Abstract
Purpose
This study examined the effects of short-term high-intensity interval training (HIT) and continuous moderate-intensity training (CMT) on cardiac function in young, healthy men.
Methods
Sixteen previously untrained men (mean age of 25.1 ± 4.1 years) were randomly assigned to HIT and CMT (n = 8 each) and assessed before and after six sessions over a 12-day training period. HIT consisted of 8–12 intervals of cycling for 60 s at 95–100 % of pre-training maximal aerobic power (\(\dot{V}\)O2max), interspersed by 75 s of cycling at 10 % \(\dot{V}\)O2max. CMT involved 90–120 min of cycling at 65 % pre-training \(\dot{V}\)O2max. Left ventricular (LV) function was determined at rest and during submaximal exercise (heart rate ~105 bpm) using two-dimensional and Doppler echocardiography.
Results
Training resulted in increased calculated plasma volume (PV) in both groups, accompanied by improved \(\dot{V}\)O2max in HIT (HIT: from 39.5 ± 7.1 to 43.9 ± 5.5 mL kg−1 min−1; CMT: from 39.9 ± 5.9 to 41.7 ± 5.3 mL kg−1 min−1; P < 0.001). Resting LV function was not altered. However, increased exercise stroke volume (P = 0.02) and cardiac output (P = 0.02) were observed, secondary to increases in end-diastolic volume (P < 0.001). Numerous Doppler and speckle tracking indices of diastolic function were similarly enhanced during exercise in both training groups and were related to changes in PV.
Conclusion
Short-term HIT and CMT elicit rapid improvements in \(\dot{V}\)O2max and LV filling without global changes in cardiac performance at rest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term endurance training elicits numerous cardiac (Blomqvist and Saltin 1983; Fagard 2003; Goodman 2000) and peripheral vascular adaptations (Blomqvist and Saltin 1983; Green et al. 2006), both of which contribute to increasing the maximal rate of oxygen delivery (\(\dot{V}\)O2max) (Blomqvist and Saltin 1983). Structural and functional adaptations of the heart secondary to exercise training have been well described (Fagard 2003; Pelliccia 1996). While evidence of improved inotropic function secondary to training in humans remains equivocal (Blomqvist and Saltin 1983; Goodman et al. 2005), it is widely accepted that adaptations to sustained aerobic training include bradycardia (Smith et al. 1989) and increased stroke volume (SV) at rest and during exercise (Blomqvist and Saltin 1983; Goodman et al. 2005; Spina et al. 1992). Training-induced bradycardia is mediated by increased parasympathetic (vagal) tone to the heart (Carter et al. 2003). The increase in resting and exercise SV is predominantly attributed to increases in left ventricular (LV) end-diastolic volume (EDV), in part attributed to plasma volume (PV) expansion (Convertino 1991; Fagard 1997; Spina et al. 1992). Left ventricular remodeling that increases both chamber size and mass accompany these functional outcomes over the course of training (Shapiro 1984), referred to so-called ‘athletic heart’ (Fagard 2003; Goodman 2000).
Although most traditional training programs have involved continuous, moderate-intensity training (CMT) sessions of long durations, high-intensity interval training (HIT) can stimulate similar, if not superior, changes in cardiovascular function in both healthy (Gibala 2007) and clinical populations (Warburton et al. 2005; Wisloff et al. 2007). The adaptive stimulus provided by HIT is often characterized by a lower total exercise volume (e.g., duration × intensity), thereby being a time-effective alternative to CMT. In contrast to ample evidence describing the effects of prolonged training, there are few data describing cardiovascular adaptations arising from short-term training interventions (e.g., ≤2 weeks), reflecting the early phase of the training process. The cardiac changes that occur with short-term training would provide information about the early time-course of adaptations, before structural remodeling is likely to occur.
Short-term CMT has significant systemic effects (Goodman et al. 2005; Green et al. 1990; Harris et al. 2003), and short-term HIT induces rapid adaptations in skeletal muscle metabolism (Gibala et al. 2006; Little et al. 2010). A limited number of studies have assessed the influence of short-term CMT on cardiovascular function (Currie et al. 2009; Goodman et al. 2005; Green et al. 1990; Harris et al. 2003; Mier et al. 1997), yet the effects of HIT on cardiac morphology and function have not been investigated during the early stages of training. Data from several investigations indicate that the benefits associated with prolonged training are intensity dependent (Swain and Franklin 2006; Wisloff et al. 2006). Given the significance of \(\dot{V}\)O2max as a key prognostic factor for cardiovascular disease (Keteyian et al. 2008), exercise intensity is an important component leading to improvements in \(\dot{V}\)O2max and reducing cardiovascular risk factors. Thus, low-volume HIT may be a practical, time-efficient intervention for inducing rapid adaptations in LV function and aerobic exercise performance, both of which are associated with improved health benefits. Therefore, the purpose of our study was to examine the effects of short-term CMT and HIT on cardiac function at rest and during exercise in young, healthy, previously untrained men. We hypothesized that HIT would be equally effective as CMT at inducing similar improvements in \(\dot{V}\)O2max. In addition, we expected short-term CMT and HIT to enhance LV filling at rest and during submaximal exercise.
Methods
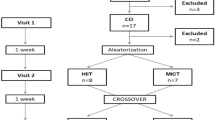
We recruited 16 young, previously untrained males ranging in age from 19 to 31 years. Individuals who were more than recreationally active, defined as participating in more than 2 h of low to moderate intensity (>6 METs) physical activity per week, were excluded. Physical activity was considered any organized or regimented activity that requires the body to expend more energy compared to resting conditions. All participants were normotensive (blood pressure <140/90 mmHg), non-obese (BMI <30 kg m−2), non-diabetic, non-smokers, and had no history of cardiovascular, pulmonary, or other metabolic or musculoskeletal disease, and were free of cardioactive medications. In addition, they were excluded if they possessed any health condition that would preclude vigorous exercise. Informed written consent was obtained from all participants and the Physical Activity Readiness Questionnaire (PAR-Q) was completed successfully prior to participation. All study procedures were reviewed and approved by the hospital and the university’s research ethics board, in full conformity with the Helsinki Declaration on the use of human participants.
Experimental design
All participants underwent an initial graded exercise test on a cycle ergometer to determine baseline \(\dot{V}\)O2max. These results were used to establish training intensities for each participant according to their training group. On a separate occasion separated by at least 1 day but not exceeding 2 weeks of baseline testing of \(\dot{V}\)O2max, participants underwent baseline assessment of cardiac function. Doppler and two-dimensional (2D) echocardiography was performed at rest and during submaximal exercise at a heart rate (HR) corresponding to ~105 beats per min (bpm) on a cycle ergometer in a semi-upright position. Following baseline assessments of \(\dot{V}\)O2max and LV function, participants were given at least 24 h to rest, after which they were randomly assigned to either HIT or CMT groups. Each supervised training protocol consisted of six sessions over a 12-day period with 1–2 days of rest in between. Following the cessation of the training program, participants returned for post-training assessments, which mirrored baseline testing. Post-training tests were performed no earlier than 48 h and no later than 72 h of the cessation of the training intervention. Participants were asked to avoid alcohol and caffeine consumption 12 h prior to their baseline and follow-up assessments.
Maximal aerobic power
\(\dot{V}\)O2max was measured using a progressive exercise test to exhaustion on an electrically braked cycle ergometer (Lode Examiner, Groningen-Holland Medical Technology) (Currie et al. 2009). Following a 3-min warm-up at 25 W, work rate increased to 50 W for 2 min, after which it increased by 50 W every 2 min until 200 W, and then increased by 25 W every minute until exhaustion. Breath-by-breath expired gases were collected using a metabolic cart (Moxus Modular System, Applied Electrochemistry Incorporated, Pittsburgh, PA) with HR monitored (Polar 810i) and recorded continuously (HRTrak II Heart Rate Tracker, Equilibrated Bio Systems Inc., New York, USA). \(\dot{V}\)O2max was confirmed by a plateau in oxygen consumption despite an increase in work rate and presence of the following secondary endpoints: (1) attainment of age-predicted maximal HR, (2) achievement of a respiratory exchange ratio (RER) that is ≥1.15, and (3) a rating of perceived exertion of >18 based on a 20-point Borg scale.
Blood sampling
Resting blood samples were drawn prior to and after training using microhematocrit capillary tubes in triplicate. Hct, the percentage/ratio of red blood cell volume to a given volume of whole blood, was determined using a microhematocrit scale and reported in %. Hct values were multiplied by 0.8736 (0.96 × 0.91) to correct for trapped plasma (0.96) and to convert venous Hct to whole body Hct (0.91) (van Beaumont et al. 1973). The percent change in PV following training was calculated from the corrected Hct readings (van Beaumont et al. 1973).
Left ventricular function
Resting and submaximal exercise (corresponding to HR of ~105 bpm). Imaging and exercise was performed on an imaging table (Model 96039, American Echo) fitted with a cycle ergometer (Supine Ergometer, model AE2, American Echo, Kansas City, MO). All participants pedaled at a cadence of 60 revolutions per min before and after training through the aid of a cadence monitor mounted on the cycle ergometer. We attempted to establish a steady-state HR of ~105 bpm during exercise to establish similar cardiac loading and imaging conditions. To accomplish this, the work rate on the cycle ergometer was increased until the target HR was achieved. After approximately 2 min of cycling and after a steady-state HR was observed (30 s without drifting of HR beyond 5 bpm), echocardiographic images were acquired. In instances where the HR began fluctuating (increasing or decreasing beyond 5 bpm) at any particular load once cardiac imaging had commenced, the work rate was adjusted to re-establish steady state. These procedures were standardized pre- and post-intervention. Subjects were positioned in a 60° semi-upright position oriented in the left lateral decubitus position that was secured to the echocardiography-imaging table. HR was monitored by a 3-lead ECG (Case 16 Exercise Testing System, Marquette Medical Systems, Milwaukee, WI).
Cardiac images were acquired by 2D and Doppler echocardiography (GE Vivid 7 Imaging System, version BT03-5; GE Healthcare, Canada) with M4S probe using optimized windows with a frame rate between 60 and 90 fps and analyzed off-line using a proprietary workstation (GE Healthcare, EchoPac, Version 7) by a single trained observer, with imaging and analyses performed in accordance with the American Society of Echocardiography guidelines (Gottdiener et al. 2004). The average of a minimum of three cardiac cycles is reported. Anatomical M-mode (AM-mode) and 2D echocardiography images were obtained from the standard parasternal and apical windows. Efforts were made to ensure subjects maintained regular breathing patterns, but it was not possible to capture images during breath-holding at end-expiration. All system settings were adjusted to produce optimal signal-to-noise ratio and endocardial delineation. AM-mode image acquisition and measurements were used to determine the LV internal dimension during diastole (LVIDd) and systole (LVIDs), the interventricular septum during diastole (IVSd), and the LV posterior wall thickness during diastole (LVPWDd). Measures of LV mass and LV mass index were then automatically derived using standard equations. LV end-diastolic (EDV) and end-systolic (ESV) volumes were measured and used to determine ejection fraction (EF) in apical two-chamber and four-chamber views, using a modified bi-plane Simpson’s method (Lang et al. 2005). SV was calculated according to the following equation: SV = EDV − ESV, and cardiac output (CO) was determined based on the following equation: CO = SV × HR. To assess LV systolic performance, myocardial strain (S) and strain rate (SR) were measured from apical four-chamber views using 2D speckle tracking analysis acquired 70 frames per second. Regional systolic and diastolic longitudinal S and SR analysis involved six segments of the LV wall (basal septum, mid septum, apical septum, apical lateral, mid lateral, and basal lateral). Global longitudinal S and SR were derived based on the mean of the six LV segments. Peak longitudinal S was defined as the greatest value on the strain curve (S-curve). Measures of LV S are reported as the percentage change in deformation from the initial value, whereas SR is the rate of deformation change per second. To assess diastolic function, early (peak E) diastolic SR (SRe) and late (peak A) diastolic SR (SRa) were assessed with 2D speckle tracking, and pulsed wave (PW) Doppler interrogation of mitral inflow velocity spectral, and Tissue Doppler imaging (TDI) were examined in the apical four-chamber view. PW Doppler recordings were performed to determine diastolic transmitral blood flow velocities for peak early (E) and peak late (A) filling and the ratio of early to late diastolic filling velocities (E/A). TDI measures of peak early (E′) and late atrial (A′) myocardial tissue velocities were acquired at the septal and lateral mitral annular sites for the determination of E′/A′ values. PW Doppler and TDI data were combined to calculate E/E′ (ratio of early diastolic filling to peak early tissue velocity), a non-invasive estimate of left atrial (LA) pressure (Gottdiener et al. 2004). The intraclass correlation coefficient (ICC) was calculated for resting and exercise CO, and E and E′ values. All data were analyzed in a non-sequential and randomized manner by a single trained observer who was blinded to group allocation or time of assessment.
Exercise training
Training interventions were modified based upon previous short-term models (Currie et al. 2009; Gibala et al. 2006; Goodman et al. 2005; Little et al. 2010). Each training regimen consisted of six sessions completed over 12 days. Thus, a minimum of 24 h of rest was given to each participant in between training days to minimize fatigue. The time of the training session each day varied slightly according to subject scheduling, but was within a similar 3–4 h window. Each session included a 3 min, low-intensity warm-up (10 % pre-training \(\dot{V}\)O2max). Water was provided to the participants ad libitum. HR and fluid intake were recorded throughout to ensure that participants were working at their specified intensity and maintained hydration.
Continuous moderate-intensity training
The CMT program required the participants to cycle for 90–120 min (1.5–2 h) during six sessions at 65 % of their pre-training \(\dot{V}\)O2max values. Training consisted of cycling for 90 min on the first and second training sessions, 105 min on the third and fourth sessions, and 120 min on the final two sessions. Based on prior reports (Currie et al. 2009; Goodman et al. 2005), it was expected that during the earlier phases of the training program, some participants would not have the capacity to cycle continuously for 90–105 min at their given training intensity. Therefore, participants were allowed to take brief (2–4 min) intermittent rests as required; however, they had to complete the required number of hours of exercise during each training session. In previous studies, subjects were able to complete 2 h of exercise without interruption by the end of a 6-day exercise training session (Goodman et al. 2005; Currie et al. 2009).
High-intensity interval training
The HIT intervention was modified from Little et al. (2010) who reported high tolerance and no complications in young, healthy, recreationally active males. This involved 8–12 intervals of cycling for repeated efforts of 60 s at an intensity corresponding to 95–100 % pre-training \(\dot{V}\)O2max (peak power output achieved during baseline testing), interspersed by 75 s of cycling at a workload corresponding to 10 % pre-training \(\dot{V}\)O2max. Participants completed 8 intervals on the first and second training sessions, 10 intervals on the third and fourth sessions, and 12 intervals on the last two sessions. Unlike previous studies that matched training volumes between HIT and CMT (Warburton et al. 2004, 2005; Wisloff et al. 2007), in the present study, the training volume for the HIT group was only ~24 % of the CMT group (1,565 vs. 6,500 kJ) with a total training time commitment over 2 weeks of ~2 h (including recovery periods), compared to the CMT group participants, who performed a total of ~10.5 h of exercise.
Statistical analysis
Data are presented as means and standard deviations. Statistical analysis was performed using a mixed model repeated measures of Analysis of Variance (ANOVA) with two factors (group and time) to determine the significance of the main effect. Independent t tests using Bonferroni post hoc analysis were used to assess differences in main effect between groups. Pearson’s simple correlation was used to study the association between changes in PV and changes in \(\dot{V}\)O2max, SV, EDV, E, E′, as well the relation between changes in SRe and changes in EDV. All comparisons were based on a 95 % confidence limit (P < 0.05). All data were analyzed using the statistical software Predictive Analytics SoftWare (PASW) Statistics, version 18.0.
Results
Subject characteristics and ventricular morphology data are presented in Table 1. All participants successfully completed the study with no adverse events. At baseline, there were no significant differences in age, height, body mass, body mass index (BMI), peak HR, \(\dot{V}\)O2max and peak power output between the two groups. All participants presented with normal \(\dot{V}\)O2max, typical of young, previously untrained males. There were no significant changes in height, body mass, BMI, and peak HR following training in either group compared to baseline. Ventricular morphology was similar between HIT and CMT groups at baseline and did not significantly change following training.
Hematocrit and plasma volume
Following training, Hct was reduced in both HIT (from 45.5 ± 2.4 % to 43.0 ± 3.4 %, P < 0.001) and CMT (from 46.1 ± 2.3 % to 43.8 ± 3.3 %, P < 0.001). The calculated changes in PV were 10.8 and 9.7 % in the HIT and CMT groups, respectively.
Maximal aerobic power
\(\dot{V}\)O2max increased significantly in HIT and CMT (main effect of time, P < 0.001) after training, reflecting improvements of 9 and 5 % in HIT and CMT, respectively. The magnitude of increase trended more favorably for the HIT group (Fig. 1), and while consistent responses were observed for subjects in both groups, the group-differences failed to reach statistical significance (P = 0.06) (Table 1). A correlation between the percent change (%∆) in PV and %∆\(\dot{V}\)O2max was observed (r = 0.71, P < 0.01). In addition, peak power output was significantly increased in HIT and CMT (main effect of time, P = 0.034) compared to baseline (Table 1).
Hemodynamic responses to training
At rest, baseline measures of systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, SV, CO, EDV, ESV, EF, and systolic blood pressure to end-systolic volume (SBP/ESV) did not significantly differ between the groups. Regardless of training modality, these variables were not changed after the training intervention (Tables 1, 2).
During exercise, EDV and SV (main effect for time, P < 0.001 and P = 0.02, respectively) were significantly elevated under comparable HRs after training with the magnitude of increase being similar between the two training groups. These changes resulted in significantly higher submaximal exercise CO values in both groups after training relative to baseline (main effect for time, P = 0.02) (Fig. 2; Table 2). There was a modest, yet significant association between %∆PV and %∆SV (r = 0.52, P < 0.05), although there was no significant relation between %∆PV and %∆EDV (P > 0.05). Exercise measures of ESV, EF, and SBP/ESV were unaltered following training in both groups. We examined the intra-rater reliability in all participants using ICCs. The ICC for CO measures was 0.91 and 0.86 at rest and during exercise, respectively.
Individual data points for changes in EDV, SV, and CO during submaximal exercise after training. Bold line indicates the mean change. *P < 0.05 for within-group comparison of pre- vs. post-training. EDV end-diastolic volume, SV stroke volume, CO cardiac output, HIT high-intensity interval training, CMT continuous moderate-intensity training
Diastolic function at rest and during submaximal exercise
The resting E/A was significantly increased after training compared to baseline in the HIT group only (P = 0.02). E/E′ ratio at rest was significantly higher at baseline (P = 0.013) and following training (P = 0.018) in the HIT group compared to CMT, although this index was unaltered by either training program. There were no differences observed for other resting PW Doppler flow and myocardial tissue velocities before and after training. However, during submaximal exercise, short-term HIT and CMT resulted in significantly increased velocities for E (main effect for time, P = 0.001) and E′ septal (main effect for time, P = 0.01). Furthermore, E, E/A ratio, and E′ lateral were significantly augmented (main effect for time, P = 0.002, P = 0.001, and P = 0.004, respectively) in the HIT group only (Table 3). Individual and mean data points demonstrating changes in E and E′ (combined septal and lateral) are shown in Fig. 3. Training-induced %∆PV was directly related to changes in E (r = 0.53, P < 0.05), while %∆PV was also correlated with %∆E′ (r = 0.84, P < 0.01). The ICC of E and E′ at rest was 0.91 and 0.85, respectively, and 0.89 and 0.87 for E and E′, respectively, during submaximal exercise.
Individual data points for changes in E, E′, and SRe during submaximal exercise after training. Bold line indicates the mean change. *P < 0.05 for within-group comparison of pre- vs. post-training. E peak early diastolic blood flow velocity across the mitral valve, E’ peak early myocardial tissue velocity, SRe early diastolic strain rate, HIT high-intensity interval training, CMT continuous moderate-intensity training
Myocardial strain and strain rate at rest and during submaximal exercise
There were no changes in resting measures of S, SR, SRe, SRa, and SRe/a in either group (P > 0.05). SRe during exercise was significantly elevated in the HIT group only after training (P = 0.002) (Table 4; Fig. 3). Furthermore, there was an association between %∆SRe and %∆EDV in both groups (r = 0.54; P < 0.05). Other exercise measures of S and SR remained unchanged in both groups following training.
Discussion
This study compared the influence of short-term HIT and CMT on LV function at rest and during submaximal exercise. Our findings of enhanced \(\dot{V}\)O2max are congruent with previous studies of short periods of training (Goodman et al. 2005; Green et al. 1990; Harris et al. 2003; Mier et al. 1997) and support observations that low-volume HIT induces rapid adaptations in exercise performance after a relatively low time commitment (Gibala et al. 2006; Little et al. 2010). Two novel observations include the improvement in LV diastolic function during exercise after short-term HIT, and similar training adaptations in each group despite significant differences in total work performed and training time. The HIT group expended approximately 1,500 kJ including the recovery periods, whereas the CMT group expended close to 6,500 kJ, almost four times the work of HIT. Furthermore, participants in the HIT group cycled for a total duration of ~120–174 min, considerably less than the CMT group (~630 min).
Aerobic power
We have shown that short-term HIT and CMT result in comparably marked improvements in \(\dot{V}\)O2max, in agreement with others using short-term CMT (Goodman et al. 2005; Green et al. 1990; Mier et al. 1997). Our group has previously reported significant augmentations in \(\dot{V}\)O2max following six consecutive days of CMT (Goodman et al. 2005). However, others have failed to demonstrate changes in \(\dot{V}\)O2max following CMT protocols (Currie et al. 2009; Harris et al. 2003). The discrepancy is likely due to differences in experimental protocols, training interventions, and participant characteristics. All post-training \(\dot{V}\)O2max measures occurred after 2 days and before 3 days of recovery to avoid detraining effects and ensure recovery before post-training assessments. Although an increase in \(\dot{V}\)O2max following interval training has been reported in athletes (Laursen et al. 2005), healthy sedentary individuals (Helgerud et al. 2007), and clinical populations (Warburton et al. 2005; Wisloff et al. 2007), the training period for the majority of these reports has been longer than in the current study. Several investigations have observed improvements in cycling time trial performance as a measure of functional exercise capacity following HIT interventions (Gibala et al. 2006; Little et al. 2010). Using a similar HIT protocol as the one employed in the present study, Little et al. (2010) reported significant enhancements in 50 kJ and 750 kJ cycling time trial performance, concomitant with increased mean power output, in recreationally active males. The present study demonstrated the effectiveness of a low-volume HIT model to elicit pronounced responses in \(\dot{V}\)O2max within 2 weeks of training. It is noteworthy that prior reports evaluating adaptations to short-term HIT (Burgomaster et al. 2008; Gibala et al. 2006; Little et al. 2010) have primarily employed recreationally active individuals as study participants, which may limit improvements.
Systolic function
Evidence surrounding the effects of short-term endurance training on cardiac contractile function is mixed. In a previous study, our group (Goodman et al. 2005) reported that EF failed to change the following six consecutive days of CMT; however, EF is highly influenced by loading conditions, and therefore EF data do not directly imply changes in LV contractile state (Carabello 2003). Changes in SBP/ESV ratio, a more sensitive index of contractility, were also unchanged (Goodman et al. 2005). Using similar indices, another study (Warburton et al. 2004) reported no changes in systolic function after 6 weeks of CMT and HIT. However, exercise LV EF, SBP/ESV, and fractional shortening ratio have increased following short-term endurance training (Ehsani et al. 1991; Mier et al. 1997). We failed to detect changes in any of these indices, particularly with less load-dependent measures of systolic performance obtained from speckle tracking imaging, such as LV S and SR. These findings demonstrate that short-term endurance training generally does not alter LV contractile performance in healthy young individuals.
Diastolic function
A consistent finding for both CMT and HIT groups increased EDV during exercise. Increases in SV after sustained endurance training are secondary to enhanced ventricular preload and recruitment of the Frank–Starling mechanism during exercise (Arbab-Zadeh et al. 2004; Levine et al. 1991); these changes were associated with enhanced diastolic filling indices, which would serve to compensate for the reduction in diastole during exercise despite increasing HR. In our study, EDV, SV, and the consequent rise in CO increased to the same magnitude in both training groups without enhanced LV emptying. More compliant ventricles, in association with hypervolemia, may explain the larger LV filling volumes observed during exercise in athletes (Arbab-Zadeh et al. 2004; Carrick-Ranson et al. 2012) with more pronounced effects observed in the upright position (Hopper et al. 1988; Kanstrup and Ekblom 1982). The training-induced increases in EDV and SV during exercise are consistent with previous reports and support the hypothesis that short-term training results in volume-mediated changes in LV performance. It has been postulated that this adaptation may be related to the intensity of training and independent of training duration (Goodman et al. 2005; Spina et al. 1992). Due to the significant influence of HR on LV loading conditions and in particular, diastolic function, we assessed all subjects at a target HR of ~105 bpm so that early and late phases of diastole could be delineated using Doppler measures of transmitral flow velocities. Of particular interest was the finding that under comparable HRs, SV and CO were augmented in both groups following training, whereas LV EF, ESV, SBP/ESV, S, and SR were unchanged. E/E′, an index of LA pressure, was unaffected by training, suggesting that a key adaptation was an increase in SV and CO despite both a similar filling pressure and time in diastasis. This is supported by an evidence indicating that the primary difference between trained individuals and their sedentary counterparts is the improved ability to recruit the Frank–Starling mechanism (Levine et al. 1991), secondary to remodeling that enhances LV compliance as evidenced by increased LV E′. This may be due to improved beta-adrenergic stimulation in association with increased rate of calcium uptake from the myocellular cytosol into the sarcoplasmic reticulum induced by phosphorylation of phospholamban (Sasaki et al. 1992). Furthermore, both pericardial remodeling (Esch et al. 2007a) and diastolic ventricular interactions favoring the unloading of the right ventricle (Esch et al. 2007b) may also contribute to improved LV compliance in endurance-trained athletes. Although the E/E′ ratio provides a reliable estimate of filling pressures in patients with cardiac disease, E′ is directly related to preload in healthy subjects (Bhella et al. 2011; Firstenberg et al. 2000). Therefore, E/E′ may not reliably reflect changes in filling pressure in healthy individuals, particularly during exercise. In addition, since E′ reflects early LV filling, it is subject to the influence of not only LV relaxation, but also LA pressure, which is most noticeable in healthy individuals (Nagueh et al. 2001). Thus, the potential effect of LA pressure is another limitation to E′, and E/E′ ratio may not detect small changes in LA pressure, which would significantly affect transmitral flow velocities during exercise. Thus, we cannot rule out that small alterations in LA pressure may have affected our results.
Alterations in blood volume may influence LV diastolic filling, as the elevated myocardial performance of endurance-trained individuals may be partly due to the training-induced expansion of blood volume (Warburton et al. 2000, 2004). Hypervolemia, mostly through PV expansion (Convertino 1991; Sawka et al. 2000), is the most pronounced adaptation to short-term training (Goodman et al. 2005; Green et al. 1984, 1990; Mier et al. 1997) and similar to the effects of acute PV expansion in untrained individuals (Coyle et al. 1990; Hopper et al. 1988). The BV expansion peaks within approximately 10–14 days of training (Convertino 1991; Sawka et al. 2000); therefore, the early enhancement of cardiovascular performance is associated and possibly triggered by hypervolemia (Convertino 1991; Green et al. 1984; Harris et al. 2003; Warburton et al. 2004). We observed a calculated PV expansion of 11 and 10 % in the HIT and CMT groups, respectively, consistent with prior observations using the same methodology (Goodman et al. 2005; Harris et al. 2003). However, the limitations of predicting PV from peripheral blood sampling preclude definitive conclusions, and this response may not be universal as a recent study using a similar HIT model as the present study did not show changes in PV or total BV (Jacobs et al. 2013). The improved LV filling (i.e., E and E′) observed in our study is possibly associated with PV expansion that may induce longer-term structural remodeling (Goodman et al. 2005), while acutely enhancing the Frank–Starling mechanism during exercise.
We were surprised to observe that SRe increased in the HIT group only and while there was an association between changes in SRe and changes in EDV, this observation was largely confined to the HIT group. Although the significance of these particular findings is unclear, they nonetheless provide further support for the effectiveness of HIT as a potent stimulus to mediate rapid improvements in diastolic performance in non-athletes. In addition, we did not observe a training-induced bradycardia in either group after short-term training, as a longer time period of training may be required to stimulate neurally-mediated changes in HR (Goodman et al. 2005). However, this suggests that the changes in EDV were independent of filling time. Longer-term training appears to induce LV remodeling and changes in vagal-sympathetic drive that induces more substantial changes in cardiac function (Blomqvist and Saltin 1983; Harris et al. 2003). In addition, such adaptations may not confer improvements in maximal CO, as was recently shown (Jacobs et al. 2013), underscoring the importance of peripheral adaptations to HIT.
Since Doppler measures of flow velocity are determined by the pressure gradient across the valves, transmitral flow velocities may be reflective of the pressure difference (i.e., driving force) between the LA and LV (Nishimura et al. 1989). However, despite their high reproducibility and common use to assess diastolic function, transmitral Doppler flow velocities remain HR and load-dependent and interpretation of these endpoints should be made with caution (Galderisi et al. 1993; Prasad et al. 2007). The E/A ratio is a non-specific composite index of both early and late transmitral filling and is influenced by both ventricular compliance and relaxation (Prasad et al. 2007). In our study, it appears that following training, the enhancement of Doppler transmitral measures (i.e., increased E) reflects a more compliant ventricle coupled with reduced atrial contribution (i.e., reduced A and increased E/A ratio). TDI data confirm these results and may have fewer limitations than Doppler flow velocities (Garcia et al. 1998; Gottdiener et al. 2004), in part due to their high amplitude and low frequency (Garcia et al. 1998). The onset of E′ occurs prior to that of E during resting supine positions, therefore E′ represents ventricular relaxation after aortic valve closure and before and during mitral inflow in early diastole. (Prasad et al. 2007). Further evidence of improved diastolic performance is provided by S and SR, which can provide an index of myocardial contractility and relaxation unaffected by transducer position, tethering, or cardiac translation. In addition, S and SR are more sensitive measures of regional systolic and diastolic function (Marwick 2006; Gilman et al. 2004), yet remain somewhat load-dependent (Choi et al. 2008; Voigt et al. 2002). Diastolic S and SR may provide a more complete assessment of LV diastolic function in healthy, athletic, and clinical populations. Therefore, our study provides corroborating evidence to support the effectiveness of short-term endurance training on improving LV diastolic performance using multiple techniques of assessment.
Limitations
Our study had certain limitations. The relatively small sample size was a major limitation and may have limited our ability to detect significant group differences. In addition, we did not include a sedentary control group, yet numerous studies using short-term training models show no placebo or time effect in non-exercising controls (Burgomaster et al. 2005, 2006; Mier et al. 1997). We cannot evaluate potential training-related and/or sex differences, and our results may not be transferable to older individuals who may have a differential training response (Pelliccia 1996). In addition, we obtained only single pre-post measurements of \(\dot{V}\)O2max and as with other reports, there is a risk of habituation. However, the use of vigorous criteria to document a valid \(\dot{V}\)O2max (i.e., achievement of plateau, maximal HR, and RER ≥ 1.15) confirmed these criteria were met. For assessment of submaximal LV performance, we chose to fix work rates to elicit a target HR (105 bpm), eliminating the confounding influence of HR on indices of diastolic performance; therefore, subjects performed a slightly lower submaximal work rate during echocardiography assessments following training. We accept that the absolute measures of CO from 2D echocardiography may be limited, but relative changes were the primary interest. We also acknowledge that our interpretation of LV performance was limited to the longitudinal and not radial, circumferential or rotational mechanics (i.e., LV twist and untwist), which may be altered with training (Lee et al. 2012).
Conclusion
The use of HIT and CMT elicits rapid improvements in maximal exercise performance and LV diastolic filling during submaximal exercise. The increased LV filling occurred without global changes in systolic performance or cardiac morphology at rest. These results suggest that HIT is an equally efficacious intervention as CMT for inducing rapid adaptations in aerobic power and LV function during exercise, with the advantage of requiring less than 25 % of the total work.
Abbreviations
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- bpm:
-
Beats per minute
- BV:
-
Blood volume
- CMT:
-
Continuous moderate-intensity training
- CO:
-
Cardiac output
- ECG:
-
Electrocardiogram
- EDV:
-
End-diastolic volume
- E:
-
Ejection fraction
- ESV:
-
End-systolic volume
- Hct:
-
Hematocrit
- HIT:
-
High-intensity interval training
- HR:
-
Heart rate
- IVSd:
-
Interventricular septum dimension during diastole
- LV:
-
Left ventricle or left ventricular
- LVIDd:
-
Internal diastolic diameter of the LV
- LVIDs:
-
Internal systolic diameter of the LV
- LVPWDd:
-
Left ventricular posterior wall thickness during diastole
- PW:
-
Pulsed wave
- PV:
-
Plasma volume
- SBP/ES:
-
Ratio of systolic blood pressure to end-systolic volume; index of contractility
- SV:
-
Stroke volume
- TD:
-
Tissue Doppler imaging
- \(\dot{V}\)O2max :
-
Maximal oxygen consumption or maximal aerobic capacity
References
Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD (2004) Effect of aging and physical activity on left ventricular compliance. Circulation 110(13):1799–1805. doi:10.1161/01.CIR.0000142863.71285.74
Bhella PS, Pacini EL, Prasad A, Hastings JL, Adams-Huet B, Thomas JD, Grayburn PA, Levine BD (2011) Echocardiographic indices do not reliably track changes in left-sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circ Cardiovasc Imaging 4(5):482–489. doi:10.1161/CIRCIMAGING.110.960575
Blomqvist CG, Saltin B (1983) Cardiovascular adaptations to physical training. Annu Rev Physiol 45:169–189. doi:10.1146/annurev.ph.45.030183.001125
Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ (2005) Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol 98(6):1985–1990. doi:10.1152/japplphysiol.01095.2004
Burgomaster KA, Heigenhauser GJ, Gibala MJ (2006) Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol 100(6):2041–2047. doi:10.1152/japplphysiol.01220.2005
Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ (2008) Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586(1):151–160. doi:10.1113/jphysiol.2007.142109
Carabello BA (2003) The ten most commonly asked questions about measuring left ventricular function. Cardiol Rev 11(2):58–59. doi:10.1097/01.CRD.0000053828.38772.DC
Carrick-Ranson G, Doughty RN, Whalley GA, Walsh HJ, Gamble GD, Baldi JC (2012) The larger exercise stroke volume in endurance-trained men does not result from increased left ventricular early or late inflow or tissue velocities. Acta Physiol (Oxf) 205(4):520–531. doi:10.1111/j.1748-1716.2012.02430.x
Carter JB, Banister EW, Blaber AP (2003) Effect of endurance exercise on autonomic control of heart rate. Sports Med 33(1):33–46. pii: 330103
Choi JO, Shin DH, Cho SW, Song YB, Kim JH, Kim YG, Lee SC, Park SW (2008) Effect of preload on left ventricular longitudinal strain by 2D speckle tracking. Echocardiography 25(8):873–879. doi:10.1111/j.1540-8175.2008.00707.x
Convertino VA (1991) Blood volume: its adaptation to endurance training. Med Sci Sports Exerc 23(12):1338–1348
Coyle EF, Hopper MK, Coggan AR (1990) Maximal oxygen uptake relative to plasma volume expansion. Int J Sports Med 11(2):116–119. doi:10.1055/s-2007-1024774
Currie KD, Thomas SG, Goodman JM (2009) Effects of short-term endurance exercise training on vascular function in young males. Eur J Appl Physiol 107(2):211–218. doi:10.1007/s00421-009-1116-4
Ehsani AA, Ogawa T, Miller TR, Spina RJ, Jilka SM (1991) Exercise training improves left ventricular systolic function in older men. Circulation 83(1):96–103
Esch BT, Bredin SS, Haykowsky MJ, Scott JM, Warburton DE (2007a) The potential role of the pericardium on diastolic filling in endurance-trained athletes under conditions of physiological stress. Appl Physiol Nutr Metab 32(2):311–317. doi:10.1139/h06-086
Esch BT, Scott JM, Haykowsky MJ, McKenzie DC, Warburton DE (2007b) Diastolic ventricular interactions in endurance-trained athletes during orthostatic stress. Am J Physiol Heart Circ Physiol 293(1):H409–H415. doi:10.1152/ajpheart.00928.2006
Fagard RH (1997) Impact of different sports and training on cardiac structure and function. Cardiol Clin 15(3):397–412
Fagard R (2003) Athlete’s heart. Heart 89(12):1455–1461
Firstenberg MS, Levine BD, Garcia MJ, Greenberg NL, Cardon L, Morehead AJ, Zuckerman J, Thomas JD (2000) Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol 36(5):1664–1669. (pii: S0735-1097(00)00909-8)
Galderisi M, Benjamin EJ, Evans JC, D’Agostino RB, Fuller DL, Lehman B, Levy D (1993) Impact of heart rate and PR interval on Doppler indexes of left ventricular diastolic filling in an elderly cohort (the Framingham Heart Study). Am J Cardiol 72(15):1183–1187 (pii: 0002-9149(93)90991-K)
Garcia MJ, Thomas JD, Klein AL (1998) New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol 32(4):865–875 (pii: S0735-1097(98)00345-3)
Gibala MJ (2007) High-intensity interval training: a time-efficient strategy for health promotion? Curr Sports Med Rep 6(4):211–213
Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA (2006) Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575(Pt 3):901–911. doi:10.1113/jphysiol.2006.112094
Gilman G, Khandheria BK, Hagen ME, Abraham TP, Seward JB, Belohlavek M (2004) Strain rate and strain: a step-by-step approach to image and data acquisition. J Am Soc Echocardiogr 17(9):1011–1020. doi:10.1016/j.echo.2004.04.039
Goodman JM (2000) The Athlete’s Heart. In: Shephard RJ, Åstrand P-O (eds) Endurance in Sport: Basic Scientific Considerations. Blackwell Science Ltd, London, pp 68–83
Goodman JM, Liu PP, Green HJ (2005) Left ventricular adaptations following short-term endurance training. J Appl Physiol 98(2):454–460. doi:10.1152/japplphysiol.00258.2004
Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ (2004) American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr 17(10):1086–1119. doi:10.1016/j.echo.2004.07.013
Green HJ, Thomson JA, Ball ME, Hughson RL, Houston ME, Sharratt MT (1984) Alterations in blood volume following short-term supramaximal exercise. J Appl Physiol 56(1):145–149
Green HJ, Jones LL, Painter DC (1990) Effects of short-term training on cardiac function during prolonged exercise. Med Sci Sports Exerc 22(4):488–493
Green DJ, Naylor LH, George K (2006) Cardiac and vascular adaptations to exercise. Curr Opin Clin Nutr Metab Care 9(6):677–684. doi:10.1097/01.mco.0000247473.49284.e3
Harris SK, Petrella RJ, Overend TJ, Paterson DH, Cunningham DA (2003) Short-term training effects on left ventricular diastolic function and oxygen uptake in older and younger men. Clin J Sport Med 13(4):245–251
Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J (2007) Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc 39(4):665–671. doi:10.1249/mss.0b013e3180304570
Hopper MK, Coggan AR, Coyle EF (1988) Exercise stroke volume relative to plasma-volume expansion. J Appl Physiol 64(1):404–408
Jacobs RA, Fluck D, Bonne TC, Burgi S, Christensen PM, Toigo M, Lundby C (2013) Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol 115(6):785–793. doi:10.1152/japplphysiol.00445.2013 (1985)
Kanstrup IL, Ekblom B (1982) Acute hypervolemia, cardiac performance, and aerobic power during exercise. J Appl Physiol 52(5):1186–1191
Keteyian SJ, Brawner CA, Savage PD, Ehrman JK, Schairer J, Divine G, Aldred H, Ophaug K, Ades PA (2008) Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J 156(2):292–300. doi:10.1016/j.ahj.2008.03.017
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18(12):1440–1463. doi:10.1016/j.echo.2005.10.005
Laursen PB, Shing CM, Peake JM, Coombes JS, Jenkins DG (2005) Influence of high-intensity interval training on adaptations in well-trained cyclists. J Strength Cond Res 19(3):527–533. doi:10.1519/15964.1
Lee LS, Mariani JA, Sasson Z, Goodman JM (2012) Exercise with a twist: left ventricular twist and recoil in healthy young and middle-aged men, and middle-aged endurance-trained men. J Am Soc Echocardiogr 25(9):986–993. doi:10.1016/j.echo.2012.05.018
Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG (1991) Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 84(3):1016–1023
Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ (2010) A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588(Pt 6):1011–1022. doi:10.1113/jphysiol.2009.181743
Marwick TH (2006) Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol 47(7):1313–1327. doi:10.1016/j.jacc.2005.11.063
Mier CM, Turner MJ, Ehsani AA, Spina RJ (1997) Cardiovascular adaptations to 10 days of cycle exercise. J Appl Physiol 83(6):1900–1906
Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS (2001) Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol 37(1):278–285 (pii: S0735-1097(00)01056-1)
Nishimura RA, Abel MD, Housmans PR, Warnes CA, Tajik AJ (1989) Mitral flow velocity curves as a function of different loading conditions: evaluation by intraoperative transesophageal Doppler echocardiography. J Am Soc Echocardiogr 2(2):79–87
Pelliccia A (1996) Determinants of morphologic cardiac adaptation in elite athletes: the role of athletic training and constitutional factors. Int J Sports Med 17(Suppl 3):S157–S163. doi:10.1055/s-2007-972918
Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD (2007) The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol 99(12):1629–1636. doi:10.1016/j.amjcard.2007.01.050
Sasaki T, Inui M, Kimura Y, Kuzuya T, Tada M (1992) Molecular mechanism of regulation of Ca2+ pump ATPase by phospholamban in cardiac sarcoplasmic reticulum. Effects of synthetic phospholamban peptides on Ca2+ pump ATPase. J Biol Chem 267(3):1674–1679
Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ (2000) Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc 32(2):332–348
Shapiro LM (1984) Physiological left ventricular hypertrophy. Br Heart J 52(2):130–135
Smith ML, Hudson DL, Graitzer HM, Raven PB (1989) Exercise training bradycardia: the role of autonomic balance. Med Sci Sports Exerc 21(1):40–44
Spina RJ, Ogawa T, Martin WH 3rd, Coggan AR, Holloszy JO, Ehsani AA (1992) Exercise training prevents decline in stroke volume during exercise in young healthy subjects. J Appl Physiol 72(6):2458–2462
Swain DP, Franklin BA (2006) Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol 97(1):141–147. doi:10.1016/j.amjcard.2005.07.130
van Beaumont W, Strand JC, Petrofsky JS, Hipskind SG, Greenleaf JE (1973) Changes in total plasma content of electrolytes and proteins with maximal exercise. J Appl Physiol 34(1):102–106
Voigt JU, Lindenmeier G, Werner D, Flachskampf FA, Nixdorff U, Hatle L, Sutherland GR, Daniel WG (2002) Strain rate imaging for the assessment of preload-dependent changes in regional left ventricular diastolic longitudinal function. J Am Soc Echocardiogr 15(1):13–19 (pii: S0894731702914732)
Warburton DE, Gledhill N, Quinney HA (2000) Blood volume, aerobic power, and endurance performance: potential ergogenic effect of volume loading. Clin J Sport Med 10(1):59–66
Warburton DE, Haykowsky MJ, Quinney HA, Blackmore D, Teo KK, Taylor DA, McGavock J, Humen DP (2004) Blood volume expansion and cardiorespiratory function: effects of training modality. Med Sci Sports Exerc 36(6):991–1000 (pii: 00005768-200406000-00012)
Warburton DE, McKenzie DC, Haykowsky MJ, Taylor A, Shoemaker P, Ignaszewski AP, Chan SY (2005) Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol 95(9):1080–1084. doi:10.1016/j.amjcard.2004.12.063
Wisloff U, Nilsen TI, Droyvold WB, Morkved S, Slordahl SA, Vatten LJ (2006) A single weekly bout of exercise may reduce cardiovascular mortality: how little pain for cardiac gain? ‘The HUNT study, Norway’. Eur J Cardiovasc Prev Rehabil 13(5):798–804. doi:10.1097/01.hjr.0000216548.84560.ac
Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T (2007) Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115(24):3086–3094. doi:10.1161/CIRCULATIONAHA.106.675041
Acknowledgments
The authors thank Ms. Joan Persaud for her helpful technical assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Carsten Lundby.
Rights and permissions
About this article
Cite this article
Esfandiari, S., Sasson, Z. & Goodman, J.M. Short-term high-intensity interval and continuous moderate-intensity training improve maximal aerobic power and diastolic filling during exercise. Eur J Appl Physiol 114, 331–343 (2014). https://doi.org/10.1007/s00421-013-2773-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2773-x