59–67
Westing SH, Seger JY (1989) Eccentric and concentric torque-velocity characteristics, torque output comparisons, and gravity effect torque corrections for the quadriceps and hamstring muscles in females. Int J Sports Med 10:175–180
Westing SH, Cresswell AG, Thorstensson A (1991) Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur J Appl Physiol Occup Physiol 62:104–108
Zwart SR, Hargens AR, Smith SM (2004) The ratio of animal protein intake to potassium intake is a predictor of bone resorption in space flight analogues and in ambulatory subjects. Am J Clin Nutr 80:1058–1065
Zwart SR, Davis-Street JE, Paddon-Jones D, Ferrando AA, Wolfe RR, Smith SM (2005) Amino acid supplementation alters bone metabolism during simulated weightlessness. J Appl Physiol 99:134–140
Zwart SR, Hargens AR, Lee SM, Macias BR, Watenpaugh DE, Tse K, Smith SM (2007) Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone 40:529–537
Zwart SR, Crawford GE, Gillman PL, Kala G, Rodgers AS, Rogers A, Inniss AM, Rice BL, Ericson K, Coburn S, Bourbeau Y, Hudson E, Mathew G, Dekerlegand DE, Sams CF, Heer MA, Paloski WH, Smith SM (2009a) Effects of 21 days of bed rest, with or without artificial gravity, on nutritional status of humans. J Appl Physiol 107:54–62
Zwart SR, Oliver SM, Fesperman JV, Kala G, Krauhs J, Ericson K, Smith SM (2009b) Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat Space Environ Med 80:A15–A22
Zwart SR, Pierson D, Mehta S, Gonda S, Smith SM (2010) Capacity of omega-3 fatty acids or eicosapentaenoic acid to counteract weightlessness-induced bone loss by inhibiting nf-kappab activation: from cells to bed rest to astronauts. J Bone Miner Res 25:1049–1057
Zwart SR, Booth SL, Peterson JW, Wang Z, Smith SM (2011a) Vitamin k status in spaceflight and ground-based models of spaceflight. J Bone Miner Res 26:948–954
Zwart SR, Mehta SK, Ploutz-Snyder R, Bourbeau Y, Locke JP, Pierson DL, Smith SM (2011b) Response to vitamin d supplementation during antarctic winter is related to bmi, and supplementation can mitigate epstein-barr virus reactivation. J Nutr 141:692–697
Zwart SR, Morgan JL, Smith SM (2013a) Iron status and its relations with oxidative damage and bone loss during long-duration space flight on the international space station. Am J Clin Nutr 98:217–223
Zwart SR, Parsons H, Kimlin M, Innis SM, Locke JP, Smith SM (2013b) A 250 µg/week dose of vitamin d was as effective as a 50 µg/d dose in healthy adults, but a regimen of four weekly followed by monthly doses of 1250 µg raised the risk of hypercalciuria. Br J Nutr 110:1866–1872
Acknowledgments
We would like to thank the subjects for their participation in this training study; Karen Paulder, Sydney Stein, and Leah Stroud for their assistance with exercise training and data collection; Mary Jane Maddocks of the NASA JSC Bone and Mineral Laboratory for acquisition and analysis of DXA scans; the NASA JSC Nutritional Biochemistry Laboratory for the collection and analysis of blood and urine samples; Dr. Mitzi Laughlin for statistical analysis; Drs. Brian Arenare, Todd Schlegel, and Linda Shackelford for medical monitoring; the NASA JSC Human Test Subject Facility for test subject recruitment; Jackie Reeves and Dr. Meghan Downs for editorial and technical review of the manuscript; and Linda Loerch, Dr. Jennifer Tuxhorn, and Dr. Clarence Sams for their programmatic support of this research. Finally, we will always be grateful for the mentoring, support, and friendship of our NASA JSC Exercise Physiology Laboratory lead, the late Dr. R. Donald Hagan.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards
The experiments described herein were conducted in compliance with the laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
The authors, K. L. English and J. A. Loehr, contributed equally to this work.
Rights and permissions
About this article
Cite this article
English, K.L., Loehr, J.A., Lee, S.M.C. et al. Early-phase musculoskeletal adaptations to different levels of eccentric resistance after 8 weeks of lower body training. Eur J Appl Physiol 114, 2263–2280 (2014). https://doi.org/10.1007/s00421-014-2951-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-2951-5