Abstract
Purpose
The aim of the study was to examine the effects of a post-prandial 20 min nap on a short-term physical exercise and subsequent sleep in athletes keeping their usual sleep schedules and in 5-h phase-advance condition.
Methods
Sixteen healthy young male athletes (age 22.2 ± 1.7 years, non-habitual nappers) participated in the study. After a baseline 8-h time in bed in normal and 5-h advanced sleep schedules, a standardized morning and lunch in a laboratory environment, subjects underwent either a nap (20 min of sleep elapsed from 3 epochs of stage 1 or 1 epoch of stage 2), or a rest without sleep by lying in a bed, between 13:00 and 14:00 hours in non-shifted condition or 08:00 and 09:00 hours in shifted condition, after which anaerobic exercises were performed twice 2 h apart. Core body temperature was recorded throughout the study period.
Results
The nap extended sleep onset latency from 6.72 ± 3.83 to 11.84 ± 13.44 min, after shifted condition but did not modify sleep architecture of the post-trial night among athletes, whether shifted or not. Moreover, napping did not improve physical performance but it delayed acrophase and batyphase of core body temperature rhythm parameters.
Conclusion
Napping showed no reliable benefit on short-term performances of athletes exercising at local time or after a simulated jet lag.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impact of transcontinental travel results in physiologic and sleep disturbances, sleepiness and intermittent fatigue. Jet lag and travel fatigue have been identified by athletes and their coaches as important but challenging problems that could benefit from practical solutions (Samuels 2012). The management of this fatigue is considered a key factor in sustaining alertness and performance (Belenky et al. 2003). Therefore, to compensate sleep debt and subsequent fatigue during travel crossing multiple time zones, or to help athletes, especially during stressful training, napping is a behavioral measure that may be used to alleviate a number of sleep-related problems, promote waking function, reduce problems with arousal and counteract the deleterious effects of jet lag.
Different types of diurnal siestas: appetitive napping for pleasure of sleeping, prophylactic napping when a future debt is expected and replacement napping in response to subjective fatigue have all been described by Dinges and Broughton (1989). But in this review of the timing of self-selected nap, Dinges reported that the afternoon is by far the most frequent period, with a nap taken closer to the afternoon circadian dip in alertness evidenced in a large-scale survey of young students. Sleep efficiency is better, sleep latency is shorter, and the amount of slow wave sleep is greater than in a nap taken during the morning or evening forbidden zone for sleep (Lavie and Zvuluni 1992).
Naitoh (1981) cautioned that naps taken at the wrong time could lead to prolonged sleep inertia. As naps are influenced by circadian rhythms, one strategy involves varying the time of day a nap takes place during sustained wakefulness. Indeed, naps taken after 6 or 18 h of wakefulness showed more benefits than those taken after 30, 43 or 54 h of wakefulness; also, naps taken after fewer hours of wakefulness prevented a mean drop in body temperature (Dinges et al. 1987).
According to several studies, healthy young adults should ideally nap for approximately 10–20 min to decrease fatigue, increase vigor, improve subjective alertness, objective alertness and cognitive performance (Brooks and Lack 2006; Tietzel and Lack 2002; Hayashi et al. 1999). These short naps would be ideal for athletes whose performance is normally required immediately upon awakening. As described in the two-process model of sleep–wake regulation, which predicts that longer sleep is required for restoration and improvements in waking performance and cognition, nap time duration is linked with the sleep-dependent process S as described by Borbely (1982).
Thus, homeostatic and circadian processes interact to determine the extent of restoration, e.g., the quality of the prior sleep period, the duration of the nap, the presence of sleep inertia, and the timing of the nap (Milner and Cote 2009).
Most of the studies on daytime nap effects have been conducted in shift workers, medical staff, drivers, and pilots on alertness, but to our knowledge, very few in athletes (Waterhouse et al. 2007). Furthermore, while advice may seem reasonable, there appears to be little hard empirical evidence as to whether a nap is indeed helpful in shifted athletes. Thus, the first aim of the study was to examine the effects of a post-prandial 20 min nap on a short-term physical exercise and subsequent sleep in athletes keeping their usual sleep schedules. The second aim was to investigate whether this nap may have different effects after a simulated jet lag.
Method
Participants
Twenty highly trained male subjects were recruited, of whom sixteen (age 22.2 ± 1.7 years, height 178.3 ± 5.6 cm, body mass 73.6 ± 7.9 kg) participated in the study. Indeed, four subjects were excluded because of reporting sleep disturbances. They were selected according to their oxygen uptake (VO2 max), measured directly during a rapidly progressive incremental exercise test until exhaustion on a cycle ergometer (Lode Excalibur, Lode B.V., Groningen, the Netherlands) 1 week before the experiment. The maximal power output and VO2 max averaged 384 ± 66 watts and 55.5 ± 9.1 ml min−1 kg−1, respectively. Subjects did not report any significant previous or present health problems, including substance abuse, and specifically, they did not have any sleep-related complaints or partial sleep restrictions. All had normal sleep–wake habits. Moreover, they were selected on the basis of their circadian typology, according to the Horne and Östberg “morningness–eveningness” questionnaire and also based on a sleeping diary that they completed for 1 month before experimental procedures (Horne and Ostberg 1976). Circadian typology score averaged 52.88 ± 5.45; all subjects had an intermediate chronotype. Their sleep diaries averaged total sleep time per night of 491.44 ± 41.12 min, sleep onset latency of 15.75 ± 12.18 min and sleep efficiency index (total sleep time/time in bed × 100) of 94.81 ± 3.02 %. All subjects used to go to bed between 22:30 and 23:00 hours. None of them habitually napped.
Ethics
The study was approved by the local ethics committee (Protocol no. T/2009/06 “WINAP”). The study procedures were explained to all participants and any questions about the protocol were answered. All volunteers provided a written informed consent and were informed about their right to withdraw from the study at any time. Upon completion, they received financial compensation.
Procedure
The experimental protocol is described in detail in Fig. 1.
Sleep conditions
The subjects spent a total of 9 nights consisting of one habituation night at the beginning of the study and 2 nights per week for each condition, for 4 consecutive weeks, in a sleep laboratory in a quiet, comfortable and soundproofed bedroom, with constant temperature (18–20 °C), noise (<40 dB), and dim light (<10 lux) conditions.
The subjects first spent one habituation night to ensure that they had no sleep disorders and to accustom them to sleeping in the laboratory. All times are expressed in “local time”.
The habituation night was followed by four randomized conditions. The conditions were counter-balanced so that participants experienced the conditions in an equal number of different orders:
-
One pre-trial night and one post-trial night in normal sleep condition (PTN1) with post-lunch rest (A),
-
One pre-trial night and one post-trial night in normal sleep condition (PTN2) with post-lunch nap (C),
-
One pre-trial night and one post-trial night in 5-h phase-advance condition (PTPAN1) with post-lunch rest (B),
-
One pre-trial night and one post-trial night in 5-h phase-advance condition (PTPAN2) with post-lunch nap (D).
For the habituation night and pre-trial nights in normal sleep condition, subjects reached the laboratory at 19:00 hours for a standardized dinner. Time in bed was at 22:30 hours and lights were turned off from 23:00 hours until 07:00 hours, corresponding to their breakfast time.
For the pre-trial nights in phase-advance condition, subjects reached the laboratory at 14:00 hours for a standardized dinner. They were asked to change their clocks and watches. Time in bed was at 17:30 hours and the lights were turned off from 18:00 to 02:00 hours; corresponding to breakfast time. Participants did not leave the laboratory all along the experiment and were monitored by the experimenter.
Subjects stayed awake sitting, watching television, reading books or listening music while being controlled by polysomnography (PSG) and also under the constant surveillance of a technician (to avoid micro-sleeping) during standardized morning, i.e., from 07:00 to 12:00 hours in normal sleep conditions and from 02:00 to 07:00 hours during 5-h phase-advance conditions. In the latter conditions, subjects were exposed to 5 h of room light once they woke up (Pharos Max, 10,000 Lux—76 cm) to keep them awake.
Identical lunches were offered either at 12:00 or 07:00 hours. Subjects filled dietary diaries, indicating their diary intakes during 4 days, 1 week before experimentations, in usual quantity or in weight. These data were analyzed by GENI software (MICRO 6, CS 90172, Villers les Nancy Cedex). In this way, all meals during the protocol were prepared to contain appropriate energy requirements for each subject based on their dietary diaries, of 2,500–3,500 Cal.
Intense physical activity and naps were prohibited during the day before experiment. To evaluate this, daily motor activity was measured during the 24 h before the subjects came to the laboratory, and during the week between each condition, by recording movements of the non-dominant wrist with an Actiwatch activity monitoring system® (Actiwatch AW7, CamNtech Ltd, Cambridge, UK).
Subjects were also required to abstain from caffeinated beverages the week before the experiments and on the days of the experimental sessions.
Sleep recordings
A camera constantly monitored subjects and EEG was transmitted in real-time to a computer manned by one of the experimenters. Sleep was assessed using standard PSG techniques using an 8-channel EEG device (i.e., F4, C4, 02, M2, F3, C3, 01, M1 electroencephalogram, right and left electrooculogram, electromyogram). PSG data were recorded directly to a data acquisition, storage, and analysis system (Schwarzer GmbH, Munich, Germany; OSG BrainRT, OSG Bvba, Rumst, Belgium). Night EEG recordings were scored using the AASM (American Academy of Sleep Medicine) standard rules to obtain the overnight pattern of sleep stages (Iber et al. 2007). Morning EEG monitoring were screened to check for micro-sleeping.
Nap recordings
Post-lunch naps were scheduled at 13:00 hours after normal sleep condition (C) and at 08:00 hours in phase-advance condition (D). They were continuously monitored and scored by PSG. Subjects were required to lie on a bed in a darkened room for 1 h and were awakened by intercom when about 20 min of sleep had elapsed from 3 epochs of stage 1 or 1 epoch of stage 2.
In post-lunch rest conditions (A and B), subjects remained lying in a bed in the same conditions as in post-lunch nap conditions, for 1 h, between 13:00 and 14:00 hours and 08:00 and 09:00 hours after normal sleep and phase-advance conditions, respectively. They were asked not to take a nap. In case of any signs of sleep, they were awakened by intercom. EEG recordings confirmed that they did not sleep throughout this period. Subjects were not submitted to darkness, contrary to nap condition.
After post-lunch nap or rest, as for standardized mornings, subjects stayed awake sitting, watching television, reading books or listening music while being controlled by PSG and under constant surveillance of a technician.
Exercise protocol
Wingate test
Following each pre-trial night, the subjects performed two Wingate tests per day, either at 15:30 and 17:30 hours, or at 10:30 and 12:30 hours, in normal sleep and phase-advance conditions, respectively (Fig. 1). These tests were conducted on a mechanically braked Monark 814 E cycle ergometer (Monark Exercise AB, Varberg, Sweden), a Wingate test consisting of a 30 s maximal sprint against constant resistance. For each subject, the load was determined according to body mass using the optimization tables of Bar-Or (1987) (0.10 kg × kg−1 body mass). After 10 min of cycling warm-up with 1 kg load, the subjects were requested to pedal as fast as possible from the start signal and to maintain maximal pedaling speed throughout the 30-s period. Strong verbal encouragements were given to subjects during the test. Active recovery lasted 15 min. The characteristics of the ergo cycle allowed the calculation of P peak, P mean and fatigue index as follows: P (W) = F (kg) × V (rpm × min−1). Peak power (P peak) is the highest mechanical power elicited during the test. This index was taken as the highest average power during any 5-s period. Mean power (P mean) is the average power sustained throughout the 30-s period. The fatigue index is determined from the decline in power; it is the difference between the highest and the lowest power divided by the highest power.
Blood lactates
Blood samples (100 μl) were collected by micro-method, in cycling position from one ear-lobe at the end of 30 s of exercise and fifth min of recovery. The samples were analyzed to determine lactate concentrations (EML 105 Radiometer, Radiometer Medical ApS, Brønshøj, Denmark).
Core body temperature
A rectal probe was self-inserted by the participant 12–15 cm past the rectal sphincter (Morris et al. 2009). Core body temperature (CBT) was continuously recorded for the duration of time in laboratory, at 8-s intervals with a rectal thermistor (YSI 400, Rectal Temperature Probe, Smiths Medical ASD, Inc., Rockland, United States) connected to a datalogger (SmartReader Plus 8, ACR Systems Inc., Canada).
Statistical analysis
Statistical analyses were performed using Stata/SE 10.1 for Windows (Stata Corp, College, and Station, TX, USA). Quantitative data are expressed as mean ± standard deviation (SD). A p value <0.05 was considered statistically significant. The effects of phase advance and nap were analyzed by two-way ANOVA measures, for sleep night parameters, Wingate variables and blood lactates and CBT values. In case of nap effect, a Newman–Keuls post hoc test was performed, to compare the data between conditions with nap vs without nap. Furthermore, the effects of time in phase-advance and nap conditions were analyzed by a three-way ANOVA for Wingate variables. In case of significant effects, a Newman–Keuls post hoc test was performed, to compare the data between trial 1 and trial 2. To calculate intra-class correlation coefficient, two-way random, single measure was used. Each rater evaluated each subject, and raters have been randomly selected.
A paired Student t test and a Wilcoxon paired test were performed for continuous variables with and without normal distribution, respectively, to evaluate differences in sleep parameters of naps.
CBT data were used to generate circadian phase estimates, using TrendReader2 software (TrendReader 2, ACR Systems Inc., Canada), Matlab (The MathWorks, Inc., version 7.11.0.584) and Cosinor analysis (Time Series Analysis Seriel Cosinor, Version 6.3, Expert Soft Technologie, Laboratory of Applied Statistics and BioMedical Computing, France). After removal of abnormal values, raw data were smoothed at the precise period of exercise, using an algorithm based on a penalized least squares method, allowing fast smoothing of data in one and more dimensions by means of the discrete cosine transform (Garcia 2010; Khayam 2003). Establishment of the cosine model was done using Cosinor methodology (Single and Population Mean Cosinor, Time Series Analysis Seriel Cosinor. http://www.eurostech.net), with an investigation of period comprised between 22 and 26 h. A least squares linear regression analysis using Cosinor analysis was used to determine the best fit of a 24-h period cosine function (Nelson et al. 1979): (Y(t i) = M + A cos ώt i + φ)), where M is the MESOR (Medline Estimating Statistic of Rhythm which corresponds to the estimated mean level of the rhythm), A is the amplitude (equal to 0.5 of the peak-to-trough variation due to rhythmicity), and φ is the acrophase (clock time at the maximal level in circadian rhythm referenced to local 00:00 hours).
Results
Polysomnographic variables
During naps
The composition of post-lunch nap was similar after normal night or phase-advance night. The subjects slept for 21–22 min in the hour they stayed in bed with sleep efficiency that approximated 70 %. Percentages of stage 2 (N2) and 3 (N3) were predominant (Table 1).
Only the number of awakenings decreased in phase-advance condition (p < 0.05). Rapid eye movement (REM) sleep occurred after normal sleep night, and tended to increase after phase-advance night (p = 0.08).
During the post-trial nights
There was a significant main effect of phase advance for total sleep time (TST; p < 0.05), sleep efficiency (SE; p < 0.05), sleep onset latency (SOL; p < 0.01), total time in rapid eye movement (TTREM; p < 0.001), and REM percentage (% REM; p < 0.001). Only a tendency in phase-advance effect was observed in N3 percentage (% N3; p = 0.054). Furthermore, there was a significant effect of naps vs no-nap for sleep onset latency (p < 0.01). The interaction of phase advance and nap was significant only for sleep onset latency (p = 0.05; Table 2).
In normal sleep condition without napping, the sleep onset latency (SOL) in post-trial night was 13.28 ± 5.52 min and increased significantly after post-lunch naps reaching higher values (24.27 ± 11.77 min; p < 0.01; Table 2).
In the 5-h phase-advance condition, SOL in post-trial night was short in no-nap condition (6.72 ± 3.83 min) and reached higher values in nap condition (11.84 ± 13.44 min; p < 0.05; Table 2).
There were no significant differences in the other recorded sleep variables.
Physical performance variables
Table 3a summarizes ANOVA results from Wingate parameters and lactate levels after both trials. No significant main effect of phase advance or nap was observed. Furthermore, there were no significant interactions between the main factors.
Peak power, mean power or fatigue index were similar after normal sleep or phase-advance condition with or without napping and whatever the trial (Table 3).
Whatever the experimental conditions, no significant difference in lactate levels was observed after nap vs no-nap in trial 1 or 2 (Table 3). Nevertheless, in shifted or unshifted condition, with or without nap, lactates levels at the end of exercise or at the fifth of recovery, decreased significantly in trial 2 (p < 0.001), compared to that measured in trial 1.
Core body temperature
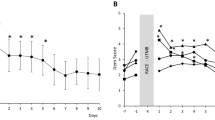
All CBT data are presented in Table 4 and Fig. 2. Times are expressed in local time.
Cosine curve of core body temperature in normal sleep condition (a), in 5-h phase advance (b), in normal sleep condition with nap (c) and in 5-h phase advance with nap (d). Filled black bars indicate sleep episodes (a–d), suns indicate period of exposure to natural light (a, c) and light bulbs indicate period of exposure to bright light (b, d). The clock time on the destination time (5-h phase advance) is indicated in the top axis (b, d). The corresponding clock time on the Paris time zone is indicated in the bottom axis (b, d). Downwards gray arrows indicate the minimum of endogenous CBT cycle. Upwards gray arrows indicate the time of post-lunch nap (c, d). The upwards black arrows indicate time of Wingate tests (a–d)
ANOVAs showed that there were significant main effects of phase-advance condition for acrophase (p < 0.001), batyphase (p < 0.001), amplitude (p < 0.001), trough (p < 0.001) and mesor (p < 0.05). There were also significant effects of nap for acrophase (p < 0.01), peak (p < 0.01), trough (p < 0.05) and mesor (p < 0.05, Table 4a). The interaction between phase advance and nap was significant on batyphase (p < 0.05), peak (p < 0.01) and mesor (p < 0.01).
In normal sleep condition, acrophase was delayed by 25 min in nap condition compared to no-nap, although the difference was not significant. But, peak, trough and mesor significantly increased by 0.20 °C (p < 0.001), 0.05 °C (p < 0.01) and 0.11 °C (p < 0.001; Table 4b), respectively, in nap vs no-nap condition.
In 5-h phase-advance condition, period, amplitude, peak, trough and mesor were similar after nap or no-nap condition. Acrophase and batyphase were 59 and 47 min delayed, respectively, in nap condition compared to no-nap (p < 0.01; Table 4).
Discussion
This study is the first in our knowledge to evaluate the effects of a 20-min nap scheduled during the post-prandial period on subsequent nocturnal sleep structure and physical performance in normal sleep or in 5-h phase-advance condition simulating home sleep or eastward transmeridian travel, respectively. The main results of the study indicate that a 20-min nap between 13:00 and 14:00 hours in non-shifted condition, and between 08:00 and 09:00 hours in shifted condition did not change sleep architecture of the post-trial night in athletes. Furthermore, naps did not improve physical performance while it modified the circadian temperature parameters. Nevertheless, the only significant data are those obtained, whatever the conditions, in lactatemia, at the end of exercise and at recovery, which are lower in trial 2 compared to those obtained in trial 1.
The composition of naps in terms of quantity and quality was very similar when it was carried out at different times. In non-shifted situation, nap occurred at the post-lunch dip in vigilance, when sleep propensity increased (Krauchi et al. 2006) and CBT fell. In shifted situation, naps took place in a forbidden zone for sleep (Lavie and Zvuluni 1992) but the sleep onset is possible, in relationship with a decrease of CBT at this time.
Furthermore, naps were composed essentially of stage 2 and stage 3 sleeps with a slight amount of REM, which increased in phase-advance condition, suggesting a good quality of daytime sleep. Milner et al. (2006) demonstrated that non-habitual young and healthy nappers, as the athletes of our study, fall asleep faster and tend to have greater sleep efficiency in contrast to usual nappers. On the other hand, McDevitt et al. (2012) showed that frequent napping appears to be associated with lighter daytime sleep and increases sleepiness during the day. These reasons explain why some athletes are sometimes unwilling to take a nap.
In shifted condition, higher REM activity may be related to a rebound due to early awakening (02:00 hours) on the pre-trial night, prohibiting the appearance of enough REM sleep (data not shown), since REM is usually concentrated at the end of night. At last, reduction of awakenings could be explained by a small sleep debt after the pre-trial phase-advance night (data not shown), causing a compensation of sleep and a lesser fragmented sleep.
Regarding sleep night structure, only sleep latency which is already long in most conditions, except during the PTPAN1, was lengthened by nap, which is rarely observed in athletes who usually fall asleep in about 5 min, as reported by Hayashi et al. (2005). These results could be explained by the change of environment (laboratory conditions) and the nap itself delaying sleep. Previous research examined the effects of napping as a way to predict the degree of benefits gained from nap in young adults after sleep restriction (Brooks and Lack 2006), or on waking function in elderly people (Milner and Cote 2008), shift workers (Schweitzer et al. 2006), and patients with sleep disorders (Reyes et al. 2013), but none in athletes. In light of our results, we may suggest that a short nap has little effect on the post-trial night’s sleep in athletes.
Moreover, to the best of our knowledge, no study to date has been specifically designed to examine the impact of napping on subsequent anaerobic performance performed either after a normal sleep or a 5-h phase advance. Most studies that have examined the effects of napping were conducted in the elderly, shift workers or young healthy subjects, on alertness (Asaoka et al. 2012), cognitive performances (Groeger et al. 2011), or motor imagery learning (Debarnot et al. 2011), demonstrating that napping was effective in enhancing performance. However, few studies have evaluated the consequences of napping on the physical performance in athletes. To date, only Waterhouse et al. (2007) investigated the effects of a 30-min post-lunch nap in partially sleep-deprived athletes and showed an improvement in sprint performance. These discrepancies with our findings are probably related to the duration of prior sleep. Indeed, in the latter study, subjects slept only 4 h during the previous night while in our study, subjects were not sleep deprived. However, the shifted athletes had a reduced duration of sleep of 1 h 20 min during the pre-trial night (data not shown). In our experimental protocol, the exercise or the duration of the nap may be too short to observe significant changes. We also speculate that the absence of napping effects would be related to the fact that our athletes were in the same temperature and posture conditions in nap condition than in rest position, and only the light exposure being different; it would be also related to the timing of the nap that coincides with the peak of alertness. Furthermore, our results could be related to changes in the secretion of cortisol and melatonin. As shown by Lemmer et al.(2002), the rhythm of these hormones are affected in their 24 h profiles and additionally modified, in well-trained athletes after an eastbound flight over 8 time zones. Similarly, Dijk et al. (2012) demonstrated an amplitude reduction and phase shifts of melatonin and cortisol after a gradual advance of sleep and light exposure in humans. Thereby, in our study, the circadian process would have a greater impact than the homeostatic process.
Nevertheless, effects of napping in athletes in phase-advance condition were found on acrophase which was 59 min delayed and on batyphase which was 47 min delayed, suggesting that nap did not facilitate readjustment of CBT. On this subject, Krauchi et al. (2006) showed close links between naps and CBT, through the relationship between the components of the three-process model of sleepiness regulation (homeostatic, circadian, and sleep inertia) and the thermoregulatory system.
Conclusion
Consequently, from a sport performance perspective, we showed that a short post-lunch nap induced more difficulties in falling asleep in subsequent nights at home or after crossing time zones. Moreover, napping had no beneficial effect on short-term physical performance and did not facilitate readjustment of CBTs in phase-advance condition. Yet, it remains to be determined if nap opportunities of 40 and 60 min would show more benefits as recommended for improvement in cognitive performance (Mulrine et al. 2012). Taking a nap at different times of the day would be also more effective (especially for athletes who have to perform twice a day, in training or competitions). Finally, it would be interesting to test our experimental procedure in longer track events and in a wider range of sporting activities.
Abbreviations
- AASM:
-
American Academy of Sleep Medicine
- CBT:
-
Core body temperature
- EEG:
-
Electroencephalogram
- P mean :
-
Mean power
- P peak :
-
Peak power
- % N1:
-
Percentage in sleep stage 1
- % N2:
-
Percentage in sleep stage 2
- % N3:
-
Percentage in sleep stage 3
- % REM:
-
Percentage in rapid eye movement
- PSG:
-
Polysomnography
- PTN1:
-
Post-tests night 1
- PTN2:
-
Post-tests night 2
- PTPAN1:
-
Post-tests night with phase advance 1
- PTPAN2:
-
Post-tests night with phase advance 2
- SE:
-
Sleep efficiency
- SOL:
-
Sleep onset latency
- TST:
-
Total sleep time
- TTN1:
-
Total time in stage 1
- TTN2:
-
Total time in stage 2
- TTN3:
-
Total time in stage 3
- TTREM:
-
Total time in stage REM
References
Asaoka S, Fukuda K, Murphy TI, Abe T, Inoue Y (2012) The effects of a nighttime nap on the error-monitoring functions during extended wakefulness. Sleep 35(6):871–878
Bar-Or O (1987) The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med (Auckland, NZ) 4(6):381–394
Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ (2003) Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 12(1):1–12
Borbely AA (1982) A two process model of sleep regulation. Hum Neurobiol 1(3):195–204
Brooks A, Lack L (2006) A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep 29(6):831–840
Debarnot U, Castellani E, Valenza G, Sebastiani L, Guillot A (2011) Daytime naps improve motor imagery learning. Cogn Affect Behav Neurosci 11(4):541–550
Dijk DJ, Duffy JF, Silva EJ, Shanahan TL, Boivin DB, Czeisler CA (2012) Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS One 7(2):e30037. doi:10.1371/journal.pone.0030037
Dinges DF, Broughton RJ (1989) Sleep and alertness: chronobiological, behavioral, and medical aspects of napping. Raven Press, New York
Dinges DF, Orne MT, Whitehouse WG, Orne EC (1987) Temporal placement of a nap for alertness: contributions of circadian phase and prior wakefulness. Sleep 10(4):313–329
Garcia D (2010) Robust smoothing of gridded data in one and higher dimensions with missing values. Comput Stat Data Anal 54(2010):1167–1178
Groeger JA, Lo JC, Burns CG, Dijk DJ (2011) Effects of sleep inertia after daytime naps vary with executive load and time of day. Behav Neurosci 125(2):252–260
Hayashi M, Watanabe M, Hori T (1999) The effects of a 20 min nap in the mid-afternoon on mood, performance and EEG activity. Clin Neurophysiol 110(2):272–279
Hayashi M, Motoyoshi N, Hori T (2005) Recuperative power of a short daytime nap with or without stage 2 sleep. Sleep 28(7):829–836
Horne JA, Ostberg O (1976) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4(2):97–110
Iber C, Ancoli-Israel S, Chesson AL Jr, SF Q (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification, 1st edn. American Academy of Sleep Medicine, Westchester
Khayam SA (2003) The discrete cosine transform (DCT): Theory and Application. Department of Electrical & Computer Engineering, Michigan State University
Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C (2006) Challenging the sleep homeostat does not influence the thermoregulatory system in men: evidence from a nap vs. sleep-deprivation study. Am J Physiol Regul Integr Comp Physiol 290(4):R1052–R1061. doi:10.1152/ajpregu.00381.2005
Lavie P, Zvuluni A (1992) The 24-hour sleep propensity function: experimental bases for somnotypology. Psychophysiology 29(5):566–575
Lemmer B, Kern RI, Nold G, Lohrer H (2002) Jet lag in athletes after eastward and westward time-zone transition. Chronobiol Int 19(4):743–764
McDevitt EA, Alaynick WA, Mednick SC (2012) The effect of nap frequency on daytime sleep architecture. Physiol Behav 107(1):40–44
Milner CE, Cote KA (2009) Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res 18(2):272–281
Milner CE, Cote KA (2008) A dose-response investigation of the benefits of napping in healthy young, middle-aged and older adults. Sleep Biol Rhythm 6(1):2–15
Milner CE, Fogel SM, Cote KA (2006) Habitual napping moderates motor performance improvements following a short daytime nap. Biol Psychol 73(2):141–156
Morris C, Atkinson G, Drust B, Marrin K, Gregson W (2009) Human core temperature responses during exercise and subsequent recovery: an important interaction between diurnal variation and measurement site. Chronobiol Int 26(3):560–575
Mulrine HM, Signal TL, Berg MJ, Gander PH (2012) Post-sleep inertia performance benefits of longer naps in simulated nightwork and extended operations. Chronobiol Int 29(9):1249–1257. doi:10.3109/07420528.2012.719957
Naitoh P (1981) Circadian cycles and restorative power of naps. In: Johnson et al. (eds) Biological rhythms: sleep and shift works. Spectrum publications, New York, pp 553–580
Nelson W, Tong YL, Lee JK, Halberg F (1979) Methods for Cosinor-rhythmometry. Chronobiologia 6(4):305–323
Reyes S, Algarin C, Bunout D, Peirano P (2013) Sleep/wake patterns and physical performance in older adults. Aging Clin Exp Res 25(2):175–181
Samuels CH (2012) Jet lag and travel fatigue: a comprehensive management plan for sport medicine physicians and high-performance support teams. Clin J Sport Med 22(3):268–273
Schweitzer PK, Randazzo AC, Stone K, Erman M, Walsh JK (2006) Laboratory and field studies of naps and caffeine as practical countermeasures for sleep-wake problems associated with night work. Sleep 29(1):39–50
Tietzel AJ, Lack LC (2002) The recuperative value of brief and ultra-brief naps on alertness and cognitive performance. J Sleep Res 11(3):213–218
Waterhouse J, Atkinson G, Edwards B, Reilly T (2007) The role of a short post-lunch nap in improving cognitive, motor, and sprint performance in participants with partial sleep deprivation. J Sports Sci 25(14):1557–1566
Acknowledgments
The authors are grateful to the athletes who participated in this study. We also thank Gaëlle Brunotte for editorial assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael Lindinger.
Rights and permissions
About this article
Cite this article
Petit, E., Mougin, F., Bourdin, H. et al. A 20-min nap in athletes changes subsequent sleep architecture but does not alter physical performances after normal sleep or 5-h phase-advance conditions. Eur J Appl Physiol 114, 305–315 (2014). https://doi.org/10.1007/s00421-013-2776-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2776-7