Abstract
The purpose of this review is to describe the unique anatomical and physiological features of the hands and feet that support heat conservation and dissipation, and in so doing, highlight the importance of these appendages in human thermoregulation. For instance, the surface area to mass ratio of each hand is 4–5 times greater than that of the body, whilst for each foot, it is ~3 times larger. This characteristic is supported by vascular responses that permit a theoretical maximal mass flow of thermal energy of 6.0 W (136 W m2) to each hand for a 1 °C thermal gradient. For each foot, this is 8.5 W (119 W m2). In an air temperature of 27 °C, the hands and feet of resting individuals can each dissipate 150–220 W m2 (male–female) of heat through radiation and convection. During hypothermia, the extremities are physiologically isolated, restricting heat flow to <0.1 W. When the core temperature increases ~0.5 °C above thermoneutral (rest), each hand and foot can sweat at 22–33 mL h−1, with complete evaporation dissipating 15–22 W (respectively). During heated exercise, sweat flows increase (one hand: 99 mL h−1; one foot: 68 mL h−1), with evaporative heat losses of 67–46 W (respectively). It is concluded that these attributes allow the hands and feet to behave as excellent radiators, insulators and evaporators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the evolution of homo sapiens, selection pressures probably favoured individuals capable of regulating body temperature, and who possessed the intellectual capacity to develop behavioural responses that resulted in protection from, and the modification of, the thermal environment (Heinrich 1977; Crompton et al. 1978). One can only speculate regarding the dominant characteristics that ensured survival, but it is reasonable to assume that these co-existed with other favourable attributes, such as a sizeable endurance capacity (Ruben 1995; Bennett et al. 2000). Integral within this natural selection was the structural and physiological modifications of the hands and feet, and it is the roles that these appendages play in temperature regulation that is the focus of this review.
Hands and feet evolved through their roles in locomotion, food gathering, tool use and the provision of sensory feedback (Lovejoy et al. 2009). Indeed, hand evolution was probably linked with tool use (Marzke and Marzke 2000; Young 2003), with hands becoming tools with which the brain manipulated objects (Putz and Tuppek 1999; Marzke 1992; van Duinen and Gandevia 2011). The continual refinement of the intricate neural, vascular and musculoskeletal structures provided appendages possessing remarkable dexterity. Similarly, the feet evolved from grasping appendages, driven by adopting bipedal locomotion (Harcourt-Smith and Aiello 2004; Preuschoft 2004; Wang and Crompton 2004). Thus, from small arboreal graspers evolved a relatively large, bipedal, homeothermic species with considerable physical endurance. However, homo sapiens could not have survived unless they also possessed effective autonomic and behavioural mechanisms for heat conservation and dissipation. Whilst much is known about these human capacities, detail pertaining to the roles of the hands and feet within these processes is fragmentary, and this review is an attempt to fill that gap as well as building upon existing knowledge. Also provided is a description of the morphological, physiological and biophysical characteristics of the hands and feet as participants within an integrated temperature regulatory system.
Morphological characteristics
The dry and evaporative exchanges of thermal energy within the body and with the thermal environment are dictated by temperature and water vapour pressure gradients, the size of each exposed surface relative to its mass, and by the convective (mass flow) and conductive delivery of heat to the superficial tissues. Thus, morphological characteristics exert a powerful influence on heat exchange.
Anatomical structures
The human hand contains 27 bones, and the foot 26, which are arranged in three groups, of which the digits can be wholly exposed to the surrounding environment (Standring 2008; for online and interactive hand and foot resources, see: Mahadevan et al. (2000) and McGrouther et al. (2000)). The bones make up ~20 % (males) and 30 % (females) of the hand mass, and about 28 % (males) and 31 % (females) of the foot mass (Table 1). These tissues behave as heat sinks.
Active skeletal muscles can liberate large amounts of thermal energy. However, since the principal muscles that control the foot reside within the leg (Standring 2008), and since the 11 intrinsic foot muscles are only responsible for fine toe movements, there is no sizeable heat source within the foot. Thus, thermal energy for the feet comes mainly from other sources which, in combination with their frequently intimate contact with large heat sinks, increases their susceptibility to cold injury (Golden et al. 2013). Nevertheless, this configuration supports survival, since incapacitation of the foot due to protracted hypothermia does not result in immobilisation. This is not so for the hand. It has more than 30 muscles (Standring 2008), with ten controlling the thumb, and the rest producing hand and finger movements. Nineteen of these muscles are found within the hand, and these are responsible for precise finger movements and object manipulation. These muscles represent 20–30 % of the hand mass (female–male dimensions); they are relatively inactive and poorly insulated (Table 1). Consequently, the hand also has limited heat production capability, and when significantly cooled, hand functions become adversely affected (Hunter et al. 1952; Brajkovic and Ducharme 2003; Zander and Morrison 2008; Daanen 2009). Thus, lower-limb function can be retained, albeit impaired, long after losing fine hand movements.
Segmental surface areas and masses
The whole-body surface area to mass ratio for an average, adult man and woman is 0.024–0.025 m2 kg−1 [male–female: assuming 79.1 kg, 1.72 m; and 66.2 kg, 1.60 m, respectively (Table 1; also see ISO/TR 7250-2 2010)], with a larger ratio associated with a greater potential for heat exchange. When separate body segments are analysed, one finds considerable variability in these ratios. For instance, the surface area to mass ratio of the hand, relative to the whole body, is 4.1–5.2 times greater (male–female), whilst that of the foot is 2.9–3.2 times greater (male–female; Table 1). Yet the combined surface areas of both hands and both feet represent only 4.4–4.6 % (female–male) and 7.1–7.4 % (female–male) of the body’s total surface area (respectively), making these appendages morphologically well suited for dissipating heat. Indeed, the feet, and especially the hands, are excellent radiators if sufficient heat can be delivered by the blood (convective delivery or mass heat flow).
Neural connections
A detailed description of the neurophysiology of the hands and feet is beyond the current scope. However, a brief overview of this topic provides important background information for understanding thermal sensory feedback, and the control of the thermoeffectors within these appendages.
Innervation of the hands and feet
The somatosensory nerves that innervate the hands and feet provide common pathways not only for autonomic and motor control, but also for sensory feedback. Three such nerves innervate each hand (Standring 2008). Firstly, and perhaps of greatest functional significance, is the ulnar nerve, which enters the hand ventrally, passing below the palmarcarpal ligament and dividing into deep and superficial segments. Since it innervates most of the intrinsic hand muscles, except those responsible for the delicate digit movements (Standring 2008), then the preservation of the functional integrity of the hand relies upon the protection of this nerve. The second major pathway is the median nerve. It also passes below the palmarcarpal ligament and is the most important sensory nerve of the hand (Standring 2008). In addition, it innervates the muscles that control fine thumb and finger movements. Finally, there is the superficial branch of the radial nerve, which enters the hand at the base of the thumb. It carries the cutaneous sensory information from the dorsolateral surface of the hand as well as from parts of the thumb and the first two fingers (Standring 2008).
Each foot has five somatosensory nerves (Standring 2008). Branches of the tibial nerve (medial calcaneal, medial and lateral plantar nerves) innervate the heel, the sole and the first four toes. These enter from behind the medial malleolus, with the lateral plantar nerve controlling the intrinsic foot muscles (Standring 2008). Secondly, the superficial fibular nerve traverses the dorsal foot, carrying most of the cutaneous sensory information for that surface. The deep fibular nerve innervates three muscles and carries some sensory afferents from the first and second toes (Standring 2008). Finally, two nerves innervate the medial ankle and upper foot (saphenous nerve) and the lateral aspect of the foot (sural nerve; Standring 2008).
Thermoafferent feedback
Thermoreceptors
Thermoreceptors are located throughout the deep and superficial structures of the body (Boulant 2011; Pierau 2011) with the latter providing our first source of thermal awareness. The warm- and cold-sensitive receptors (free nerve endings) are not homogeneously distributed across the skin surface (Hardy and Oppel 1938), and all are found in a three-dimensional configuration within the lower epidermis (Iggo 1969; Hensel et al. 1974; Ivanov et al. 1986).
One generally considers the volar (glabrous) aspects of the fingers to possess greater thermosensitivity, though this is perhaps more due to habitual hand use to obtain sensory feedback than it is to their thermoreceptor density. Indeed, Zotterman (1959) identified 7–9 cold-sensitive spots per cm2 within the skin of the dorsal fingers and hands, but only 2–5 spots cm−2 for the volar skin. The feet have a similar distribution (dorsal: 5.6 spots cm−2; volar: 3.4 spots cm−2), whilst the warm-sensitive sensors are more evenly distributed (0.4–1.7 spots cm−2 across all sites; Zotterman 1959). Furthermore, the cold-sensitive receptors respond to temperatures ranging from −5 to 43 °C, whilst warm-sensitive sensors operate over 28–48 °C (Hensel 1981). This greater response range, in combination with their greater density, is of considerable functional significance during cold exposure and defence.
Thermoafferent pathways and thermosensitivity
Most cutaneous sensory flow from the hand occurs through the radial and median nerves, whilst that from the foot is carried in the tibial (plantar surface), superficial fibular (dorsal surface) and saphenous nerves (laterodorsal ankle surface; Standring 2008). Of these two appendages, the nerves of the hands are better protected, with those of the feet being more vulnerable. For instance, the tibial and sural nerves pass behind the malleoli (Standring 2008), and are relatively well protected. However, poorly designed shoes that come into firm contact with the malleoli may impinge upon these nerves. Moreover, the superficial fibular nerve passes across the upper surface of the foot, so shoes that are tight across that surface may also adversely affect neural function.
Cutaneous thermoafferents travel to the spinal cord, with neurons from the hands entering the dorsal (sensory) roots of the sixth, seventh and eighth cervical spinal nerves, and those from the foot feeding into the first sacral and the fifth lumbar spinal nerves (Michael-Titus et al. 2007; Standring 2008). This arrangement means that anatomically related skin regions share common neural pathways (dermatomes). The spinal cord is thermosensitive, as well as being a relay through which signals pass and undergo some synaptic modification and convergence (Simon 1974; Simon et al. 1998).
Within the spinal cord, second-order thermal afferents ascends via the lateral spinothalamic tract (Willis et al. 1973; Brück and Hinkel 1990), eventually reaching the somatosensory cortex and the hypothalamus. We consciously perceive our environment from sensory feedback to the former, the organisation of which enables discrimination among different skin surfaces. Indeed, the sensory acuity of some surfaces is much greater than that of others, due to variations in peripheral innervation density and also the volume of the somatosensory cortex assigned to those regions. Thus, sites with superior acuity have a greater innervation density as well as a greater cortical representation (Penfield and Rasmussen 1952). These are both characteristics of the hands and fingers, which, along with the face, are our most sentient of structures. The feet and toes also have considerable sensory importance. Accordingly, feedback from any of these surfaces heavily influences thermal sensation, which appears to be age dependent (Taylor et al. 1995). In the hypothalamus, sensory neurons synapse with both the warm- and cold-sensitive hypothalamic neurons (Boulant and Hardy 1974), and the integration of these signals gives rise to the autonomic regulation of body temperature by controlling heat loss, heat conservation and thermogenesis (Werner et al. 2008).
At this point, it is necessary to briefly review the relative importance of thermal feedback from the hands and feet (physiological thermosensitivity), since animal research has established regional differences in cutaneous thermosensitivity (Hales and Hutchinson 1971; Ingram and Legge 1972; Necker 1977). In humans, however, differential cutaneous thermosensitivities have not been convincingly demonstrated, despite several attempts (Nadel et al. 1973; Crawshaw et al. 1975; Werner and Heising 1990; Bothorel et al. 1991). The problem was that thermosensitivity was evaluated using closed-loop methods, in which selected (relatively small) skin sites were heated and cooled, but the temperatures of the untreated surfaces were not controlled. Under such conditions, local heating or cooling will elicit generalised thermoeffector responses which, in turn, modify the temperatures of larger non-treated surfaces, modifying thermal feedback and altering thermoeffector function. For example, forearm heating can increase whole-body sweating and evaporative heat loss. This lowers the mean skin temperature and can counteract the sudomotor responses induced by the initial treatment.
To address this limitation, Cotter and Taylor (2005) examined cutaneous thermosensitivities for sweating in supine, resting individuals during the local warming and cooling of ten skin sites. Throughout each manipulation, the deep-body (core) and remaining skin temperatures were clamped above the sweat threshold (Cotter et al. 1995; Patterson et al. 1998), opening feedback loops from untreated skin and ensuring that changes in sweating could be assigned solely to feedback arising from the treated region. With respect to the hands and feet, neither site differed significantly in thermosensitivity from each other, or from any other treated site (Cotter and Taylor 2005). That is, local thermal stimulation displayed equivalent and minimal autonomic impact on whole-body sweating. However, these open-loop experiments have not yet been performed to evaluate local cutaneous thermosensitivies with respect to cutaneous blood flow.
Thermoefferent pathways
Sweating and cutaneous vasomotor responses are driven by both thermal (Kuno 1938; Johnson and Kellogg 2010; Roddie 2011) and non-thermal stimulations (Kenny and Journeay 2010; Kondo et al. 2010). Thus, whilst whole-body heating induces sweating and cutaneous vasodilatation, the subsequent initiation of exercise is accompanied by a reduction in cutaneous blood flow and a further elevation in sweat secretion (Christensen and Nielsen 1942; van Beaumont and Bullard 1963). In this case, neural feedforward (central command) emanating from the rostral brain simultaneously activates the motor and sympathetic neurons (Kondo et al. 2010). These generalisations apply also to the thermoeffectors of the hands and feet, with the thermal sudomotor and cutaneous vascular responses being controlled by the preoptic anterior hypothalamus (Teague and Ranson 1936; Hardy et al. 1964; Boulant et al. 1989).
The thermally activated sudomotor efferents descend through the brain stem and spinal tract, terminating in the lateral horn region and synapsing with neurons that innervate the eccrine sweat glands (Sato 1977). The classical work of List and Peet (1938) first revealed the spinal segments through which these efferents left the spine, with thoracic segments T2–T8 innervating sweat glands of the upper limbs, and the lower thoracic and lumbar segments (T11–L2) relaying sudomotor efferents to the lower extremities. These neurons are post-ganglionic, sympathetic fibres producing the neurotransmitter acetylcholine (Schotzinger and Landis 1988) which targets the muscarinic receptors of the clear cells. Efferent signals reach the sweat glands in waves, such that each sudomotor unit secretes sweat in a pulsatile fashion, with a period of 0.60–0.74 s (Bini et al. 1980; Nilsson et al. 1980). This secretion pattern occurs in synchrony with motor units innervating other skin regions (Nakayama and Takagi 1959; van Beaumont et al. 1966), thus confirming their autonomic linkage with the hypothalamus. Moreover, secretions from the volar and dorsal (non-glabrous) surfaces of the hands and feet are also synchronised (van Beaumont et al. 1966; Taylor and Machado-Moreira 2013), although this is not always observed (Nakayama 1969).
An absence of this sudomotor synchronisation between the volar and dorsal surfaces of the hands and feet is consistent with different pathways innervating each area, which may, in turn, be activated by different central mechanisms. Furthermore, it might indicate that different control centres modulate sweating from each skin surface, and some have hypothesised that dorsal secretion is driven by the hypothalamic thermoregulatory centre whilst a separate centre controls psychological sweating from the volar surfaces (Darrow 1937; Kuno 1956; Ogawa 1975). It has even been proposed that these psychological responses were noradrenergically mediated (Robertshaw 1977; Nakazato et al. 2004). Indeed, this hypothetical modulation of thermal and non-thermal sweating has become the frequently accepted teaching (Iwase et al. 1997), although those views are not held by the current authors, and these theories are explored and challenged within a subsequent section of this communication.
It has long been known that two separate neural mechanisms modify the dilatation of cutaneous arterial blood flow (passive and active dilatation: Kellogg 2006; Roddie 2011). Indeed, the research legacy behind this knowledge can be traced to Bernard (1852), and readers are directed to reviews by Rowell (1993), Charkoudian (2003), Kellogg (2006) and Roddie (2011), with the following text providing a distillation specific to the control of blood vessels that perfuse the skin of the hands and feet.
For the volar surfaces of the hands, and by default, also those of the feet, only one control mechanism modulates cutaneous blood flow (Roddie 2011), with this pathway first being identified through the research of Lewis and Pickering (1931) and then by Roddie et al. (1957b). This mechanism operates via active constriction during both thermoneutral and cold states, with strong vasoconstriction also seen during various non-thermal stresses, such as exercise (Blair et al. 1961). Therefore, in thermoneutral conditions, blood flow to the volar skin surfaces is minimal, like that for the nose, lips and ears (Roddie 2011). Thus, the vasoconstricted state is normal for these areas, and venous pooling is also minimal. Constriction is brought about through the tonic release of noradrenaline from the sympathetic fibres that innervate these blood vessels (Roddie 2011). Noradrenaline activates both the alpha 1 and alpha 2 receptors that respectively dominate the arterial and venous vessels of the volar skin surfaces (Bodelsson et al. 1990). In warm–hot conditions, this sympathetic vaso- and venomotor tone is withdrawn, leading to a pressure-mediated (passive) dilatation (Blair et al. 1960).
On the dorsal surfaces of the hands, and again presumably the feet, there exist active vasodilatory mechanisms (Johnson et al. 1995). When the body is heated, the cutaneous arterioles actively dilate, with the corresponding smooth muscle activation reducing the vascular transmural pressure, and facilitating a cutaneous blood flow elevation (Roddie et al. 1957a). Under thermoneutral conditions, there is some tonic constrictor tone to these skin regions; however, the active dilatory pathways are silent (Roddie 2011). Cooling induces greater noradrenergic constriction, but does not influence the vasodilatory mechanism (Roddie 2011).
Vasomotor characteristics
Blood vessels of the hands and feet
The arteries
Blood flow to the hand is provided by the brachial artery, which terminates below the elbow, dividing into the radial and ulnar arteries (Standring 2008). The former passes over the wrist to the dorsal hand, then down through the first dorsal interosseous muscle to the palm. Near the fifth metacarpal, the radial artery forms the deep palmar arch and joins the deep branch of the ulnar artery. The superficial palmar branch of the radial artery provides blood to the thenar muscles. On the palmar aspect, the radial artery also gives rise to smaller vessels that feed the thumb and index finger. The deep palmar arch is formed by vessels from the radial and ulnar arteries. Three metacarpal arteries run along the second, third and fourth interossei, and join the digital branches of the superficial palmar arch (Standring 2008). The latter divides to form the common palmar digital branches that descend on the lumbricals and join the corresponding palmar metacarpal artery. The main blood vessels for the fingers now arise and run down both sides of each digit before eventually terminating in the subcutaneous tissues of the finger tips (Standring 2008).
Each foot receives blood from arteries that enter behind each malleolus (medial and lateral malleolar arteries), and across the dorsal foot surface (dorsalis pedis artery; Standring 2008), and their positioning, with respect to the bones, has significant implications for shoe design. Thus, poorly designed or incorrectly fitted footwear may reduce blood flow through these arteries. Along the dorsal foot, dorsalis pedis forms the medial and lateral tarsal arteries, the arcuate artery and the first dorsal metatarsal artery (Standring 2008). This last vessel supplies blood to the first and second toes, whilst the second, third and fourth dorsal metatarsal arteries supply both their respective digits plus one neighbour. Eventually, each gives rise to two dorsal digital arteries. On the plantar surface, the medial plantar artery (a sub-division of the posterior tibial artery) enters below the medial malleolus (Standring 2008). This vessel supplies the foot muscles, the skin of the medial sole and the three medial toes. The lateral plantar artery travels to the calcaneus, the adjacent muscles, the plantar tarsal and tarso-metatarsal joints, and the lateral sole. Eventually it joins dorsalis pedis, forming the plantar arch deep in the foot. The four plantar metatarsal arteries are now formed, feeding the digital arteries as per the dorsal metatarsal arteries. However, the plantar digital arteries are the major arterial source for the toes (Standring 2008).
Capillaries and arteriovenous anastomoses
Cutaneous capillaries typically have internal diameters of ~10 µm (Molyneux and Bryden 1981), and it is through the walls of these vessels that exchanges occur between the blood and the interstitial compartment (Starling 1896). In the skin, capillaries are in the papillary region, looping up towards the surface before descending to the superficial venous plexus (Standring 2008). In the fingers and toes, the density of these capillaries is quite variable. For instance, Zhong et al. (2000) reported a fingernail capillary density of ~65 vessels mm−2, whilst Bukhari et al. (2000) found 6.5 vessels mm−2 on the nailfold. However, perhaps the most extensive work for the hand still remains that of Grant and Bland (1931), and these data are presented in Table 2. In the foot, Mørk et al. (2002) observed toenail bed capillary densities of 54 vessels mm−2, and Zhong et al. (2000) reported 38 vessels mm−2 on the big toe. For the whole foot, Lamah et al. (1999) found average densities of 34 vessels mm−2. From this evidence, one may conclude that capillary densities of the hands and feet are in the range 40–70 vessels mm−2.
These papillary capillaries are aligned perpendicularly to their arterial (rete subpapillare) and venous connections (Standring 2008), optimising the gradient for heat exchange. Since the papillae are not well perfused, then epidermal temperatures are heavily influenced by ambient temperature, and heat exchange with the blood is maximised (Conrad 1971). Moreover, since capillary flow is very slow (Hales 1985), then the blood rapidly equilibrates with tissue temperature.
However, whilst the papillary capillaries facilitate heat dissipation, this function is enhanced in a multiplicative manner by the cutaneous arteriovenous anastomoses that exist within the hands and feet. These vessels, first described by Sucquet (1862) and Hoyer (1877), are found in the skin of the hands, feet, ears, lips and nose, and almost exclusively on the glabrous surfaces (Clark 1938; Nagasaka et al. 1987a). The most complex and largest anastomoses are believed to exist within the palms and the soles (Clark 1938; Abramson 1965). Although, Grant and Bland (1931) found that anastomoses were most abundant in the nail beds of the hands and feet (500–600 anastomoses cm2), with the volar surfaces of the second toes being the next most plentiful sites (290 anastomoses cm2), followed by the volar aspect of distal phalanges of the fingers (150 anastomoses cm2). The palmar and plantar surfaces seem to possess widely variable distributions, ranging from 30 to 200 anastomoses cm2. However, most of the dorsal surfaces of the hands and feet appear not to have these arteriovenous anastomoses (Grant and Bland 1931). Readers are directed to Masson (1937) and Clark (1938) for both a critique of, and elaboration upon, these observations.
These anastomotic vessels are found deeper than the papillary capillaries, and they behave as capillary by-pass vessels and have internal diameters ranging from 25 to 125 µm (Hales 1985). These characteristics appear counter-intuitive, given the function of anastomoses in promoting heat loss. However, the paradox disappears when one considers volar blood flow during heat-induced vasodilatation. In this state, papillary blood flow rises, and the anastomotic vessels passively dilate, producing a much larger elevation in cutaneous blood flow. This occurs because dilatation of a vessel with a radius that is perhaps tenfold larger than a capillary, would, for the same pressure head, elicit a 10,000-fold greater blood flow elevation over the same tube length (Poiseuille’s law: Nelms 1963; Molyneux and Bryden 1981). Therefore, whilst this blood by-passes the more superficial papillary capillaries, it actually delivers more blood to the slower moving, deep venous plexus. Since the tissues surrounding these plexuses are poorly insulated, then heat exchanges with the ambient medium are enhanced (Midtgåtrd 1980; Nuzzaci et al. 1999).
Conversely, maximal constriction of the arterioles and the anastomoses reduces acral cutaneous blood flow to levels less than required to support basal metabolism (Abramson 1965), and heat is conserved. Thus, extremes of cold can result in protracted under-perfusion, giving rise to potentially debilitating consequences, including disturbances to manual dexterity, peripheral pain, work performance, and non-freezing and freezing cold injuries (Enander and Hygge 1990; Heus et al. 1995; Stocks et al. 2004; Imray et al. 2011; Golden et al. 2013). However, the anastomoses will intermittently open (cold-induced vasodilatation), with each blood flow surge helping to protect the surrounding tissues (Wilson and Goldman 1970; Nuzzaci et al. 1999; Daanen 2003). Readers are directed to supplementary resources for further discussion on cold-induced vasodilatation (Edwards and Burton 1960; Livingstone 1976; Bergersen et al. 1999; Daanen and Ducharme 1999; O’Brien 2005; van der Struijs et al. 2008; Cheung and Mekjavic 2007; Flouris and Cheung 2009; Keramidas et al. 2010).
The veins
The veins are the vascular capacitance vessels and may contain 70–80 % of the total blood volume in thermoneutral individuals (Pang 2001; Mertz 2004). Thus, changes to venous tone can have a pronounced influence on central blood volume and systemic blood pressure (Rowell 1993; Halliwill et al. 2013). The cutaneous veins similarly have a considerable capacity (Rowell 1993; Roddie 2011), and are well innervated and quite responsive to sympathetic stimulation (Zimmerman 1966; Webb-Peploe and Shepherd 1968). These veins participate principally in temperature regulation by reducing the volume of blood within the cutaneous tissue beds (Rothe 1983; Rowell 1983). In addition, the close proximity of veins and arteries within the hands and feet, but perhaps more importantly within the forearms and legs, means that, during cold exposures, heat carried in the arterial blood is transferred to the veins. This counter-current heat exchange reduces peripheral heat loss, and it was first recognised by Bernard (1876), with Forster et al. (1946), Scholander and Krog (1957) and Weinbaum et al. (1984) elaborating on this heat conservation mechanism. However, it was the classical work of Scholander and Schevill (1955) that demonstrated its significance in heat conservation for diving mammals.
Each finger possesses a venous network, with most blood passing to the dorsal surface, and through the metacarpal veins and the dorsal venous arch (Standring 2008). This latter structure is the largest superficial venous network of the hand and provides tributaries to the cephalic and basilic veins (Schmidt and Lanz 2004). On the palmar surface and within the limbs in general, there are three drainage routes: the deep and superficial palmar venous arches (draining into the radial and ulnar veins, respectively) and the more superficial venous plexus that feeds the median antebrachial vein (Schmidt and Lanz 2004). However, there exist numerous communicating conduits (perforator veins) between the palmar and dorsal vessels of the carpo-metacarpal region. These vessels pass through the interosseous spaces, permitting palmar blood to flow to the dorsal surface, but since 70 % of these vessels have valves, this flow is predominantly unidirectional (Zhang and Schmidt 1993). Thus, the perforator veins facilitate venous return that is assisted by muscle pumping during finger and hand flexion (Pegum and Fegan 1967b; Simons et al. 1996).
In the foot, blood drains from the toes through superficial veins on the dorsal and plantar surfaces (Standring 2008). The feet have several plantar and dorsal veins, the most significant of which are the two venous arches: the deep plantar arch and the more superficial dorsal arch (Standring 2008). The former runs the length of the metatarsal region and, as well as feeding into the posterior tibial vein, these vessels connect to the dorsal veins by perforating conduits at the foot margins (Pegum and Fegan 1967a). Perforating veins also join the plantar and dorsal veins, again with about half containing valves (Pegum and Fegan 1967a). Thus, venous blood from the plantar surface can return via the tibial veins and also through the foot fascia, mixing with blood from the dorsal surface, before leaving through the saphenous veins. This latter route is activated during lower-limb loading (compression pumping).
Since the veins are very compliant, a significant volume of blood within each appendage may be retained within these vessels (Levy et al. 1985), particularly when in a dependent position at rest. The resulting venous pooling increases blood vessel diameter and slows blood transit time, both of which optimise heat exchange. However, generalised or cutaneous venoconstriction shifts significant blood volumes away from the skin, preventing pooling and reducing vessel transit times. Therefore, the combination of these anatomical structures with the morphological configuration of the hands and feet allows these regions to potentially behave as radiators and insulators.
Mechanical modulation of hand and foot blood flow
Muscle pumping assists venous return from dependent regions (Halliwill et al. 2013), and this is particularly important for the hands and feet, both of which are susceptible to oedema. In addition, pressure loading and load bearing also modify arterial inflow and venous stasis (Pegum and Fegan 1967b; Broderick et al. 2010).
Activation of the forearm flexors when gripping objects elevates venous pressure and flow in the hand (Simons et al. 1996). Similarly, standing compresses the plantar venous arch, as does foot flexor activation (Broderick et al. 2010). Thus, whilst the anastomoses increase blood flow to the palmar and plantar skin of supine, resting individuals, compression pumping diverts this blood through the perforating veins to the dorsal skin. One might therefore expect these surfaces to become prime heat loss avenues during physical activity, perhaps with the overall contributions from these appendages being reduced relative to the resting state.
If these local pressures are excessive, blood flow is impeded. For instance, finger flows can be reduced by 85 % when local pressures of 30–52 kPa are applied, whilst palmar flow can tolerate pressures up to 100 kPa before experiencing a similar decline (Johansson et al. 2002). However, a 70-kg individual bearing the full body mass on both hands will only experience a palmar pressure of ~16 kPa, or a plantar pressure of only ~12 kPa when standing. Furthermore, the hands, and presumably the feet, resist compression through a reactive, pressure-induced vasodilatation of the cutaneous vasculature (Abraham et al. 2001), and this sustains tissue perfusion, albeit at a reduced level.
Thermal modulation of cutaneous blood flow
Hand and foot blood flows are rarely stable (Grant and Pearson 1938; Blair et al. 1961). Indeed, it is often when people are thermally comfortable that appendage flows are the most variable, with the vaso- and venomotor tone to these appendages constantly changing within the thermoneutral zone (Mekjavic and Eiken 2006; Werner et al. 2008). These changes modify the convective delivery of thermal energy to the skin, and with the whole-body cutaneous blood flow in thermoneutral males averaging ~350 mL min−1 (Rowell 1974, 1993), then ~5 kJ min−1 (83 W) of thermal energy will be transferred to the skin for the 4 °C core-skin gradient that typically obtains under these conditions. In fact, in suitably clothed people resting at 22–27 °C, thermal homeostasis can be achieved entirely through subtle changes in cutaneous blood flow, particularly to the hands, face and feet.
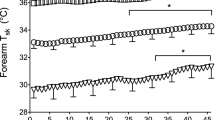
Blood flow to the skin of the hands and feet is modified centrally (Boulant 2011), with deep-body temperature dominating this modulation (Wenger et al. 1975; Proppe et al. 1976; Jessen 2011). This also applies to sweating, but with cutaneous feedback rising in importance during thermal adaptation (Regan et al. 1996; Tipton et al. 2013; Taylor 2014) or when the skin temperature is rapidly changed (Libert et al. 1978). With the exception of the arteriovenous anastomoses (Hales 1985), cutaneous blood vessels are also powerfully affected by local thermal changes (Taylor et al. 1984; Pérgola et al. 1993; Johnson and Kellogg 2010), the influence of which is related to the existing level of sympathetic tone (Spealman 1945; Pérgola et al. 1993; Caldwell et al. 2014). For instance, Spealman (1945) demonstrated that hand blood flow was always greatest when the deep-body tissues were warm (Fig. 1), and this deep-body dominance in the control of blood flow has recently been further verified for the foot (Caldwell et al. 2014).
Volume-specific hand blood flow during hand immersion at different water temperatures, but with the body uncomfortably warm (air temperature 32 °C), thermoneutral (air temperature 24 °C) or uncomfortably cold (air temperature 16 °C). Blood flow was powerfully modulated by the thermal state of the deep-body tissues when this temperature was elevated, regardless of local tissue temperatures. Data digitised from Spealman (1945) and used with permission
At the hands or feet, local cooling can elicit strong vasoconstriction (Grant and Pearson 1938; Blair et al. 1961; Pérgola et al. 1993; Caldwell et al. 2014), with blood flow in previously cooled individuals decreasing to below that needed for normal cellular function (0.8 mL 100 mL−1 min−1: Abramson 1965). Local skin heating of thermoneutral subjects first produces a large flow increase (rapid vasodilatation), followed by a slight constriction, then a gradual dilatation over 25–30 min (Pérgola et al. 1993; Minson et al. 2001). However, whilst powerful, these local thermal influences can neither abolish nor dominate central autonomic drive (Johnson et al. 1976; Pérgola et al. 1993; Caldwell et al. 2014). Thus, whilst it is generally assumed that (local) heating the skin to 42 °C induces maximal vasodilatation (Taylor et al. 1984), maximal hand and foot blood flows can only be obtained if one first induces some level of hyperthermia (Spealman 1945; Caldwell et al. 2014).
In a thermoneutral state, vasoconstrictor tone within the hands and feet dictates blood flow, with active dilatation being minimal (Blair et al. 1961). During whole-body cooling, cutaneous venoconstriction is activated, driving a significant blood volume back to the deep-body tissues. Simultaneously, a generalised vasoconstriction reduces arterial flow to the hands and feet. This is associated with a relatively stronger constriction of the sub-papillary veins and the intermediate venous plexuses, which are closer to the skin surface, and this means that venous blood is directed into the deeper and better insulated vessels. The arteriovenous anastomoses also close, and tissue insulation increases to conserve heat. Indeed, under these influences, a temperature gradient is established along the skin surface (Freeman and Nickerson 1938; Werner and Reents 1980), and also through cutaneous cross-sections (Pennes 1948; Webb 1992). Thus, the appendages behave as a protective barrier, with a physiological amputation of the extremities conserving deep-body heat as one becomes progressively colder (Forster et al. 1946; Scholander and Krog 1957; Caldwell et al. 2014). Without this insulating capacity, primitive humans would probably not have migrated beyond the temperate regions, unless they possessed heavily insulated hand and footwear.
In warmer conditions, veno- and vasoconstrictor tones may be abolished and pressure-induced, passive dilatation occurs. During maximal heating, whole-body cutaneous blood flow can reach 7–8 L min−1 (Rowell et al. 1970). Thus, for a 1 °C core-skin temperature gradient, this flow can now deliver thermal energy at a rate of 27 kJ min−1 (450 W). This elevates the skin temperature, which has three consequences, the first two of which are beneficial. Firstly, it buffers against external heat gains and helps to defend the temperatures of the deep-body tissues. Secondly, there is an elevation in cutaneous water vapour pressure which increases evaporation without the need to increase sweat flow. Finally, it reduces the convective and conductive heat flow from the deep-body tissues.
These skin temperature increases are most dramatic at the extremities (Maddock and Coller 1933; Werner and Reents 1980; Webb 1992). For the hands and feet, passive dilatation occurs along the volar surfaces, reinforced by opening the arteriovenous anastomoses. On the dorsal surfaces, active vasodilatation can also be initiated (Lewis and Pickering 1931; Greenfield 1963; Johnson et al. 1995), and the resulting cutaneous blood flow is much greater than would accompany vasoconstrictor tone withdrawal. Indeed, approximately 80–95 % of the flow increase within non-glabrous (dorsal) skin occurs through active vasodilatation (Kellogg 2006; Johnson and Proppe 2011), and these mechanisms mean that the hands and feet have a considerable capacity to both reduce and elevate cutaneous blood flow.
It has been estimated that the minimal blood flow to support cutaneous tissues is ~0.8 mL 100 mL−1 min−1 (Abramson 1965). In resting, thermoneutral males, basal (volume-specific) hand blood flow is approximately 6.7 mL 100 mL−1 min−1, whilst that of the foot is about 2.8 mL 100 mL−1 min−1 [Caldwell et al. 2014 (males)]. When normalised to segmental surface areas (Table 1), basal hand blood flow (550 mL m−2 min−1) is more than three times greater than might observed for the rest of the body [79.1 kg, 1.72 m (Table 1), absolute blood flow 350 mL min−1 (Rowell 1974, 1993); 183 mL m−2 min−1], and 1.6 times that observed in the foot (340 mL m−2 min−1). Such basal, area-specific flows emphasise the extent to which these appendages, particularly the hand, can function as radiators under thermoneutral states (Grant and Pearson 1938; Forster et al. 1946; Ferris et al. 1947).
Table 3 provides an historical summary of hand and foot blood flows across a range of treatments, revealing that the hands have an enormous potential for increasing blood flow and heat dissipation. If one assumes that volume-specific flows of 30 (hand) and 18 mL 100 mL−1 min−1 (foot) are maximal, with the respective minimal flows both approximating 0.2 mL 100 mL−1 min−1 (Table 3), then the hand supports a 150-fold blood flow increase over these extremes; for the foot, this is a 90-fold elevation. Indeed, both extremities can dramatically elevate tissue insulation by reducing blood flow to <25 % of their metabolic requirement. However, for lightly clad individuals resting in the cold, blood flows of ~0.4 (hand) and 0.2 mL 100 mL−1 min−1 are more typical (Table 3). Finger flows reveal even greater minimal-to-maximal ranges (0.2–120 mL 100 mL−1 min−1; Wilkins et al. 1938; Nagasaka et al. 1987b). Moreover, flow variations exist between the distal (higher) and middle phalanges of the fingers (Wilkins et al. 1938), and this is consistent with the distributions of both the arteriovenous anastomoses and eccrine sweat glands.
Notwithstanding the number of experiments in which hand and foot blood flows were investigated, until recently, a detailed description of these flows, across a range of whole-body thermal states, was not available. Indeed, most investigations focussed upon states approximating thermoneutrality. Therefore, the current authors developed water-displacement plethysmographs for the hand and foot that permitted independent control over local tissue temperatures (Caldwell and Taylor 2014). In combination with pre-experimental, whole-body cooling and heating (water immersion: Booth et al. 2001), followed by whole-body thermal clamping (Cotter and Taylor 2005), three steady-state thermal states were investigated: mild hypothermia [deep-body (oesophageal) temperature: 36.1 °C; mean skin temperature: 22.2 °C], thermoneutral (deep-body: 37.0 °C; mean skin: 33.6 °C) and moderate hyperthermia (deep-body: 38.5 °C; mean skin: 37.7 °C). Under each of these clamped conditions, five thermal treatments (5, 15, 25, 33, 40 °C) were applied to the hand and foot of supine, resting participants using rapid changes in the water temperature of each plethysmograph (Caldwell et al. 2014). These data are summarised in Table 4. From this experiment, three-dimensional surfaces were created that described the interactions of mean body and local tissue temperatures on segmental blood flows (Fig. 2a, b).
Three-dimensional surfaces for hand (a) and foot blood flows (b) across mean body temperatures from 31.2 to 38.3 °C and segmental temperatures from 5 to 40 °C. Semi-nude, supine subjects (resting N = 8) were pre-treated (water immersion) and clamped in three thermal states (separate trials): mild hypothermia, thermoneutral and moderate hyperthermia. Mean body temperatures were calculated using the weighted sum of oesophageal and mean skin temperatures using the following deep-body coefficients (Vallerand et al. 1992): 0.65 (mild hypothermia), 0.70 (thermoneutral) and 0.80 (moderate hyperthermia). Five hand and foot thermal treatments were applied (5, 15, 25, 33, 40 °C). Segmental blood flows were measured using water-displacement, venous-occlusion plethysmography. Data are averages for the 15 three-dimensional coordinates with points between derived through extrapolation. The colour spectra indicate flow graduations (red for highest), whilst the transparent (white) planes are thermoneutral blood flows for the opposite limb segment (oesophageal temperature 37.0 °C a, b, mean skin temperature 33.6 °C a, b, and respective foot and hand skin temperatures of 33.7 and 33.4 °C). From Caldwell et al. (2014) and reproduced with permission
Volume-specific hand blood flows exceeded those of the foot during all treatments (Fig. 2), and flow elevations with increments in segmental temperature (sensitivity) were significantly greater within each thermal state. When mildly hypothermic, these thermal treatments were barely able to override vasoconstriction. However, when hyperthermic, both segments demonstrated considerable sensitivity to these local temperature changes. Indeed, both appendages act as radiators when humans are hot, with the hands performing significantly better. This cutaneous vascular dilatation means that heat delivery to the skin is sustained even when hot individuals are immersed in cool, but not cold water (Caldwell et al. 2014), and this centrally mediated resistance to vasoconstriction provides a vascular mechanism that explains why immersion cooling of hyperthermic individuals can be achieved quite rapidly, even when using temperate water (26 °C; Taylor et al. 2008; Casa et al. 2010).
The descriptions above largely relate to blood flows measured during supine rest. However, during upright locomotion in the heat, it is highly likely that dilatation of the arteriovenous anastomoses within the feet will be much less effective, with load bearing redirecting plantar venous return to the dorsal foot surfaces, which may well be covered. When the hands are used for work, the contribution of the palmar surfaces is similarly reduced, and in both instances, the appendages are generally in dependent positions. Thus, whilst the hands and feet of unclothed, resting individuals can radiate large amounts of heat, this capacity is compromised during exercise and work.
Restoration of blood flow following hypothermia
Foot and hand blood flow within hypothermic individuals cannot be instantaneously restored (Wyndham and Wilson-Dickson 1951; Savard et al. 1985). In the early stage of recovery from acute hypothermia, this delay is due to the progressive reduction in deep-body temperature following rescue (afterdrop: Currie and Percival 1792; Currie 1797; Alexander 1945; Golden and Hervey 1977). Beyond this point, the combined influence of deep-body and peripheral cooling results in the maximal constriction of the arteriolar and venous vessels. If mean arterial pressure is normal, then peripheral blood flow restoration initially involves a release of this generalised constrictor tone. However, this only occurs if the deep-body tissues have first been warmed (Ferris et al. 1947; Savard et al. 1985; Brajkovic et al. 1998; Caldwell et al. 2014). The process involves sympathetic withdrawal (Freeman 1935), with a more complete disengagement occurring at the hands than at the feet (Pickering and Hess 1933; Caldwell et al. 2014). Thus, the feet retain constrictor tone, not only when thermoneutral (Caldwell et al. 2014), but also during rewarming, and even after the hands have dilated (Pickering and Hess 1933).
Cold-induced cutaneous vasoconstriction is maximal, even in moderate air temperatures (Bittel et al. 1988), and if this constriction has been protracted, then peripheral tissue temperatures will approximate ambient temperature. In this circumstance, smooth muscle relaxation will be impaired, even after constrictor tone has eased. Since the surrounding tissues have a relatively poor thermal conductivity, they will remain cool for some time, and this local impediment to the restoration of cutaneous blood flow is not immediately corrected (Bazett 1968). Indeed, blood flow is only fully restored when deep-body thermoneutral tissue temperatures have been re-established (Savard et al. 1985; Caldwell et al. 2014). However, the return of normal blood flow occurs faster to the hands than to the feet for an equivalent level of cold, physiological strain (Pickering and Hess 1933), with the application of external heating accelerating this process (Savard et al. 1985).
Sudomotor characteristics
Human skin in not impermeable, with both water and water vapour moving through the epidermis. In this section, the focus is primarily on eccrine sweating, but the hands and feet lose more water through transepidermal (insensible) water loss than any other body segment (Taylor and Machado-Moreira 2013).
In resting, thermoneutral individuals, whole-body transepidermal water loss occurs at approximately 20–43 mL h−1 (Taylor and Machado-Moreira 2013). The stratum corneum has a uniform thickness (10–20 µm), except at the volar surfaces of the hands and feet (both 400–600 µm: Rushmer et al. 1966; Scheuplein and Blank 1971). This thickness appears not to dictate vapour diffusion. In fact, water vapour flux through abdominal skin, for instance, is only about 10 % of that observed from the sole, and 30 % that of the palm (Scheuplein and Blank 1971), and water loss from the hands and feet appears to be 2–4 times greater than that from all other skin surfaces. Thus, the dorsal hands and feet lose ~0.05 mg cm−2 min−1, whilst the corresponding volar surfaces lose 0.13 and 0.10 mg cm−2 min−1 (respectively: Taylor and Machado-Moreira 2013).
Thermal sweating
Eccrine sweat gland distribution
Recent analysis of investigations involving over 320 data sets has shown that a reference adult (70.0 kg, 1.702 m, body surface area 1.807 m2: Miller et al. 1980) will possess some 2.06 million functional eccrine sweat glands (Taylor and Machado-Moreira 2013). On the non-glabrous surfaces of the hand and feet, these glands are found at the intersections of the skin creases (Johnson et al. 1970) and participate extensively in thermal sweating. Conversely, the epidermal ridges of the palmar and plantar surfaces contain glands at much greater densities (Johnson et al. 1970; Taylor and Machado-Moreira 2013). These glands are powerfully activated by non-thermal influences (e.g. psychological sweating; Kuno 1956; Machado-Moreira and Taylor 2012a, b), although they also respond strongly to thermal stimulation (Taylor et al. 2006; Machado-Moreira et al. 2008a), and in a manner very similar to the torso and head (Machado-Moreira et al. 2008b, c). The distribution of sweat glands across the hands and feet is summarised within Table 5, with minimal evidence of ethnic differences (Taylor 2006). These gland counts were determined following thermal, exercise, psychological and pharmacological stimulations, and if one assumes that the volar surfaces represent 39 % of the total hand surface area (Hsu and Yu 2010), and 79 % of the total foot surface area (Yu et al. 2010), then one may predict local sweat gland densities (glands cm−2) for each hand and foot from these data (Taylor and Machado-Moreira 2013) from a knowledge of total body surface area and the relative contribution of each appendage to that total area. Thus:
One could expect to find our reference person (1.8 m2) to have about 270,000 eccrine glands across both hands, 67 % of which would occur on the palmar surfaces. For the feet, the corresponding values would be 410,000 glands, with 77 % on the plantar surfaces. Since these glands typically have a mass of ~35 µg (Sato 1977) and an approximate length of 6.1 mm (Kuno 1956; Sato and Sato 1983), then the hands and feet of this individual would contain glands with a combined mass of ~24 g, and if laid end-to-end, their combined length would be approximately 4.1 km.
Sweat composition
Eccrine sweat gland activation initiates a calcium flux into the clear cells, followed by an active pumping of sodium into those cells and the passive influx of chloride and water (Sato 1977; Morimoto 1978). On the luminal sides of these cells, sodium–potassium pumps increase their activity, now transporting sodium ions into the glandular lumen, with chloride and water again following passively (Hashimoto 1978). This primary (precursor) sweat rapidly accumulates, eventually elevating the intra-luminal pressure to the point that sweat starts to flow through the coiled duct. En route, the surrounding cells actively extract sodium, with an obligatory reabsorption of chloride and water (Sato 1973). The resulting hypotonic fluid that reaches the skin surface forms the discharged sweat, with its volume and composition dictated by its transit time within the duct (Schwartz and Thaysen 1956). Indeed, across the physiological range of secretion rates, sweat sodium concentrations can change at least twofold in some people (Allan and Wilson 1971; Costill 1977). Thus, the composition of sweat is widely variable across (Dill et al. 1966; Maughan and Shirreffs 2008) and within individuals (Dill et al. 1967; Patterson et al. 2000).
Four groups have studied the composition of discharged sweat from the hands and feet (Collins 1962; Emrich et al. 1968; Yousef and Dill 1974; Patterson et al. 2000). At secretion rates of about 0.05 mg cm−2 min−1, one may expect palmar sweat to contain 20.9 mmol L−1 of sodium, 17.4 mmol L−1 of chloride and 18.8 mmol L−1 of potassium. On the dorsal surfaces of the hands, for which the corresponding sweat rate may be 0.50 mg cm−2 min−1, the respective ion losses would be 28.8, 28.3 and 5.7 mmol L−1. Plantar sweat composition seems not to have been reported. However, when the dorsal foot sweats at 0.56 mg cm−2 min−1, its sodium, chloride and potassium compositions approximate 24.3, 18.1 and 6.8 mmol L−1 (respectively). For comparative purposes, whole-body electrolyte losses, derived across sweat rates from 0.72 to 3.65 mg cm−2 min−1, would range from 26.5 to 49.7 mmol L−1 for sodium (95 % confidence interval), and chloride loss would be 26.8–36.7 mmol L−1, with a potassium loss of 2.7–4.5 mmol L−1 (Taylor and Machado-Moreira 2013). The palmar surfaces lose relatively small amounts of sodium and chloride, but potassium loss appears to be about five times greater than for the rest of the body. However, since only one data set was available for this electrolyte (Collins 1962), these data need to be verified and perhaps treated with caution at this time.
Sweat secretion during passive heating
The hydration state of the skin is essential for its normal function, and the stratum corneum contains about 0.9 mL water g−1 dry tissue (Scheuplein and Blank 1971). This content is sustained through vapour fluxes from deeper layers as well as reabsorption from sweat ducts and plays an important role in protecting the skin from injury (Wilcott 1966). These generalisations also apply to the hands and feet, and in the former instance, hydration affects contact friction and grip (Adelman et al. 1975) as well as the tactile and thermal sensitivities of the volar surfaces (Edelberg 1961). Thus, these water fluxes interact with tool use, locomotion and temperature regulation, and it is assumed that these attributes evolved simultaneously as co-selected characteristics (Montagna and Parakkal 1974; Folk and Semken 1991).
During whole-body (passive) heating of resting individuals, a considerable range of sweat secretion may be observed across the body surface (Weiner 1945; Hertzman et al. 1952; Smith and Havenith 2011; Taylor and Machado-Moreira 2013). Readers may see a critique of the sweat-measurement techniques within Taylor and Machado-Moreira (2013). Herein, the focus centres entirely upon passive thermal sweating from the hands and feet, and data for these regions were collected from three studies, and reported in Fig. 3. Each project was undertaken under identical conditions (climatic chamber: 36 °C, 60 % relative humidity; water-perfusion suit: 40–46 °C), with local sweat rates measured using ventilated capsules positioned at 14 sites over the dorsal and volar surfaces of the hands, fingers, feet and toes (Taylor et al. 2006; Machado-Moreira et al. 2008a; Smith et al. 2013). This thermal load elevated deep-body temperature about 0.5 °C, and resulted in a mean skin temperature of 37.1 °C and resting heart rates of 84 beats min−1 (see original manuscripts for details). Under these conditions, a threefold variation in the steady-state sweating was observed across the hand surfaces [dorsal finger (highest) to palm], with a twofold range across the feet [dorsal toe (highest) to sole]. These changes occurred without introducing reactive errors, such as the cooling of skin below each sweat capsule.
Distributions of steady-state thermal sweating (ventilated capsules) across 14 sites within the hands and feet (shaded bars) of resting individuals (seated: air temperature 36 °C, 60 % relative humidity) wearing a heated, water-perfusion suit (40–46 °C). Data are means with standard deviations. Sources: Taylor et al. (2006), Machado-Moreira et al. (2008a), Smith et al. (2013)
These data confirm the presence of thermal sweating from the volar surfaces of both appendages, although some have suggested otherwise (Kerassidis 1994; Tronstad et al. 2008). Certainly the palms and soles are the least prolific sites (Fig. 3), as first described by Grew (1684), but this is not a universal attribute of the glabrous surfaces, with the volar fingers secreting only slightly, although not significantly less sweat than the dorsal hand (Machado-Moreira et al. 2008a). When the fingers and toes are included with the metacarpal and metatarsal surfaces, the glabrous sites are seen to secrete 60–67 % (foot-hand) of the thermal sweat produced from the dorsal surfaces. However, when the digits are excluded, these relative flows become 40 % (hand) and 66 % (foot), reflecting the greater thermoresponsiveness of the fingers. When all sites were considered, along with typical hand and foot surfaces areas (Table 1), then one may anticipate that, for a deep-body temperature elevation of ~0.5 °C above its thermoneutral level, a resting individual would lose approximately 20 mL h−1 of sweat from each hand and about 27 mL h−1 from each foot. Since whole-body heating significantly increases hand and foot blood flow (Caldwell et al. 2014), and skin temperatures (Maddock and Coller 1933; Werner and Reents 1980; Webb 1992), then cutaneous water vapour pressure rises, elevating evaporation. Thus, these appendages also behave as physiological evaporators, with these secretion patterns resembling segmental blood flow variations.
Sweat secretion during exercise in the heat
When exercising, whole-body water losses of 10–16 L day−1 can occur under challenging climatic conditions (Eichna et al. 1945; Ladell 1945; Latzka and Montain 1999), with the hands and, to a lesser extent, the feet sweating copiously. In the trials reported above (Taylor et al. 2006; Machado-Moreira et al. 2008a; Smith et al. 2013), subjects exercised (cycling) in the same conditions after resting data were collected, and this was incremental in nature (25-W increase every 15 min), terminating at volitional fatigue. This forcing function resulted in final deep-body temperatures of 38.5–39.0 °C. Sweat data were averaged across all work rates to provide integrated responses for the same 14 sites (Fig. 4), representing flows when performing about 100 W of external work (36 °C, 60 % relative humidity). To facilitate inter- and intra-segmental comparisons, Figure 4 includes only those data collected using ventilated sweat capsules, although qualitatively similar outcomes have been obtained from most skin surfaces using the sweat-patch technique (Fogarty et al. 2007; Smith et al. 2013). Relative to the resting phase, sweat rates increased 1.5-fold (volar toe) to 9.4-fold (dorsal surface, proximal phalanx of index finger). Across the entire hand, sweating varied by a factor of 5.7 (lowest-highest), whilst for the foot there was a 2.8-fold variation.
Sweat secretion (ventilated capsules) from 14 sites on the hands and feet (shaded bars) during incremental exercise in the heat (cycling: air temperature 36 °C, 60 % relative humidity) whilst wearing a heated water-perfusion suit (40–46 °C). Data are means (with standard deviations) averaged across the entire exercise period, and equate with sweat rates that would obtain when working at 100 W. Sources: Taylor et al. (2006), Machado-Moreira et al. (2008a), Smith et al. (2013)
If one now combines the morphological data from Table 1 with these sweat rates, mean total sweat rates for each hand (80.7 mL h−1 or 1.52 mg cm−2 min−1) and each foot can be obtained (64.8 mL h−1 or 0.75 mg cm−2 min−1) for individuals exercising in the heat at an average external work rate of ~100 W. Within each segment, the volar surfaces produced 43–57 % (foot-hand) of the sweat secreted from the dorsal regions, or 33–44 % (foot-hand) of total sweat from each segment. The relationship between the volar and dorsal surfaces is not identical for the hands and feet. For example, secretions from the sole and the volar surface of the big toe were uniformly lower than both the average dorsal foot and dorsal toe sweat rates. However, this generalisation did not apply to the hand, with the dorsal and volar surfaces of the fingers secreting significantly more sweat than the palm, but not the dorsal hand (Machado-Moreira et al. 2008a).
Finally, one may combine the regional glandular densities with these data to derive sweat gland outputs: an approximation of discharged sweat from individual glands. Within each appendage, the surfaces with the lowest glandular densities (dorsal hand and foot) produced the greatest glandular flows: 12.7 and 7.6 µg gland−1 min−1 (respectively). The palms and soles possess intermediate gland densities, but displayed the lowest flows when exercising in the heat: 1.2 and 1.0 µg gland−1 min−1 (respectively).
Psychological (non-thermal) sweating
The eccrine sweat glands are stimulated by both thermal and non-thermal mechanisms, and readers are directed to recent reviews on the latter (Kenny and Journeay 2010; Kondo et al. 2010). However, a particular form of non-thermal sweating that was once believed to dominate secretion from the hands and feet is that which accompanies changes in emotional and affect states (Harrison and MacKinnon 1966; Homma et al. 2001); psychological (psychogenic) sweating.
The widely accepted view has been that such sweating is modulated by a separate nervous system centre (Ogawa 1975) with its own neural pathways (Chalmers and Keele 1952), possibly innervating only the glabrous surfaces of the hands and feet (Darrow 1937; Kuno 1956; Ogawa 1975), and quite probably through a noradrenergic sympathetic pathway (Robertshaw 1977; Nakazato et al. 2004). These combined hypotheses have intrigued the current authors for some time, precipitating a series of experiments, three of which are relevant to the current topic (Machado-Moreira and Taylor 2012a, b; Machado-Moreira et al. 2012).
In the first experiment, passive, whole-body heating was used to elicit steady-state sweating in 30 individuals (0.5 °C deep-body temperature elevation above thermoneutral: Machado-Moreira and Taylor 2012a). Secretion was measured from 38 sites (ventilated capsules), with an emphasis upon glabrous and non-glabrous surfaces. Following this priming of sweat secretion, cognitive challenges and a painful stimulus (palmar pressure) were used to evoke psychological sweating. These stimuli always elevated sweating, with >70 % of sites revealing significant increases, but with no change in body temperatures. Sweating from both glabrous and non-glabrous skin increased significantly, with no consistent differences between skin types. Furthermore, these whole-body trends were equally apparent at the hands and feet (Fig. 5), and appeared to refute the possibility of independent control mechanisms for thermal and psychological sweating from these surfaces.
Relative changes in sweat secretion (ventilated capsules) from the glabrous (volar) and non-glabrous (dorsal) skin surfaces of the hands and feet (shaded bars) following the superimposition of cognitive (open bars) and painful stimulations (hatched bars) upon steady-state, thermal sweating. These stimuli were applied after passive heating (climatic chamber: 36 °C, 60 % relative humidity; water-perfusion suit: 40–46 °C) had elicited a deep-body temperature elevation of ~0.5 °C and fully primed thermal sweating. Data are mean changes relative to the pre-stimulus thermal sweating (with standard errors of the means), and were extracted from Machado-Moreira and Taylor (2012a) and used with permission
Since most research on psychological sweating was undertaken in thermoneutral conditions, and since thermal loading potentiates sudomotor function, it was necessary to verify these observations without first priming the sweat glands (Machado-Moreira and Taylor 2012b). Eccrine sweating was therefore evaluated when thermoneutral individuals were challenged to perform 10 min of mental arithmetic (26 °C). Sudomotor function was first quantified using ventilated sweat capsules. Very low, but significant secretion was observed not only from the palm, the volar surfaces of third finger and the sole, but also from the dorsal aspects of the finger and foot (Fig. 6), with only the dorsal hand failing to yield significant sweat. In addition, changes in skin conductance were measured at the volar and dorsal aspects of the first and second fingers. This technique was used because the reabsorption of primary sweat can prevent low-intensity (subliminal) secretion from reaching the skin surface, thus remaining undetected via sweat capsule methods. Indeed, psychogenic sweat can remain within the duct for 150–200 s before being fully absorbed (Ohmi et al. 2009), but it can easily be detected from changes in skin conductance (Thomas and Korr 1957). Both the glabrous and non-glabrous surfaces of the fingers were found to produce significant primary sweat. Collectively, these observations demonstrated that psychological sweating is ubiquitous, further contesting the hypothesis that it might be restricted to the glabrous skin surfaces of the hands and feet.
Psychological sweating (ventilated capsules) from the glabrous (volar) and non-glabrous (dorsal) surfaces of the hands and feet (shaded bars) during mental arithmetic (10 min) performed under thermoneutral conditions (26 °C, 50 % relative humidity). Data are mean changes in sweating (peak minus baseline secretion with standard errors of the means), extracted from Machado-Moreira and Taylor (2012b) and used with permission
In the most recent project from this series, the hypothesis was tested that psychological sweating was not mediated via noradrenergic neural pathways (Machado-Moreira et al. 2012). Subjects were exposed to mental arithmetic, palmar pain and isometric handgrip stimulations under each of three different conditions, applied in series and within one trial: thermoneutral rest (27–28 °C), passive whole-body heating (water-perfusion garment and foot bath) and a systemic atropine infusion (0.04 mg kg−1) with deep-body temperature still elevated, but now clamped (Cotter and Taylor 2005). Sweating responses were measured using ventilated capsules on the dorsal and volar surfaces of one hand (plus forehead, dorsal forearm and calf), and from changes in skin conductance at the same hand sites (plus forehead and dorsal forearm). When thermoneutral, these non-thermal treatments elicited significant discharged sweat only from the palm, but following passive heating, significant secretions were also induced from the dorsal and volar sites, verifying previous observations (Machado-Moreira and Taylor 2012a). When the atropine blockade was established and sweating was completely suppressed, the non-thermal stimuli were repeated, with no site producing either primary or discharged sweat. These data demonstrated that sweating during these thermal, psychological and static exercise stimulations was exclusively cholinergically mediated. Whilst eccrine sweat glands can respond to catecholamines (Robertshaw 1977; Nakazato et al. 2004), these observations were not consistent with the existence of functionally relevant noradrenergic pathways to the glands of the glabrous hand surfaces.
Palmar and plantar hyperhidrosis
Primary hyperhidrosis is a pathological state in which discharged sweating exceeds that required for the maintenance of normal skin function or that which might normally be observed during thermal and non-thermal stimulations (Kuno 1956; Quinton 1983; Vorkamp et al. 2010). It can be a whole-body phenomenon, but is more frequently observed at the palms, soles and axillae. Epidemiological evidence indicates that perhaps 1 % of the population suffers from this condition (Adar et al. 1977; Vorkamp et al. 2010), with about one-fifth of these people being affected only at the palms (Strutton et al. 2004). Hyperhidrosis usually occurs early during adolescence (Edmondson et al. 1992), with most patients being young women (Quraishy and Giddings 1993). In some individuals, palmar and plantar sweating can be so excessive that it can handicap the performance of tasks for which hand grip may be essential. A range of treatments exist from topical medication and the administration of cholinergic antagonists, through to injections of botulinum toxin to block sudomotor neurotransmitter release (Vorkamp et al. 2010). In more severe cases, surgical interventions may be used (Kux 1978; Edmondson et al. 1992; Rieger et al. 2011).
Thermal biophysics
When a warm object is moved from one thermal steady state to a colder state, its energy content changes, with the outermost layer of molecules losing thermal energy to the environment, forming a cooler shell. A series of thermal layers soon form, with heat continuously moving away from the centre until thermal equilibration occurs. Between adjacent layers, thermal gradients are formed, and each remains until heat transfer ceases (Burton and Bazett 1936; Golden and Hervey 1977). The energy exchanges during this dynamic phase are determined by the physical properties of the object and its environment, and these first principles apply to the hands and feet. However, these appendages also produce heat, and they secrete sweat and are variably perfused.
In thermally comfortable individuals, temperature gradients favouring heat loss exist along and below the surfaces of both appendages (Pennes 1948; Werner and Reents 1980; Webb 1992). These result from local metabolism and heat transfers among adjacent tissues, including its convective delivery in the blood. Since the hands and feet have a relatively limited capacity for heat production, then local tissue temperatures are principally altered through vasomotor and sudomotor changes, and the corresponding dry and evaporative heat transfers. Tables 1 and 6 summarise the biophysical characteristics of the hands and feet that support heat conservation and dissipation, allowing one to predict heat exchange across different thermal environments. Readers are directed to hand and foot models that may supplement this information (Lotens 1992; Kuklane et al. 2000). In cool-cold conditions, skin temperatures track changes in cutaneous blood flow quite well, but this relationship becomes weaker under thermoneutral states and is almost non-existent in the heat (Fetcher et al. 1949).
Powerful vasoconstriction reduces blood flows to 0.2–0.4 mL 100 mL−1 min−1 (foot-hand), theoretically delivering <0.1 W of heat to each appendages for a 1 °C thermal gradient. Conversely, maximal segmental flows of 18–30 mL 100 mL−1 min−1 (foot-hand) increase heat mass flow to 8.5 and 6.0 W (respectively) for the same thermal gradient, elevating dry and evaporative heat losses. At the hand, maximal blood flows are typically 4.5-fold greater than in the thermoneutral state, and ~75 times greater than may be expected during maximal vasoconstriction; the corresponding comparisons for the foot are 6.4- and 90-fold larger.
To evaluate the impact of these vascular changes on the dry heat exchange of the hands and feet in air, three environments (15, 27, 45 °C) were modelled using data from Tables 1 and 4, first-principle equations (Goldman and Kampman 2007) and skin temperatures observed for unclothed people in these conditions (Webb 1992). The results of these computations are presented in Table 6.
Whilst the combined radiative and convective transfers of the hands and feet were not high, when normalised to segmental and gender-specific surface areas, these became impressive due to the large surface area to mass ratios of these segments. For instance, at 27 °C, each hand and foot could lose 150–220 W m2 (male–female); Chen et al. (1999) reported losses of 50–300 W m−2 in cold air from the hand. This gender difference is potentially quite important and needs to be explored to help explain differences in thermoeffector function. However, when considered across genders, and in combination with the arteriovenous anastomoses found in these limb segments, these capacities make the hands and feet important sites for heat dissipation, and this attribute has been investigated during water immersion (Wade et al. 1979; Livingstone et al. 1989; Kuklane et al. 1999). Indeed, others have taken advantage of this characteristic to rapidly extract heat from the hands of hyperthermic individuals (Tipton et al. 1993; House et al. 1997, 2003b; House 2003). When both hands were placed into cold water (~10 °C), heat extraction approximated 70–85 W, whilst the feet lost 90–95 W.
For heated, resting subjects secreting sweat from the hand at 22 mL h−1 (as described above), complete evaporation would dissipate a further 15 W (rest: 336 W m−2) from each hand. Sweat from each foot under identical conditions (33 mL h−1) would remove another 22 W (313 W m−2). During exercise in the heat (~100 W of external work), the potential contributions from each appendage increase to 67 W (hand: 1,506 W m−2) and 46 W (foot: 642 W m−2) using corresponding sweat rates of 99 and 68 mL h−1. Thus, these body segments, and in particular the hands, have a considerable thermolytic potential.
Since the hands and feet have such area-specific heat transfer potentials, then a brief reflection on gloves and socks appears warranted. These garments add insulation, but do not modify local temperature in the absence of either external heating or a significant change in cutaneous circulation (House et al. 2003a). However, since such garments do reduce heat loss and help defend deep-body temperature, then they add effective insulation that can enhance thermal comfort. In states close to thermoneutrality, blood will perfuse both appendages, and the hands and feet will become warmer and more comfortable. Thus, within this zone, hand and footwear can have a significant impact on thermal comfort. For instance, if an individual with cold hands or feet, but normal deep-body temperature, puts on gloves or socks, and rapidly feels a warming of the extremities, then one could assume that person was losing heat just slightly faster than its local delivery to these limb segments. One can also assume that cold discomfort preceded a fall in deep-body temperature. In this case, clothing increased total insulation to match or exceed that required for thermal homeostasis, and the thermoneutral state was rapidly restored. In another example, one often observes people removing hand or footwear without modifying torso insulation. In this instance, the upper limit of thermal comfort was crossed, prompting an effective and learned behavioural response to facilitate a subtle increase in heat removal. However, if the deep-body tissue temperature has fallen sufficiently, adding gloves and socks will have a negligible impact upon local blood flow (Figs. 1, 2), deep-body temperature or thermal comfort.
Concluding remarks
The surface area to mass ratios of the hands and feet are ideally suited to conserving and dissipating heat. This characteristic is supported by counter-current heat exchanges and the arteriovenous anastomoses that permit large local blood flow increase, with maximal hand blood flow rising to be 4.5 times greater than in the thermoneoutral state. At the foot, these flows differ by a factor of 6.4. Thus, the theoretical maximal heat deliveries for the hand and foot are 6.0 and 8.5 W (respectively) for a 1 °C thermal gradient. Maximal vaso- and venoconstriction reduces blood flows to levels less than the metabolic requirement, physiologically isolating the extremities to conserve heat, and restricting the heat flow for each segment to <0.1 W. In addition, the production of eccrine sweat from each hand (22 mL h−1) and foot (33 mL h−1) in heated, resting individuals could dissipate a further 15 and 22 W (respectively), assuming 100 % evaporation. Such attributes allow these regions to behave as excellent radiators, insulators and evaporators in resting and unclothed individuals, characteristics that are shared with the large, well-perfused and poorly insulated ears of elephants (Phillips and Heath 1992) and bills of toucans (Tattersall et al. 2009). However, during upright locomotion and tool use, vascular dilatation is much less effective, with load bearing and muscle activation redirecting plantar and palmar venous return to the dorsal surfaces, possibly reducing heat dissipation. In addition, whilst sweat flows are elevated during work in the heat [e.g. 99 (hand) and 68 mL h−1 (foot)], the thermolytic potential of these appendages is often prevented by the use of hand and footwear.
References
Abraham P, Fromy B, Merzeau S, Jardel A, Saumet JL (2001) Dynamics of local pressure-induced cutaneous vasodilation in the human hand. Microvasc Res 61:122–129
Abramson DI (1965) Pathophysiology of arteriovenous shunts in the extremities. J Cardiovasc Surg 5:217–230
Adar R, Kurchin A, Zweig A, Mozes M (1977) Palmar hyperhidrosis and its surgical treatment. Ann Surg 186:34–41
Adelman S, Taylor CR, Heglund NC (1975) Sweating on paws and palms: what is its function? Am J Physiol 229:1400–1402
Alexander L (1945) The treatment of shock from prolonged exposure to cold, especially in water. Combined intelligence objectives subcommittee, item no 24, file no 26–37, pp 1–228
Allan JR, Wilson CG (1971) Influence of acclimatization on sweat sodium concentration. J Appl Physiol 30:708–712
Allwood MJ, Burry HS (1954) The effect of local temperature on blood flow in the human foot. J Physiol 124:345–357
Amano T, Kato Y, Machado-Moreira CA, Taylor NAS, Inoue Y, Nishiyasu T, Kondo N (2011) Changes in eccrine sweating on the glabrous skin of the palm and finger during isometric exercise. Acta Physiol 202:649–655
Bar-Or O, Lundegren HM, Magnusson LI, Buskirk ER (1968) Distribution of heat-activated sweat glands in obese and lean men and women. Hum Biol 40:235–248
Bazett HC (1968) The regulation of body temperatures. In: Newburgh LH (ed) Physiology of heat regulation and the science of clothing. Hafner Publ. Co., New York, pp 109–192
Bennett AF, Hicks JW, Cullum AJ (2000) An experimental test of the thermoregulatory hypothesis for the evolution of endothermy. Int J Org Evol 54:1768–1773
Bergersen TK, Hisdal J, Walløe L (1999) Perfusion of the human finger during cold-induced vasodilatation. Am J Physiol 276:R731–R737
Bernard C (1852) Sur les effets de la section de la portion céphalique du grand sympathique. Comptes Rendus des Séances et Mémoires de la Société de Biologie 4:168–170
Bernard C (1876) Leçons sur la chaleur animale, sur les effets de la chaleur et sur la fièvre. J.-B Baillière et Fils, Paris
Bini G, Hagbarth K-E, Hynninen P, Wallin BG (1980) Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol 306:537–552
Bittel JHM, Nonotte-Varly C, Livecchi-Gonnot GH, Savourey GLMJ, Hanniquet AM (1988) Physical fitness and thermoregulatory reactions in a cold environment in men. J Appl Physiol 65:1984–1989
Blair DA, Glover WE, Roddie IC (1960) Vasomotor fibres to skin in the upper arm, calf and thigh. J Physiol 153:232–238
Blair DA, Glover WE, Roddie IC (1961) Vasomotor responses in the human arm during leg exercise. Circ Res 9:264–274
Bodelsson M, Arneklo-Nobin B, Nobin A, Owman C, Sollerman C, Törnebrandt K (1990) Cooling enhances alpha2-adrenoceptor-mediated vasoconstriction in human hand veins. Acta Physiol Scand 138:283–291
Booth JD, Wilsmore BR, MacDonald AD, Zeyl A, McGhee S, Calvert D, Marino FE, Storlien LH, Taylor NAS (2001) Whole-body pre-cooling does not alter human muscle metabolism during sub-maximal exercise in the heat. Eur J Appl Physiol 84:587–590
Bothorel B, Dewasmes G, Hoeft A, Candas V (1991) Temperature and sweating responses in one-legged and two-legged exercise. Eur J Appl Physiol 63:157–164
Boulant JA (2011) Hypothalamic neurons regulating body temperature. Comprehensive physiology, Handbook of physiology, environmental physiology, suppl 14, pp 105–126. First published in print 1996. doi:10.1002/cphy.cp040106
Boulant JA, Hardy JD (1974) The effect of spinal and skin temperatures on the firing rate and local thermosensitivity of preoptic neurons. J Physiol 216:1371–1374
Boulant JA, Curras MC, Dean JB (1989) Neurophysiological aspects of thermoregulation. In: Wang LCH (ed) Advances in comparative and environmental physiology. Springer, Berlin, pp 117–160
Brajkovic D, Ducharme MB (2003) Finger dexterity, skin temperature, and blood flow during auxiliary heating in the cold. J Appl Physiol 85:758–770
Brajkovic D, Ducharme MB, Frim J (1998) Influence of localized auxiliary heated on hand comfort during cold exposure. J Appl Physiol 85:2054–2065
Broderick BJ, Corley GJ, Quondamatteo F, Breen PP, Serrador J, ÓLaighin G (2010) Venous emptying from the foot: influences of weight bearing, toe curls, electrical stimulation, passive compression, and posture. J Appl Physiol 109:1045–1052
Brück K, Hinkel P (1990) Thermoafferent networks and their adaptive modifications. In: Schönbaum E, Lomax P (eds) Thermoregulation: physiology and biochemistry. Pergamon Press, New York, pp 129–153
Bukhari M, Hollis S, Moore T, Jayson MIV, Herrick AL (2000) Quantification of microcirculatory abnormalities in patients with Raynaud’s phenomenon and systemic sclerosis by video capillaroscapy. Rheumatology 39:506–512
Burton AC, Bazett HC (1936) A study of the average temperature of the tissues, of the exchanges of heat and vasomotor responses in man by means of a bath calorimeter. Am J Physiol 117:36–54
Caldwell JN, Matsuda-Nakamura M, Taylor NAS (2014) Three-dimensional interactions of core and local skin temperatures in the control of hand and foot blood flows. Eur J Appl Physiol. doi:10.1007/s00421-014-2894-x
Caldwell JN, Taylor NAS (2014) Water-displacement plethysmography: a technique for the simultaneous thermal manipulation and measurement of whole-hand and whole-foot blood flows. Physiol Measurement (in press)
Casa DJ, Kenny GP, Taylor NAS (2010) Immersion treatment for exertional hyperthermia: cold or temperate water? Med Sci Sports Exerc 42:1246–1252
Catania JJ, Thompson LW, Michalewski HA, Bowman TE (1980) Comparisons of sweat gland counts, electrodermal activity, and habituation behavior in young and old groups of subjects. Psychophysiology 17:146–152
Chalmers TM, Keele CA (1952) The nervous and chemical control of sweating. Br J Dermatol 64:43–54
Charkoudian N (2003) Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 78:603–612
Chen F, Nilsson H, Holmér I (1999) Evaluation of hand and finger heat loss with heated hand model. Appl Human Sci 18:135–140
Cheung SS, Mekjavic IB (2007) Cold-induced vasodilatation is not homogenous or generalizable across the hand and feet. Eur J Appl Physiol 99:701–705
Christensen EH, Nielsen M (1942) Investigations of the circulation in the skin at the beginning of muscular work. Acta Physiol Scand 4:162–170
Clark ER (1938) Arterio-venous anastomoses. Physiol Rev 18:229–247
Clark E, Lhamon RH (1917) Observations on the sweat glands of tropical and northern races. Anat Rec 12:139–147
Collins KJ (1962) Composition of palmar and forearm sweat. J Appl Physiol 17:99–102
Conrad MC (1971) Functional anatomy of the circulation to the lower extermities. Year Book, Chicago
Costill DL (1977) Sweating: its composition and effects on body fluids. Ann N Y Acad Sci 301:160–174
Cotter JD, Taylor NAS (2005) Distribution of cutaneous sudomotor and alliesthesial thermosensitivity in mildly heat-stressed humans: an open-loop approach. J Physiol 565:335–345
Cotter JD, Patterson MJ, Taylor NAS (1995) A method for clamping human skin and body core temperatures. Proc Aust Physiol Pharmacol Soc 26:204P
Crawshaw LI, Nadel ER, Stolwijk JAJ, Stamford BA (1975) Effect of local cooling on sweating rate and cold sensation. Pflügers Arch 354:19–27
Crompton AW, Taylor CR, Jagger JA (1978) Evolution of homeothermy in mammals. Nature 272:333–336
Cunningham DJ, Stolwijk JAJ, Wenger CB (1978) Comparative thermoregulatory responses of resting men and women. J Appl Physiol 45:908–915
Currie J (1797) Medical reports on the effects of water, cold and warm as a remedy in fever, and febrile diseases; whether applied to the surface of the body, or used as a drink: with observations on the nature of fever; and on the effects of opium, alcohol, and inanition. Cadell and Davies, London
Currie J, Percival T (1792) An account of the remarkable effects of a shipwreck on the mariners; with experiments and observations on the influence of immersion in fresh and salt water, hot and cold, on the powers of the living body. Philos Trans Royal Soc Lond 82:199–224
Daanen HAM (2003) Finger cold-induced vasodilation: a review. Eur J Appl Physiol 89:411–426
Daanen HAM (2009) Manual performance deterioration in the cold estimated using the wind chill equivalent temperature. Ind Health 47:262–270
Daanen HAM, Ducharme MB (1999) Finger cold-induced vasodilation during mild hypothermia, hyperthermia and at thermoneutrality. Aviat Space Environ Med 70:1206–1210
Darrow CW (1937) Neural mechanisms controlling the palmar galvanic skin reflex and palmar sweating. Arch Neurol Psychiatry 37:641–663
Dill DB, Hall FG, van Beaumont W (1966) Sweat chloride concentration: sweat rate, metabolic rate, skin temperature, and age. J Appl Physiol 21:99–106
Dill DB, Horvath SM, van Beaumont W, Gehlsen G, Burrus K (1967) Sweat electrolytes in desert walks. J Appl Physiol 23:746–751
Edelberg R (1961) The relationship between galvanic skin response, vasoconstriction, and tactile sensitivity. J Exp Psychol 62:187–195
Edmondson RA, Banerjee AK, Rennie JA (1992) Endoscopic transthoracic sympathectomy in the treatment of hyperhidrosis. Ann Surg 215:289–293
Edwards M, Burton AC (1960) Correlation of heat output and blood flow in the finger, especially in cold-induced vasodilation. J Appl Physiol 15:201–208
Eichna LW, Ashe WF, Bean WB, Shelley WB (1945) The upper limits of environmental heat and humidity tolerated by acclimatized men working in hot environments. J Ind Hyg Toxicol 27:59–84
Emrich HM, Stoll E, Friolet B, Colombo JP, Richterich R, Rossi E (1968) Sweat composition in relation to rate of sweating in patients with cystic fibrosis of the pancreas. Pediatr Res 2:464–478
Enander AE, Hygge S (1990) Thermal stress and human performance. Scand J Work Environ Health 16(suppl 1):44–50
Ferris BG, Forster RE, Pillion EL, Christensen WR (1947) Control of peripheral blood flow: responses in the human hand when extremities are warmed. Am J Physiol 150:304–314
Fetcher ES, Hall JF, Shaub HG (1949) The skin temperature of an extremity as a measure of its blood flow. Science 110:422–423
Flouris AD, Cheung SS (2009) Influence of thermal balance on cold-induced vasodilation. J Appl Physiol 106:1264–1271
Fogarty AL, Barlett B, Ventenat V, Havenith G (2007) Regional foot sweat rates during a 65-minute uphill walk with a backpack. In: Mekjavic IB, Kounalakis SN, Taylor NAS (eds) Environmental ergonomics XII. Biomed d.o.o, Ljubljana, pp 283–284
Folk GE, Semken HA (1991) The evolution of sweat glands. Int J Biometeorol 35:180–186
Forster RE, Ferris BG, Day R (1946) The relationship between total heat exchange and blood flow in the hand at various ambient temperatures. Am J Physiol 146:600–609
Freeman NE (1935) The effect of temperature on the rate of blood flow in the normal and in the sympathectomized hand. Am J Physiol 113:384–398
Freeman H, Nickerson RF (1938) Skin and body temperatures of normal individuals under cold conditions. J Nutr 15:597–606
Golden FStC, Hervey GR (1977) The mechanism of the afterdrop following immersion hypothermia in pigs. J Physiol 272:P26–P27
Golden FStC, Francis TJR, Gallimore D, Pethybridge R (2013) Lessons from history: morbidity of cold injury in the Royal Marines during the Falklands Conflict of 1982. Extreme Physiol Med 2:23
Goldman RF, Kampman B (2007) Handbook on clothing. Biomedical effects of military clothing and equipment systems. Report of NATO Research Study Group 7 on Bio-medical research aspects of military protective clothing. 2nd edn. International Society for Environmental Ergonomics. http://www.environmental-ergonomics.org. Accessed 15 Nov 2013
González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B (2000) Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol 524:603–615
Grant RT, Bland EF (1931) Observations on arteriovenous anastomoses in human skin and in the birds foot with special references to the reaction to cold. Heart 15:385–411
Grant RT, Pearson RSB (1938) The blood circulation in the human limb; observations on the differences between the proximal and distal parts and remarks on the regulation of body temperature. Clin Sci 3:119–173
Gray H (1918) Anatomy of the human body. Lea & Febiger, Philadelphia. Bartleby.com, 2000. www.bartleby.com/107/
Greenfield ADM (1963) The circulation through the skin. In: Hamilton WF, Dow P (eds) Handbook of physiology. Circulation. Sect 2, vol II, American Physiological Society, Washington DC, pp 1325–1351
Grew N (1684) The description and use of the pores in the skin of the hands and feet. Philos Trans 14:566–567
Hales JRS (1985) Skin arteriovenous anastomoses, their control and role in thermoregulation. In: Johansen K, Burggren WW (eds) Cardiovascular shunts. Alfred Benzon Symposium. pp 433–448
Hales JRS, Hutchinson JCD (1971) Metabolic, respiratory and vasomotor responses to heating the scrotum of the ram. J Physiol 212:353–375
Halliwill JR, Sieck DC, Romero SA, Buck TM, Ely MR (2013) Blood pressure regulation X: what happens when the muscle pump is lost? Post-exercise hypotension and syncope. Eur J Appl Physiol (online first). doi:10.1007/s00421-013-2761-1
Harcourt-Smith WE, Aiello LC (2004) Fossils, feet and the evolution of human bipedal locomotion. J Anat 204:403–416
Hardy JD, Oppel TW (1938) Studies in temperature sensation IV: the stimulation of cold sensation by radiation. J Clin Investig 17:771–778
Hardy JD, Hellon RF, Sutherland K (1964) Temperature-sensitive neurones in the dog’s hypothalamus. J Physiol 175:242–253
Harrison J, MacKinnon PCB (1966) Physiological role of the adrenal medulla in the palmar anhidrotic response to stress. J Appl Physiol 21:88–92
Hashimoto K (1978) The eccrine sweat gland. In: Jarrett A (ed) The physiology and pharmacology of the skin. Academic Press, London, pp 1543–1573
Heinrich B (1977) Why have some animals evolved to regulate a high body temperature? Am Nat 111:623–640
Hellon RF, Lind AR (1956) Observations on the activity of sweat glands with special reference to the influence of ageing. J Physiol 133:132–144
Henriques FC, Moritz AR (1947) Studies of thermal injury I: the conduction of heat to and through skin and the temperatures attained therein. A theoretical and an experimental investigation. Am J Pathol 23:531–549
Hensel H (1981) Thermoreception and temperature regulation. Monographs of the Physiological Society, vol 38, Academic Press, London
Hensel H, Andres KH, Düring MV (1974) Structure and function of cold receptors. Pflügers Arch 352:1–10
Hertzman AB, Randall WC, Peiss CN, Seckendorf R (1952) Regional rates of evaporation from the skin at various environmental temperatures. J Appl Physiol 5:153–161
Heus R, Daanen HAM, Havenith G (1995) Physiological criteria for functioning of hands in the cold: a review. Appl Ergon 26:5–13
Homma S, Matsunami K, Han XY, Deguchi K (2001) Hippocampus in relation to mental sweating response evoked by memory recall and mental calculation: a human electroencephalography study with dipole tracing. Neurosci Lett 305:1–4
House JR (2003) Modelling the effectiveness of techniques for reducing heat strain in Royal Navy nuclear, biological and chemical cleansing stations’ teams. J Royal Navy Med Serv 89:19–26
House JR, Holmes C, Allsopp AJ (1997) Prevention of heat strain by immersing the hands and forearms in water. J Royal Navy Med Serv 83:26–30
House CM, Lloyd K, House JR (2003a) Heated socks maintain toe temperature but not always skin blood flow as mean skin temperature falls. Aviat Space Environ Med 74:891–893
House JR, Lunt H, Magness A, Lyons J (2003b) Testing the effectiveness of techniques for reducing heat strain in Royal Navy nuclear, biological and chemical cleansing stations’ teams. J Royal Navy Med Serv 89:27–34
Hoyer H (1877) Über unmittlebare einmündung kleinster arterien in gefässäste venösen charakters. Archiv fur Mikroskopische Anatomie 13:606–644
Hsu Y-W, Yu C-Y (2010) Hand surface area estimation formula using 3D anthropometry. J Occup Environ Hyg 7:633–639
Hunter J, Kerr EH, Whillans MG (1952) The relation between joint stiffness upon exposure to cold and the characteristics of synovial fluid. Can J Med Sci 30:367–377
Iggo A (1969) Cutaneous thermoreceptors in primates and sub-primates. J Physiol 200:403–430
Imray CH, Richards P, Greeves J, Castellani JW (2011) Nonfreezing cold-induced injuries. J R Army Med Corps 157:79–84
Ingram DL, Legge KF (1972) The influence of deep body and skin temperatures on thermoregulatory responses to heating of the scrotum in pigs. J Physiol 224:477–487
ISO/TR 7250-2 (2010) Basic human body measurements for technological design part 2: statistical summaries of the body measurements from individual ISO populations. International Standard Organisation, Geneva
Ivanov K, Konstantinov V, Danilova N, Sleptchuck N, Rumiantsev G (1986) Thermoreceptors distribution in different skin layers and its significance for thermoregulation. J Therm Biol 11:25–29
Iwase S, Ikeda T, Kitazawa H, Hakusui S, Sugenoya J, Mano T (1997) Altered response in cutaneous sympathetic outflow to mental and thermal stimuli in primary palmoplantar hyperhidrosis. J Auton Nerv Syst 64:65–73
Jessen C (2011) Interaction of body temperatures in control of thermoregulatory effector mechanisms. Comprehensive physiology, Handbook of physiology, environmental physiology, suppl 14, pp 127–138 (first published in print 1996)
Johansson L, Hägg M, Fischer T (2002) Skin blood flow in the human hand in relation to applied pressure. Eur J Appl Physiol 86:394–400
Johnson JM, Kellogg DL (2010) Local thermal control of the human cutaneous circulation. J Appl Physiol 109:1229–1238
Johnson JM, Proppe DW (2011) Cardiovascular adjustments to heat stress. Comprehensive physiology, Handbook of physiology, environmental physiology, suppl 14, pp 215–243 (first published in print 1996). doi:10.1002/cphy.cp040111
Johnson C, Dawber R, Shuster S (1970) Surface appearance of the eccrine sweat duct by scanning electron microscopy. Br J Dermatol 83:655–660
Johnson JM, Brengelmann GL, Rowell LB (1976) Interactions between local and reflex influences on human forearm skin blood flow. J Appl Physiol 41:826–831
Johnson JM, Pérgola PE, Liao FK, Kellogg DL, Crandall CG (1995) Skin of the dorsal aspect of human hands and fingers possesses an active vasodilator system. J Appl Physiol 78:948–954
Juniper K, Dykman RA (1967) Skin resistance, sweat-gland counts, salivary flow, and gastric secretion: age, race, and sex differences, and intercorrelations. Psychophysiology 4:216–222
Karmani S (2006) The thermal properties of bone and the effects of surgical intervention. Curr Orthop 20:52–58
Kellogg DL (2006) In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol 100:1709–1718
Kenny GP, Journeay WS (2010) Human thermoregulation: separating thermal and nonthermal effects on heat loss. Front Biosci 15:259–290
Keramidas ME, Musizza B, Kounalakis SN, Mekjavic IB (2010) Enhancement of the finger cold-induced vasodilation response with exercise training. Eur J Appl Physiol 109:133–140
Kerassidis S (1994) Is palmar and plantar sweating thermoregulatory? Acta Physiol Scand 152:259–263
Knip AS (1969) Measurement and regional distribution of functioning eccrine sweat glands in male and female Caucasians. Hum Biol 41:380–387
Knip AS (1972) Quantitative considerations on functioning eccrine sweat glands in male and female migrant Hindus from Surinam. Proc Koninklijke Nederlandse Akademie van Wetenschappen Ser C Biol Med Sci 75:44–54
Kondo N, Nishiyasu T, Inoue Y, Koga S (2010) Non-thermal modification of heat-loss responses during exercise in humans. Eur J Appl Physiol 110:447–458
Kuklane K, Afanasieva R, Burmistrova O, Bessonova N, Holmér I (1999) Determination of heat loss from the feet and insulation of the footwear. Int J Occup Saf Ergon 5:465–476
Kuklane K, Holmér I, Havenith G (2000) Validation of a model for prediction of skin temperatures in footwear. J Physiol Anthropol Appl Hum Sci 19:29–34
Kunkel P, Stead EA (1938) Blood flow and vasomotor reactions in the foot in health, in arteriosclerosis, and in thrombo-angiitis obliterans. J Clin Investig 17:715–723
Kunkel P, Stead EA, Weiss S (1939) Blood flow and vasomotor reactions in the hand, forearm, foot and calf in response to physical and chemical stimuli. J Clin Investig 18:225–238
Kuno Y (1938) Variations in secretory activity of human sweat glands. Lancet 1:299–303
Kuno Y (1956) Human perspiration. C.C. Thomas, Springfield
Kux M (1978) Thoracic endoscopic sympathectomy in palmar and axillary hyperhidrosis. Arch Surg 113:264–266
Ladell WSS (1945) Thermal sweating. Br Med Bull 3:175–179
Lamah M, Mortimer PS, Dormandy JA (1999) Quantitative study of capillary density in the skin of the foot in peripheral vascular disease. Br J Surg 86:342–348
Latzka WA, Montain SJ (1999) Water and electrolyte requirements for exercise. Clin Sports Med 18:513–524
Levy JM, Hessel SJ, Joseph RB, Bodell LS (1985) Venous “lakes” of the hands. Radiology 156:603–605
Lewis T, Pickering GW (1931) Vasodilatation in the hands in response to warming the body, with evidence for sympathetic vasodilator nerves in man. Heart 16:33–51
Libert JP, Candas V, Vogt JJ (1978) Sweating response in man during transient rises of air temperature. J Appl Physiol 44:284–290
List CF, Peet MM (1938) Sweat secretion in man II. Anatomic distribution of disturbances in sweating associated with lesions of the sympathetic nervous system. Arch Neurol Psychiatry 40:27–43
Livingstone SD (1976) Changes in cold-induced vasodilation during Arctic exercises. J Appl Physiol 40:455–457
Livingstone SD, Nolan RW, Cattroll SW (1989) Heat loss caused by immersing the hands in water. Aviat Space Environ Med 60:1166–1171
Lotens WA (1992) Simulation of hand cooling due to touching cold materials. Eur J Appl Physiol 65:59–65
Lovejoy CO, Simpson SW, White TD, Asfaw B, Suwa G (2009) Careful climbing in the Miocene: the forelimbs of Ardipithecus ramidus and humans are primitive. Science 326:70e1–70e8
Machado-Moreira CA, Taylor NAS (2012a) Sudomotor responses from glabrous and non-glabrous skin during cognitive and painful stimulations following passive heating. Acta Physiol 204:571–581
Machado-Moreira CA, Taylor NAS (2012b) Psychological sweating from glabrous and non-glabrous skin surfaces under thermoneutral conditions. Psychophysiology 49:369–374
Machado-Moreira CA, Caldwell JN, Mekjavic IB, Taylor NAS (2008a) Sweat secretion from palmar and dorsal surfaces of the hands during passive and active heating. Aviat Space Environ Med 79:1034–1040
Machado-Moreira CA, Smith FM, van den Heuvel AMJ, Mekjavic IB, Taylor NAS (2008b) Sweat secretion from the torso during passively-induced and exercise-related hyperthermia. Eur J Appl Physiol 104:265–270
Machado-Moreira CA, Wilmink F, Meijer A, Mekjavic IB, Taylor NAS (2008c) Local differences in sweat secretion from the head during rest and exercise in the heat. Eur J Appl Physiol 104:257–264
Machado-Moreira CA, McLennan PL, Lillioja S, van Dijk W, Caldwell JN, Taylor NAS (2012) The cholinergic blockade of both thermally and non-thermally induced human eccrine sweating. Exp Physiol 97:930–942
MacKinnon PCB (1954) Variations with age in the number of active palmar digital sweat glands. J Neurol Neurosurg Psychiatry 17:124–126
Maddock WG, Coller FA (1933) The role of the extremities in the dissipation of heat. Am J Physiol 106:589–596
Mahadevan V, Anderson R, Williams L, Renwick P (2000) Interactive foot and ankle. Primal Pictures Ltd., London
Martin AD (1984) An anatomical basis for assessing human body composition: evidence from 25 dissections. Doctor of Philosophy, Simon Fraser University
Marzke MW (1992) Evolutionary development of the human thumb. Hand Clin 8:1–8
Marzke MW, Marzke RF (2000) Evolution of the human hand: approaches to acquiring, analysing and interpreting the anatomical evidence. J Anat 197:121–140
Masson P (1937) Les glomus neuro-vasculaires. Histophysiologie 4, Hermann et Cie, Paris
Maughan RJ, Shirreffs SM (2008) Development of individual hydration strategies for athletes. Int J Sports Nutr Exerc Metab 18:457–472
Mayrovitz HN, Sims N, Litwin B, Pfister S (2005) Foot volume estimates based on a geometric algorithm in comparison to water displacement. Lymphology 38:20–27
Mayrovitz HN, Sims N, Hill CJ, Hernandez T, Greenshner A, Diep H (2006) Hand volume estimates based on a geometric algorithm in comparison to water displacement. Lymphology 39:95–103
McGrouther DA, Colditz JC, Harris JM, Stoller DW (2000) Interactive hand. Primal Pictures Ltd., London
Mekjavic IB, Eiken O (2006) Contribution of thermal and nonthermal factors to the regulation of body temperature in humans. J Appl Physiol 100:2065–2072
Mertz L (2004) The circulatory system. Greenwood Press, Westport
Michael-Titus A, Revest P, Shortland P (2007) The nervous system. Churchill Livingstone, Edinburgh
Midtgåtrd U (1980) Arteriovenous anastomoses and vascularity in the feet of eiders and gulls (aves). Zoomorphology 96:263–270
Miller R, Ross WD, Rapp A, Roede M (1980) Sex chromosome aneuploidy and anthropometry: a new proportionality assessment using the phantom stratagem. Am J Med Genet 5:125–135
Minson CT, Berry LT, Joyner MJ (2001) Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91:1619–1626
Molyneux GS, Bryden MM (1981) Comparative aspects of arteriovenous anastomoses. In: Harrison RJ, Holmes RL (eds) Progress in anatomy. Cambridge University Press, Cambridge, pp 207–227
Montagna W, Parakkal PF (1974) The structure and function of skin. Academic Press, New York
Morimoto T (1978) Sweat secretion. In: Jarrett A (ed) The physiology and pharmacology of the skin. Academic Press, London, pp 1611–1621
Mørk C, Kvernebo K, Asker CL, Salrud EG (2002) Reduced skin capillary density during attacks of erythromelalgia implies arteriovenous shunting as pathogenetic mechanism. J Invest Dermatol 119:949–953
Nadel ER, Mitchell JW, Stolwijk JAJ (1973) Differential thermal sensitivity in the human skin. Pflügers Arch 340:71–76
Nagasaka T, Cabanac M, Hirata K, Nunomura T (1987a) Control of local heat gain by vasomotor response of the hand. J Appl Physiol 63:1335–1338
Nagasaka T, Hirata K, Nunomura T (1987b) Contribution of arteriovenous anastomoses to vasoconstriction induced by local heating of the human finger. Jpn J Physiol 37:425–433
Nakayama T (1969) A further investigation on the nature of sweat discharge in man. Tohoku J Exp Med 98:265–272
Nakayama T, Takagi K (1959) Minute pattern of human perspiration observed by a continuously recording method. Jpn J Physiol 9:359–364
Nakazato Y, Tamura N, Ohkuma A, Yoshimaru K, Shimazu K (2004) Idiopathic pure sudomotor failure: anhidrosis due to deficits in cholinergic transmission. Neurology 63:1476–1480
Necker R (1977) Thermal sensitivity of different skin areas in pigeons. J Comp Physiol 116:239–246
Nelms JD (1963) Functional anatomy of skin related to temperature regulation. Fed Proc 22:933–936
Nilsson AL, Nilsson GE, Öberg PÅ (1980) A note on periodic sweating. Acta Physiol Scand 108:189–190
Nuzzaci G, Evangelisti A, Righi D, Giannico G, Nuzzaci I (1999) Is there any relationship between cold-induced vasodilatation and vasomotion? Microvasc Res 57:1–7
O’Brien C (2005) Reproducibility of the cold-induced vasodilation response in the human finger. J Appl Physiol 98:1334–1340
Ogata K (1935) Functional variations in human sweat glands, with remarks upon the regional differences of the amount of sweat. J Orient Med 23:98–101
Ogawa T (1975) Thermal influence on palmar sweating and mental influence on generalized sweating in man. Jpn J Physiol 25:525–536
Ohmi M, Tanigawa M, Yamada A, Ueda Y, Haruna M (2009) Dynamic analysis of internal and external mental sweating by optical coherence tomography. J Biomed Opt 41:014026
Pang CCY (2001) Autonomic control of the venous system in health and disease, effects of drugs. Pharmacol Ther 90:179–230
Patterson MJ, Cotter JD, Taylor NAS (1998) Human sudomotor responses to heating and cooling upper body skin surfaces: differential cutaneous thermal sensitivity. Acta Physiol Scand 163:289–296
Patterson MJ, Galloway SDR, Nimmo MA (2000) Variations in regional sweat composition in normal human males. Exp Physiol 85:869–875
Pegum JM, Fegan WG (1967a) Anatomy of venous return from the foot. Cardiovasc Res 1:241–248
Pegum JM, Fegan WG (1967b) Physiology of venous return from the foot. Cardiovasc Res 1:249–254
Penfield W, Rasmussen T (1952) The cerebral cortex of man. MacMillan Press, New York
Pennes HH (1948) Analysis of tissue and arterial blood temperature in the human forearm. J Appl Physiol 1:93–122
Pérgola PE, Kellogg DL, Johnson JM, Kosiba WA, Solomon DE (1993) Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol 265:H785–H792
Phillips PK, Heath JE (1992) Heat exchange by the pinna of the African elephant (Loxodonta africana). Comp Biochem Physiol Comp Physiol 101:693–699
Pickering GW, Hess W (1933) Vasodilatation in the hands and feet in response to warming the body. Clin Sci 1:213–223
Pierau F-K (2011) Peripheral thermosensors. Comprehensive physiology, Handbook of physiology, environmental physiology, suppl 14, pp 85–104 (first published in print 1996). doi:10.1002/cphy.cp040105
Preuschoft H (2004) Mechanism for the acquisition of habitual bipedality: are there biomechanical reasons for the acquisition of upright bipedal posture? J Anat 204:363–384
Proppe DW, Brengelmann GL, Rowell LB (1976) Control of baboon limb blood flow and heart rate: role of skin vs. core temperature. Am J Physiol 231:1457–1465
Putz RV, Tuppek A (1999) Evolution der hand. Handchir Mikrochir Plast Chir 31:357–361
Quinton PM (1983) Sweating and its disorders. Ann Rev Med 34:429–452
Quraishy MS, Giddings AEB (1993) Treating hyperhidrosis. Br Med J 306:1221–1222
Randall WC (1946) Quantitation and regional distribution of sweat glands in man. J Clin Investig 25:761–767
Regan JM, Macfarlane DJ, Taylor NAS (1996) An evaluation of the role of skin temperature during heat adaptation. Acta Physiol Scand 158:365–375
Rieger R, de Paula Loureiro M, Pedevilla S, de Oliveira RA (2011) Endoscopic lumbar sympathectomy following thoracic sympathectomy in patients with palmoplantar hyperhidrosis. World J Surg 35:49–53
Roberts DF, Salzano FM, Willson JOC (1970) Active sweat gland distribution in Caingang Indians. Am J Phys Anthropol 32:395–400
Robertshaw D (1977) Neuroendocrine control of sweat glands. J Investig Dermatol 69:121–129
Roddie IC (2011) Circulation to skin and adipose tissue. Comprehensive physiology. Handbook of physiology, The cardiovascular system, peripheral circulation and organ blood flow. suppl 8, pp 285–317 (first published in print 1983). doi:10.1002/cphy.cp020310
Roddie IC, Shepherd JT (1956) The blood flow through the hand during local heating, release of sympathetic vasomotor tone by indirect heating, and a combination of both. J Physiol 131:657–664
Roddie IC, Shepherd JT, Whelan RF (1957a) The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J Physiol 136:489–497
Roddie IC, Shepherd JT, Whelan RF (1957b) A comparison of the heat elimination from the normal and nerve-blocked finger during body heating. J Physiol 138:445–448
Rothe CF (1983) Reflex control of veins and vascular capacitance. Physiol Rev 63:1281–1342
Rowell LB (1974) Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54(75):159
Rowell LB (1983) Cardiovascular aspects of human thermoregulation. Circ Res 52:367–379
Rowell LB (1993) Human cardiovascular control. Oxford University Press, New York
Rowell LB, Brengelmann GL, Blackmon JR, Murray JA (1970) Redistribution of blood flow during sustained high skin temperature in resting man. J Appl Physiol 28:415–420
Ruben JA (1995) The evolution of endothermy in mammals and birds: from physiology to fossils. Annu Rev Physiol 57:69–95
Rushmer RF, Buettner KJK, Short JM, Odland GF (1966) The skin. Science 154:343–348
Sato K (1973) Sweat induction from an isolated eccrine sweat gland. Am J Physiol 225:1147–1152
Sato K (1977) The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev Physiol Biochem Pharmacol 79:51–131
Sato K, Sato F (1983) Individual variations in structure and function of human eccrine sweat gland. Am J Physiol 245:R203–R208
Savard GK, Cooper KE, Veale WL, Malkinson TJ (1985) Peripheral blood flow during rewarming from mild hypothermia in humans. J Appl Physiol 58:4–13
Schaefer O, Hildes JA, Greidanus P, Leung D (1974) Regional sweating in Eskimos compared to Caucasians. Can J Physiol Pharmacol 52:960–965
Scheuplein RJ, Blank IH (1971) Permeability of the skin. Physiol Rev 51:702–747
Schmidt H-X, Lanz U (2004) Surgical anatomy of the hand. Thieme, New York
Scholander PF, Krog J (1957) Countercurrent heat exchange and vascular bundles in sloths. J Appl Physiol 10:405–411
Scholander PF, Schevill WE (1955) Counter-current vascular heat exchange in the fins of whales. J Appl Physiol 8:279–282
Schotzinger RJ, Landis SC (1988) Cholinergic phenotype developed by noradrenergic sympathetic neurons after innervation of a novel cholinergic target in vivo. Nature 335:637–639
Schwartz IL, Thaysen JH (1956) Excretion of sodium and potassium in human sweat. J Clin Investig 35:114–120
Shana G, Bohn C (2003) Anthropometrical data and coefficients of regression related to gender and race. Appl Ergon 34:327–337
Silver A, Montagna W, Karacan I (1964) Age and sex differences in spontaneous, adrenergic and cholinergic human sweating. J Investig Dermatol 43:255–265
Simon E (1974) Temperature regulation: the spinal cord as a site of extrahypothalamic thermoregulatory function. Rev Physiol Biochem Pharmacol 71:1–76
Simon E, Schmid HA, Pehl U (1998) Spinal neuronal thermosensitivity in vivo and in vitro in relation to hypothalamic neuronal thermosensitivity. In: Sharma HS, Westman J (eds) Progress in brain research, vol 115. Elsevier, Amsterdam, pp 25–47
Simons P, Coleridge Smith P, Lees WR, McGrouther DA (1996) Venous pumps of the hand: their clinical importance. J Hand Surg 21B:595–599
Smith CJ, Havenith G (2011) Body mapping of sweating patterns in males athletes in mild exercise-induced hyperthermia. Eur J Appl Physiol 111:1391–1404
Smith CJ, Machado-Moreira CA, Plant G, Hodder S, Havenith G, Taylor NAS (2013) Design data for footwear: sweating distribution on the human foot. Int J Cloth Sci Technol 25:43–58
Spealman CR (1945) Effect of ambient air temperature and of hand temperature on blood flow in hands. Am J Physiol 145:218–222
Standring S (2008) Gray’s anatomy: the anatomical basis of clinical practice, 40th edn. Churchill Livingstone/Elsevier, Edinburgh
Starling EH (1896) On the absorption of fluids from the connective tissue spaces. J Physiol 19:312–326
Stocks JM, Taylor NAS, Tipton MJ, Greenleaf JE (2004) Human physiological responses to cold exposure. Aviat Space Environ Med 75:444–457
Strutton DR, Kowalski JW, Glaser DA, Stang PE (2004) US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol 51:241–248
Sucquet JP (1862) Anatomie et physiologie. Circulation du sang. D’une circulation derivative dans les membres et dans la tête chez l’homme. Delahaye, Paris
Tattersall GJ, Andrade DV, Abe AS (2009) Heat exchange from the toucan bill reveals a controllable vascular thermal radiator. Science 325:468–472
Taylor NAS (2006) Ethnic differences in thermoregulation: genotypic versus phenotypic heat adaptation. J Therm Biol 31:90–104
Taylor NAS (2014) Human heat adaptation. Compr Physiol 4:325–365
Taylor NAS, Machado-Moreira CA (2013) Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extrem Physiol Med 2:4
Taylor WF, Johnson JM, O’Leary D, Park MK (1984) Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57:191–196
Taylor NAS, Allsopp NK, Parkes DG (1995) Preferred room temperature of young versus aged males: the influence of thermal sensation, thermal comfort and affect. J Gerontol A Biol Sci Med Sci 50:M216–M221
Taylor NAS, Caldwell JN, Mekjavic IB (2006) The sweating foot: local differences in sweat secretion during exercise-induced hyperthermia. Aviat Space Environ Med 77:1020–1027
Taylor NAS, Caldwell JN, van den Heuvel AMJ, Patterson MJ (2008) To cool, but not too cool: that is the question: immersion cooling for hyperthermia. Med Sci Sports Exerc 40:1962–1969
Teague RS, Ranson SW (1936) The role of the anterior hypothalamus in temperature regulation. Am J Physiol 117:562–570
Thomas PE, Korr IM (1957) Relationship between sweat gland activity and electrical resistance of the skin. J Appl Physiol 10:505–510
Thompson ML (1954) A comparison between the number and distribution of functioning eccrine glands in Europeans and Africans. J Physiol 123:225–233
Tipton MJ, Allsopp A, Balmi PJ, House JR (1993) Hand immersion as a method of cooling and rewarming: a short review. J Royal Navy Med Serv 79:125–131
Tipton MJ, Wakabayashi H, Barwood MJ, Eglin CM, Mekjavic IB, Taylor NAS (2013) Habituation of the metabolic and ventilatory responses to cold-water immersion in humans. J Therm Biol 38:24–31
Toda Y (1967) Measurement and regional distribution of active sweat glands in Indonesians. Kobe J Med Sci 13:157–164
Tronstad C, Gjein GE, Grimnes S, Martinsen ØG, Krogstad A-L, Fosse E (2008) Electrical measurement of sweat activity. Physiol Meas 29:S407–S415
Vallerand AL, Savourey G, Hanniquet A, Bittel JHM (1992) How should body heat storage be determined in humans: by thermometry or calorimetry? Eur J Appl Physiol 65:286–294
van Beaumont W, Bullard RW (1963) Sweating: its rapid response to muscular work. Science 141:643–646
van Beaumont W, Bullard RW, Banerjee MR (1966) Observations on human sweating by resistance hygrometry. Dermatol Dig pp 75–87
van der Struijs NR, van Es EM, Raymann RJ, Daanen HAM (2008) Finger and toe temperatures on exposure to cold water and cold air. Aviat Space Environ Med 79:941–946
van Duinen H, Gandevia SC (2011) Constraints for control of the human hand. J Physiol 589:5583–5593
Vorkamp T, Foo FJ, Khan S, Schmitto JD, Wilson P (2010) Hyperhidrosis: evolving concepts and a comprehensive review. Surgeon 8:287–292
Wade CE, Dacanay S, Smith RN (1979) Regional heat loss in resting man during immersion in 25.2 °C water. Aviat Space Environ Med 50:590–593
Wang WJ, Crompton RH (2004) Analysis of the human and ape foot during bipedal standing with implications for the evolution of the foot. J Biomech 37:1831–1836
Webb P (1992) Temperatures of skin, subcutaneous tissue, muscle and core in resting men in cold, comfortable and hot conditions. Eur J Appl Physiol 64:471–476
Webb-Peploe MM, Shepherd JT (1968) Response of large hindlimb veins of the dog to sympathetic nerve stimulation. Am J Physiol 215:299–307
Weinbaum S, Jiji LM, Lemons DE (1984) Theory and experiment for the effect of vascular microstructure on surface tissue heat transfer part I: anatomical foundation and model conceptualization. J Biomech Eng 106:321–330
Weiner JS (1945) The regional distribution of sweating. J Physiol 104:32–40
Wenger CB, Roberts MF, Nadel ER, Stolwijk JAJ (1975) Thermoregulatory control of finger blood flow. J Appl Physiol 38:1078–1082
Werner J, Heising M (1990) Consequences of partial body warming and cooling for the drives of local sweat rate. Eur J Appl Physiol 60:300–304
Werner J, Reents T (1980) A contribution to the topography of temperature regulation in man. Eur J Appl Physiol 45:87–94
Werner J, Mekjavic IB, Taylor NAS (2008) Concepts in physiological regulation: a thermoregulatory perspective. In: Taylor NAS, Groeller H (eds) Physiological bases of human performance during work and exercise. Churchill Livingstone Elsevier, Edinburgh, pp 325–340
Wilcott RC (1966) Adaptive value of arousal sweating and the epidermal mechanism related to skin potential and skin resistance. Psychophysiology 2:249–262
Wilkins RW, Doupe J, Newman HW (1938) The rate of blood flow in normal fingers. Clin Sci 3:403–411
Willis I, Harris DR, Moretz W (1973) Normal and abnormal variations in eccrine sweat gland distribution. J Investig Dermatol 60:98–103
Wilson O, Goldman RF (1970) Role of air temperature and wind in the time necessary for a finger to freeze. J Appl Physiol 29:658–664
Wyndham CH, Wilson-Dickson WG (1951) Physiological responses of hands and feet to cold in relation to body temperature. J Appl Physiol 4:199–207
Young RW (2003) Evolution of the human hand: the role of throwing and clubbing. J Anat 202:165–174
Yousef MK, Dill DB (1974) Sweat rate and concentration of chloride in hand and body sweat in desert walks: male and female. J Appl Physiol 38:82–85
Yu C-Y, Tu H–H (2009) Foot surface area database and estimation formula. Appl Ergon 40:767–774
Yu C-Y, Lin C-H, Yang Y-H (2010) Human body surface area database and estimation formula. Burns 36:616–629
Zander J, Morrison JB (2008) Effects of pressure, cold and gloves on hand skin temperature and manual performance of divers. Eur J Appl Physiol 104:237–244
Zhang S-X, Schmidt H-M (1993) Clinical anatomy of the subcutaneous veins in the dorsum of the hand. Ann Anat 175:381–384
Zhong J, Asker CL, Salerud EG (2000) Imaging, image processing and pattern analysis of skin capillary ensembles. Skin Res Technol 6:45–57
Zimmerman BG (1966) Separation of responses of arteries and veins to sympathetic stimulation. Circ Res 18:429–436
Zotterman Y (1959) Thermal sensations. In: Field J (ed) Handbook of physiology, vol 1. Neurophysiology, American Physiological Society, Washington DC, pp 431–458
Acknowledgments
Christiano Machado-Moreira was supported by a Doctoral scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Ministry of Education, Brazil). Joanne Caldwell and Anne van den Heuvel were supported by Australian Postgraduate Awards (Department of Innovation, Industry, Science and Research, Australia). The research and associated scholastic activities summarised within this review were supported, in part, by grants from the Australian Research Council, Defence Science and Technology Organisation (Australia), the Ministry of Defence (Republic of Slovenia) and W.L. Gore and Associates GmbH (Germany).
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by George Havenith.
Rights and permissions
About this article
Cite this article
Taylor, N.A.S., Machado-Moreira, C.A., van den Heuvel, A.M.J. et al. Hands and feet: physiological insulators, radiators and evaporators. Eur J Appl Physiol 114, 2037–2060 (2014). https://doi.org/10.1007/s00421-014-2940-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-2940-8