Abstract
Purpose
Increased oxygen uptake and utilisation during exercise depend on adequate adaptations of systemic and pulmonary vasculature. Recent advances in magnetic resonance imaging techniques allow for direct quantification of aortic and pulmonary blood flow using phase-contrast magnetic resonance angiography (PCMRA). This pilot study tested quantification of aortic and pulmonary haemodynamic adaptations to moderate aerobic supine leg exercise using PCMRA.
Methods
Nine adult healthy volunteers underwent pulse gated free breathing PCMRA while performing heart rate targeted aerobic lower limb exercise. Flow was assessed in mid ascending and mid descending thoracic aorta (AO) and main pulmonary artery (MPA) during exercise at 180 % of individual resting heart rate. Flow sequence analysis was performed by experienced operators using commercial offline software (Argus, Siemens Medical Systems).
Results
Exercise related increase in HR (rest: 69 ± 10 b min−1, exercise: 120 ± 13 b min−1) resulted in cardiac output increase (from 6.5 ± 1.4 to 12.5 ± 1.8 L min−1). At exercise, ascending aorta systolic peak velocity increased from 89 ± 14 to 122 ± 34 cm s−1 (p = 0.016), descending thoracic aorta systolic peak velocity increased from 104 ± 14 to 144 ± 33 cm s−1 (p = 0.004), MPA systolic peak velocity from 86 ± 18 to 140 ± 48 cm s−1 (p = 0.007), ascending aorta systolic peak flow rate from 415 ± 83 to 550 ± 135 mL s−1 (p = 0.002), descending thoracic aorta systolic peak flow rate from 264 ± 70 to 351 ± 82 mL s−1 (p = 0.004) and MPA systolic peak flow rate from 410 ± 80 to 577 ± 180 mL s−1 (p = 0.006).
Conclusion
Quantitative blood flow and velocity analysis during exercise using PCMRA is feasible and detected a steep exercise flow and velocity increase in the aorta and MPA. Exercise PCMRA can serve as a research and clinical tool to help quantify exercise blood flow adaptations in health and disease and investigate patho-physiological mechanisms in cardio-pulmonary disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiovascular adaptations to aerobic exercise are mediated through an interplay of cardiac, hormonal, metabolic and muscular mechanisms. The increase in cardiac output to enhance oxygen delivery plays the dominant role (Bassett and Howley 2000); however, increased demand for oxygen uptake and utilisation during exercise also depends on adequate adaptations of aortic and pulmonary vasculature, as well as, ventilatory responses. Respiratory adaptations to increased oxygen demand during exercise have been extensively studied using cardio-pulmonary exercise testing (CPET) with oxygen consumption measurement (reviewed in Balady et al. 2010). A limitation of CPET is its inability to provide data on vascular adaptations to exercise. This is important in particular in patient groups with a primary cardiac pathology that might result in insufficient pulmonary perfusion during stress and exercise with subsequent ventilation/perfusion mismatch. The physiological role of pulmonary vascular adaptations, e.g. pulmonary artery (PA) pressure and pulmonary vascular resistance (PVR) changes during exercise has not been fully resolved (Naeije and Chesler 2012). Direct assessment of pulmonary vascular flow parameters during exercise could contribute to this discussion in particular if flows, global and regional, in addition to the response of the ventricle, were simultaneously assessed.
Phase-contrast magnetic resonance angiography (PCMRA) has been extensively used to measure flow parameters at rest (Gatehouse et al. 2005) and results have a high accuracy and reproducibility (Ley et al. 2008; Lotz et al. 2002; Beerbaum et al. 2005) with wide clinical applications in assessing cardiovascular disease (Arheden et al. 1999; Varaprasathan et al. 2002). Over the last years, advances in image acquisition technology and a renewed interest in exercise physiology have led to a number of proofs of principle studies using magnetic resonance imaging (MRI) during various modes of exercise. The focus of these studies was either feasibility (Gusso et al. 2012; Lurz et al. 2009; Steeden et al. 2010), or assessing ventricular response to exercise (La Gerche et al. 2012b; Holverda et al. 2006) and measurement of the ascending aortic and MPA flow (Weber et al. 2011). We have designed a protocol and performed a pilot study using aerobic heart rate targeted supine modified bicycle exercise to assess blood flow parameters in the ascending and descending thoracic aorta (Ao) and main pulmonary artery (MPA). Our aim was to develop a protocol that allows assessment of cardio-pulmonary physiology during exercise that can be used in a clinical setting.
Methods
Participants and exercise protocol
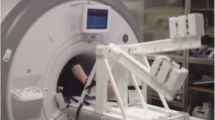
Nine healthy subjects (6 males and 3 females, mean age: 37.4 ± 8.2 years) volunteered to undergo MRI at rest and while performing heart rate targeted supine aerobic lower limb exercise using an MRI compatible braked cycle ergometer (Lode BV Groningen) mounted onto a whole body MRI system (Siemens Avanto 1.5T; Siemens Medical Solutions; Erlangen Germany) (Fig. 1). Moderate aerobic exercise was performed in a supine position in the MRI scanner using a modified cycling technique involving the lower limb muscle systems (Supplementary Video 1). Participants’ resting HR was measured in supine position before exercise. Participants started unloaded cycling for 2 min before resistance (up to 50 W) was increased to reach a 2 min steady state at 180 % of individual resting heart rate before flow measurements were taken. Steady state at 180 % of individual resting heart rate was reached after 2–3 min. Heart rate, blood pressure and oxygen saturations were monitored non-invasively at rest, exercise at 180 % of resting HR and 2 min recovery. Ethical approval for the study was obtained from the UK National Research Ethics Service (NRES). All subjects signed NRES approved consent prior to participation.
MRI acquisition and analysis
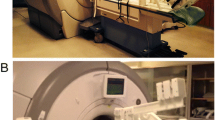
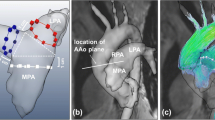
At rest and during heart rate targeted exercise, we assessed blood flow in the ascending and descending thoracic aorta and MPA. Ascending aortic flow was additionally reassessed post-exercise after 2 min rest to monitor recovery. All flow parameters were assessed by MRI both at rest and during exercise during retrospective heart rate gated (via pulse oximetry) free breathing PCMRA in the mid ascending and descending thoracic aorta and MPA (Fig. 2; Supplementary Videos 2, 3). We used a single manufacturer’s body matrix coil (Siemens Medical Solutions; Erlangen Germany). Imaging of Ao and MPA was performed during different acquisitions accounting for different imaging planes. Ascending and descending thoracic aortic images were obtained from axial HASTE images, to ensure both ascending and descending thoracic Ao were at isocentre. MPA flow data was acquired by obtaining two long axis views of the MPA and positioning the MPA flow plane perpendicular to the two long axis images of the mid MPA at isocentre (Fig. 3).
PCMRA images of aorta and MPA. a Aortic flow images at rest (upper panel) and exercise (lower panel); Ascending aorta marked as AscAo, thoracic descending aorta as DA. b MPA flow images at rest (upper panel) and exercise (lower panel); Main pulmonary artery is marked as MPA. Magnitude image on the left, phase image on the right
Demonstration of alignment for MPA flow imaging a axial HASTE. Projected axis along MPA (arrow) for cine SSFP 1 image in b. b Image produced by a; Projected axis along MPA (arrow) for next cine SSFP 2 image shown in c. c SSFP 2 cine image produced by axial alignment of b. d Perpendicular alignment to cines SSFP 1 b and SSFP 2 c defines plane for MPA flow imaging
Flow measurements were acquired as follows: standard field of view (FOV) 320 mm (adapted to patient size), base resolution 256, FOV phase 75 %, voxel size 1.3 × 1.3 × 5.0 mm for a standard FOV–TE 2.2 ms, TR 29.9 ms, slice thickness 5 mm, flip angle 30°, 3 averages, 30 reconstructed phases, Venc 150 cm s−1 at rest, 300 cm s−1 during exercise. Acquisition of a single exercise flow data set was conducted over approximately 105 heart beats and acquisition time therefore depended on HR. For example at 80 b min−1 it was 80 s, at 112 b min−1 it was 56 s. Overall imaging time during exercise was approximately 6 min. One aortic flow acquisition within this cohort had to be repeated due to significant subject movement during exercise and unsatisfactory pulse oximetry led to a delay of acquisition in one subject. Images were analysed manually contouring the vessel walls using commercial offline software (Argus, Siemens Medical Systems) by three individuals trained in MRI flow evaluation (MH, GP, CL) and these were all checked and edited by MH. Pulmonary and aortic blood flow was determined from the flow velocities in individual voxels in the region of interest. Stroke volume (SV) was measured as the ascending aortic forward flow of one cardiac cycle averaged from approximately 100 cardiac cycles during PCMRA. Q was calculated as an ascending aortic forward flow of one cardiac cycle × HR. Ascending aorta measurements were obtained distal to coronary artery origin. All results were independently counter-checked and corrected by an experienced cardiac radiologist (MH). Exercise data sets of three volunteers were independently re-analysed for intra-observer variability (MH) and inter-observer variability (MH and GP). We additionally validated the use of a single body matrix coil imaging protocol in data sets of three volunteers. Flow sequence analysis was performed using Argus offline software. Flow analysis took 30 min per subject.
Statistics
Statistical analysis was performed using SPSS V. 19 and Stata V.12.1. Differences of measured parameters at rest and during exercise were analysed using a paired t test and significance was attributed to a p value of <0.05. All measurements are mean value from nine volunteers. For reliability analysis intra-observer and inter-observer reliability were tested in three data sets using a two-way mixed effects model intra-class correlation coefficient. Correlation of two imaging techniques was tested using the same model.
Results
Nine healthy volunteers completed the exercise protocol with acquisition of images suitable for flow quantification at rest and during exercise. Resting heart rate as measured by infrared spectroscopy showed an exercise intensity related increase from 69 ± 9 b min−1 at rest to 117 ± 12 b min−1 during exercise. Oxygen saturation remained above 92 % during all exercise stages in all participants. Exercise duration was between 12 and 16 min depending on the time needed to reach a steady state at target heart rate. HR increase to 180 % of resting HR resulted in a significant increase in cardiac output (Q) as measured by aortic flow min−1 from 6.5 ± 1.4 to 12.5 ± 1.8 L min−1 (p < 0.001, 95 % CI = 5/7). Similar values were obtained for the MPA with 6.9 ± 1.0 L min−1 at rest and 14.1 ± 3.1 L min−1 (p < 0.001, 95 % CI = 5.0/9.5). Increase in cardiac output was a result of HR increase but also a significant increase in SV in the ascending aorta from 90.8 ± 15.6 to 112.3 ± 27.4 ml (p < 0.016, 95 % CI = 5.1/37.9). Q in the thoracic descending aorta showed a significant increase from rest to exercise from 4.4 ± 1.0 to 9.3 ± 1.5 L min−1 (p < 0.001, 95 % CI = 4.2/5.4). SV increase to exercise in the descending thoracic aorta was significant from 64.1 ± 13.5 to 83.4 ± 21.3 ml (p = 0.003, 95 % CI = 8.8/29.6). The ratio of Q and SV (mean) distribution between ascending and descending aorta changed towards the descending aorta during exercise with a ratio 1:0.68 at rest to 1:0.74 during exercise. SV in the MPA increased from 102.2 ± 19.7 to 130.3 ± 42.0 ml (p < 0.016, 95 % CI = 8.2/58.0). To accommodate increased SV, aortic and pulmonary systolic flow rate and systolic velocity increased significantly to the following values; in the ascending aorta systolic peak velocity increased from 89 ± 14 to 122 ± 34 cm s−1 (p = 0.016, 95 % CI = 8/58), average systolic velocity from 33 ± 5 to 38 ± 7 cm s−1 (p = 0.005, 95 % CI = 2/9) and systolic peak flow rate from 415 ± 83 to 550 ± 135 ml s−1 (p = 0.002, 95 % CI = 66/205) (Table 1; Fig. 4). A similar increase was measured in the MPA with MPA systolic peak velocity increasing from 86 ± 18 to 140 ± 48 cm s−1 (p = 0.007, 95 % CI = 20/89), average systolic velocity from 34 ± 6 to 53 ± 19 cm s−1 (p = 0.031, 95 % CI = 2/35) and MPA systolic peak flow rate from 410 ± 80 to 577 ± 180 ml s−1 (p = 0.006, 95 % CI = 62/273) (Table 2). In the descending thoracic aorta systolic peak velocity increased from 104 ± 14 to 144. ± 33 cm s−1, (p = 0.004, 95 % CI = 16/63), average systolic velocity increased from 41 ± 9 to 47 ± 7 cm s−1, but this was statistically not significant (p = 0.08, 95 % CI = −1/15) and systolic peak flow rate from 264 ± 70 to 351 ± 82 ml s−1 (p = 0.004, 95 % CI = 37/137) (Table 2). Diastolic flow in aorta and MPA was negligible. At 2 min recovery time all parameters showed a trend to normalisation apart from aortic average velocity which remained at exercise level (Table 1). Figures 5, 6, 7 show the measured parameters for each individual volunteer. All observed exercise changes were statistically significant apart from an average systolic velocity increase in the descending thoracic aorta.
Flow curves obtained from PCMRA data. Flow over one cardiac cycle is shown as averaged over one flow image acquisition (~100 heart beats) at rest (HR 49 b min−1 upper panel) and during exercise (HR 100 b min−1 lower panel); left column: ascending aorta; middle column: descending aorta, right column: MPA
Intra- and inter-observer reliability data for PCMRA at rest have been published (Beerbaum et al. 2005). We provide intra- and inter-observer reliability data during exercise (Table 3). Intra-observer variability during exercise was satisfactory with an intraclass correlation coefficient between 0.824 and 0.993, mean difference between 0 and 9.6 % (Table 3), the intraclass correlation coefficient for inter-observer variability was between 0.744 and 1, mean difference between 0 and 9.3 % (Table 4). We have performed all measurements using only one surface matrix coil removing the spine coil. A reliability test in three subjects showed good correlation between measurements with and without the additional spine coil with intra-class correlation for Q = 0.998 (mean difference 0.5 %), for SV = 0.995 (mean difference 0.64 %) and for systolic peak velocity = 0.976 (mean difference 4.8 %).
Conclusion
This study has investigated the suitability of PCMRA to quantify exercise-induced changes of aortic and PA blood flow using a physiological and practical exercise model combined with an MRI scanning protocol that can easily be used in the clinical setting. Our exercise protocol resulted in a statistically significant increase in heart rate, SV and cardiac output as measured by aortic flow. We have shown an exercise-induced increase of the ascending and descending thoracic aortic and main pulmonary flow parameters. We used a similar protocol as previously published by (Weber et al. 2011) and our results lend further validity to the findings of the above study. Beyond that we have provided data on reproducibility of flow measurements during exercise. We have additionally investigated Q, SV and blood flow response in the descending thoracic aorta and showed that exercise not only results in an overall SV increase but also leads to a decreased ratio of the ascending to descending aortic blood flow. This indicates that overall increase in cardiac output during exercise is driven by increased local demand of perfusion and oxygenation in the muscle groups involved. Ascending to descending thoracic aortic blood flow ratio during exercise can potentially be used as an indirect measure of local compliance and capacitance, thus demonstrating intact local physiological response to lower limb exercise. The measured flow rate increase during exercise is likely to be facilitated by increased SV but also by an increase in compliance and capacitance of the systemic and pulmonary vascular bed resulting in a fall of systemic and PVR. In healthy volunteers velocity and flow increase both contribute to facilitate the cardiac output increase with positive correlation between them. The non-invasive assessment of pulmonary flow parameters can therefore offer new quantitative insights into pulmonary exercise physiology in health but particularly cardio-pulmonary disease, where it can complement information on PA pressures which do not well correlate with exercise capacity (Kovacs et al. 2012) or differentiate between health and disease (Kovacs et al. 2009).
Measurement of aortic flow parameters during exercise has wide applications too. It could aid in studying and quantifying the physiological adaptive mechanisms to exercise, but more importantly could become an assessment tool in follow-up and risk stratification in patients with inherited aortic vasculopathies (e.g. Marfans syndrome) or congenital heart disease (coarctation, truncus arteriosus) where resting changes in aortic flow as assessed by MRI are well described (Secchi et al. 2009). The most significant advantage could be gained if PCMRA flow imaging during exercise as described here could be combined with the recently developed ventricular volume quantification by real time ungated CMR. This would enable simultaneous assessment of ventricular and vascular exercise response and enhance our limited understanding of ventricular–arterial coupling during exercise, in particular of the RV and pulmonary circulation La Gerche et al. (2011, 2012a).
Methodological considerations
Exercise-induced cardiac stress testing is superior to pharmaceutically induced stress testing as the latter does not result in physiological changes in systemic and PVR and systemic venous return which are key mechanisms in the exercise response. Our study used an exercise protocol that comprised aerobic exercise in supine position using up and down leg movement. Differences between cardiac parameters measured in upright and supine exercise are well documented (Bevegard et al. 1963; Granath et al. 1964) particularly in amount of recruited venous blood volume at start of exercise (preload) (Bestler et al. 1992; Parker and Thadani 1979) but also in resting and exercise maximal heart rate, cardiac output, end-diastolic volume, maximal power output, and absolute SV (Warburton et al. 2002; Groepenhoff et al. 2007). These studies have, however, also highlighted that sufficient exercise effort and cardiac stress can be achieved using a supine exercise model, and although supine and upright exercise shows a difference in initial PVR, pulmonary blood flow relationships are identical during supine and erect exercise (Naeije and Chesler 2012) making our supine exercise model a suitable tool. Finally, current clinical MRI systems do not allow for upright exercise during image acquisition. A physiological advantage of our study shared with previous published work (La Gerche et al. 2012b; Weber et al. 2011) is that imaging was performed during free breathing exercise. This is superior to exercise MRI protocols with breath held acquisition (Steding-Ehrenborg et al. 2013) which can influence venous return and particularly pulmonary blood flow (Naeije and Chesler 2012).
Our study has used heart rate targeted exercise as this represents the most practical form of defining individual exercise effort and is widely used and validated. It can also be used to judge exercise effort and work rate at higher exercise stages in non-athletes as an increase in cardiac output is, generally, only achieved by a further heart rate increase (Poliner et al. 1980; Flamm et al. 1990; Higginbotham et al. 1986). It does, however, not allow for an objective quantification of exercise intensity. In future studies we propose to perform a CPET prior to imaging to correlate individual metabolic work rates during exercise to heart rate. This would allow better quantitative estimation of exercise intensity. In our study cardiac output, SV and flow parameters did not return to normal resting values at 2 min recovery (Table 1) but this is expected in non-trained individuals after moderate aerobic exercise. We have not measured flow parameters at recovery in MPA, however, this might be very informative in patients with (Pulmonary arterial hypertension) PAH.
Technical limitations
Respiration introduces a change in MPA flow (Pinsky 1984) and as such data acquired with breath holding is not representative of the physiological changes induced by exercise. Our data acquisition was over approximately 100 cardiac cycles and thus multiple respiratory cycles, so this would not have been a source of error. Ascending aorta measurements were obtained distal to coronary artery origin. Coronary artery flow accounts for approximately 5 % of total Q at rest (Mymin and Sharma 1974) and increases during exercise. This might explain differences in the measured mean Q between the ascending aorta and MPA at rest and exercise as coronary artery flow was not accounted for in our aortic flow calculation. ECG gated PCMRA protocols are in our experience unsuitable for flow imaging even at the modest exercise levels due to muscular and magnet-induced interference with the ECG. Our study used retrospective oximetry pulse gating to define the cardiac cycle. This proved reasonably reliable in our study though care was needed with optimising the pulse trace before exercise. In our experience gated PCMRA is unsuitable to reliably measure blood flow at maximal exercise intensity. Real time PCMRA sequences may overcome this limitation and have been validated in the research setting (Steeden et al. 2010) but are presently not commercially available. Our intra-observer and inter-observer variability analysis show, however, the robustness of the protocol used. We have also calculated SV (mean) from MPA flow measurements as a potential internal control of accuracy and protocol robustness, but it has to be considered that flow based SV quantification of the MPA has imperfect accuracy at rest (Mauritz et al. 2008) and this can be assumed to be accentuated during exercise. Aortic and MPA flows were not recorded simultaneously and this can be assumed to lead to variation of aortic and MPA flow values. A further adjustment from standard PCMRA scanning protocols on the Siemens Avanto unit is the use of only one surface matrix coil in our protocol due to space incompatibility of the mounted exercise ergometer requiring the spine array to be removed. We have, however, shown that this does not appear to affect data quality in our reliability analysis.
Summary
Quantitative analysis of ascending and descending thoracic aortic and pulmonary blood flow during exercise is feasible using retrospective pulse gated free breathing phase-contrast PCMRA. Moderate aerobic exercise leads to a statistically significant increase in blood flow and flow velocities in the ascending and descending thoracic Ao, and MPA facilitating increased oxygen uptake and delivery. Exercise PCMRA could help in diagnosis, assessment of treatment response and follow-up in patient groups with pulmonary and cardiovascular pathology. Simultaneous assessment during exercise of aortic and pulmonary flow and left and right ventricular function by MRI would further increase validity and application.
Abbreviations
- Ao:
-
Aorta
- CPET:
-
Cardio-pulmonary exercise test
- FOV:
-
Field of view
- MRI:
-
Magnetic resonance imaging
- PA:
-
Pulmonary artery
- PAH:
-
Pulmonary arterial hypertension
- PCMRA:
-
Phase-contrast magnetic resonance angiography
- PVR:
-
Pulmonary vascular resistance
- RV:
-
Right ventricle
- TE:
-
Echo time
- TR:
-
Repetition time
- VENC:
-
Velocity encoding
References
Arheden H, Holmqvist C, Thilen U, Hanseus K, Bjorkhem G, Pahlm O, Laurin S, Stahlberg F (1999) Left-to-right cardiac shunts: comparison of measurements obtained with MR velocity mapping and with radionuclide angiography. Radiology 211(2):453–458
Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV (2010) Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122(2):191–225. doi:10.1161/CIR.0b013e3181e52e69
Bassett DR Jr, Howley ET (2000) Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 32(1):70–84
Beerbaum P, Korperich H, Gieseke J, Barth P, Peuster M, Meyer H (2005) Blood flow quantification in adults by phase-contrast MRI combined with SENSE––a validation study. J Cardiovasc Magn Reson 7(2):361–369
Bestler M, Alt E, Montoya P, Schandry R (1992) Effect of body posture on heart rate and cardiocirculatory parameters in stress––implications for frequency-adapted pacemaker systems. Z Kardiol 81(1):25–29
Bevegard S, Holmgren A, Jonsson B (1963) Circulatory studies in well trained athletes at rest and during heavy exercise. with special reference to stroke volume and the influence of body position. Acta Physiol Scand 57:26–50
Flamm SD, Taki J, Moore R, Lewis SF, Keech F, Maltais F, Ahmad M, Callahan R, Dragotakes S, Alpert N et al (1990) Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation 81(5):1550–1559
Gatehouse PD, Keegan J, Crowe LA, Masood S, Mohiaddin RH, Kreitner KF, Firmin DN (2005) Applications of phase-contrast flow and velocity imaging in cardiovascular MRI. Eur Radiol 15(10):2172–2184. doi:10.1007/s00330-005-2829-3
Granath A, Jonsson B, Strandell T (1964) Circulation in healthy old men, studied by right heart catheterization at rest and during exercise in supine and sitting position. Acta Med Scand 176:425–446
Groepenhoff H, Holverda S, Marcus JT, Postmus PE, Boonstra A, Vonk-Noordegraaf A (2007) Stroke volume response during exercise measured by acetylene uptake and MRI. Physiol Meas 28(1):1–11
Gusso S, Salvador C, Hofman P, Cutfield W, Baldi JC, Taberner A, Nielsen P (2012) Design and testing of an MRI-compatible cycle ergometer for non-invasive cardiac assessments during exercise. Biomed Eng Online 11:13. doi:101186/1475-925X-11-13[pii]
Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR (1986) Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res 58(2):281–291
Holverda S, Gan CT, Marcus JT, Postmus PE, Boonstra A, Vonk-Noordegraaf A (2006) Impaired stroke volume response to exercise in pulmonary arterial hypertension. J Am Coll Cardiol 47(8):1732–1733. doi:10.1016/j.jacc.2006.01.048S0735-1097(06)00295-6[pii]
Kovacs G, Berghold A, Scheidl S, Olschewski H (2009) Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 34(4):888–894. doi:10.1183/09031936.00145608
Kovacs G, Olschewski A, Berghold A, Olschewski H (2012) Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J 39(2):319–328. doi:10.1183/09031936.00008611[pii]
La Gerche A, Heidbuchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, Inder WJ, Macisaac AI, Prior DL (2011) Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc 43(6):974–981. doi:10.1249/MSS.0b013e31820607a3
La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbuchel H, Prior DL (2012a) Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 33(8):998–1006. doi:10.1093/eurheartj/ehr397[pii]
La Gerche A, Claessen G, Van De Bruaene A, Pattyn N, Van Cleemput J, Gewillig M, Bogaert J, Dymarkowski S, Claus P, Heidbuchel H (2012b) Cardiac magnetic resonance imaging: a new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging. doi:10.1161/CIRCIMAGING.112.980037
Ley S, Unterhinninghofen R, Ley-Zaporozhan J, Schenk JP, Kauczor HU, Szabo G (2008) Validation of magnetic resonance phase-contrast flow measurements in the main pulmonary artery and aorta using perivascular ultrasound in a large animal model. Invest Radiol 43(6):421–426. doi:10.1097/RLI.0b013e318169015d
Lotz J, Meier C, Leppert A, Galanski M (2002) Cardiovascular flow measurement with phase-contrast MR imaging: basic facts and implementation. Radiographics 22(3):651–671
Lurz P, Muthurangu V, Schievano S, Nordmeyer J, Bonhoeffer P, Taylor AM, Hansen MS (2009) Feasibility and reproducibility of biventricular volumetric assessment of cardiac function during exercise using real-time radial k-t SENSE magnetic resonance imaging. J Magn Reson Imaging 29(5):1062–1070. doi:10.1002/jmri.21762
Mauritz GJ, Marcus JT, Boonstra A, Postmus PE, Westerhof N, Vonk-Noordegraaf A (2008) Non-invasive stroke volume assessment in patients with pulmonary arterial hypertension: left-sided data mandatory. J Cardiovasc Magn Reson 10:51. doi:10.1186/1532-429X-10-51[pii]
Mymin D, Sharma GP (1974) Total and effective coronary blood flow in coronary and noncoronary heart disease. J Clin Invest 53(2):363–373. doi:10.1172/JCI107568
Naeije R, Chesler N (2012) Pulmonary Circulation at Exercise. Compr Physiol 2(1):711–741. doi:10.1002/cphy.c100091
Parker JO, Thadani U (1979) Cardiac performance at rest and during exercise in normal subjects. Bull Eur Physiopathol Respir 15(5):935–949
Pinsky MR (1984) Determinants of pulmonary arterial flow variation during respiration. J Appl Physiol 56(5):1237–1245
Poliner LR, Dehmer GJ, Lewis SE, Parkey RW, Blomqvist CG, Willerson JT (1980) Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation 62(3):528–534
Secchi F, Iozzelli A, Papini GD, Aliprandi A, Di Leo G, Sardanelli F (2009) MR imaging of aortic coarctation. Radiol Med 114(4):524–537. doi:10.1007/s11547-009-0386-6
Steding-Ehrenborg K, Jablonowski R, Arvidsson PM, Carlsson M, Saltin B, Arheden H (2013) Moderate intensity supine exercise causes decreased cardiac volumes and increased outer volume variations: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 15(1):96. doi:10.1186/1532-429X-15-96[pii]
Steeden JA, Atkinson D, Taylor AM, Muthurangu V (2010) Assessing vascular response to exercise using a combination of real-time spiral phase contrast MR and noninvasive blood pressure measurements. J Magn Reson Imaging 31(4):997–1003. doi:10.1002/jmri.22105
Varaprasathan GA, Araoz PA, Higgins CB, Reddy GP (2002) Quantification of flow dynamics in congenital heart disease: applications of velocity-encoded cine MR imaging. Radiographics 22(4):895–905 discussion 905–896
Warburton DE, Haykowsky MJ, Quinney HA, Blackmore D, Teo KK, Humen DP (2002) Myocardial response to incremental exercise in endurance-trained athletes: influence of heart rate, contractility and the Frank-Starling effect. Exp Physiol 87(5):613–622
Weber TF, von Tengg-Kobligk H, Kopp-Schneider A, Ley-Zaporozhan J, Kauczor HU, Ley S (2011) High-resolution phase-contrast MRI of aortic and pulmonary blood flow during rest and physical exercise using a MRI compatible bicycle ergometer. Eur J Radiol 80(1):103–108. doi:10.1016/j.ejrad.2010.06.045
Acknowledgments
The study was supported and hosted by the Bristol NIHR Biomedical Research Unit for Cardiovascular Disease. GEP holds a NIHR/University of Bristol Academic Clinical Lectureship. We express our gratitude to C. Rogers for statistical support.
Conflict of interest
None of the authors declare any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Massimo Pagani.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 26264 kb)
Supplementary material 2 (AVI 3366 kb)
Supplementary material 3 (AVI 3395 kb)
Rights and permissions
About this article
Cite this article
Pieles, G.E., Szantho, G., Rodrigues, J.C.L. et al. Adaptations of aortic and pulmonary artery flow parameters measured by phase-contrast magnetic resonance angiography during supine aerobic exercise. Eur J Appl Physiol 114, 1013–1023 (2014). https://doi.org/10.1007/s00421-014-2833-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-2833-x