Abstract
We investigated effects of 6 days of endurance exercise training [cycling at 65% of peak oxygen consumption (VO2peak) for 2 h a day on six consecutive days] on vascular function in young males. Measures of VO2peak, arterial stiffness, calf vascular conductance and heart rate variability were obtained pre- and post-training. Indices of arterial stiffness were obtained by applanation tonometry to determine aortic augmentation index normalized to a heart rate of 75 bpm (AI x @75 bpm), and central and peripheral pulse wave velocity (CPWV, PPWV). Resting and maximal calf vascular conductances were calculated from concurrent measures of blood pressure and calf blood flow using venous occlusion strain-gauge plethysmography. Time and frequency domain measures of heart rate variability were obtained from recording R–R intervals during supine and standing conditions. Both CPWV (5.9 ± 0.8 vs. 5.4 ± 0.8 m/s) and PPWV (9.7 ± 0.8 vs. 8.9 ± 1.3 m/s) were reduced following the training program. No significant changes were observed in AI x @75 bpm, vascular conductance, heart rate variability or VO2peak. These data indicate that changes in arterial stiffness independent of changes in heart rate variability or vascular conductance can be achieved in healthy young males following only 6 days of intense endurance exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The positive effects of exercise training on cardiovascular performance have been well documented (DeSouza et al. 2000; Hautala et al. 2004; Tanaka et al. 2000). Several long-term studies provide evidence of reductions in central and peripheral arterial stiffness with endurance exercise training in both young (Cameron and Dart 1994; Kakiyama et al. 2005) and older (Collier et al. 2008; Tanaka et al. 2000) populations. An increase in stiffness increases blood velocity, leading to an earlier reflected arterial wave, augmenting the systolic wave form and increasing cardiac load (Nichols and Edwards 2001). As a result, arterial stiffness is a risk factor for cardiovascular morbidity and mortality (Laurent et al. 2001). The training-induced attenuation of arterial stiffness has been attributed to improved endothelial function (DeSouza et al. 2000; Martin et al. 1990) and reductions in sympathetic tone (Hautala et al. 2004; Melanson and Freedson 2001) rather than structural alterations and remodeling given the relatively short time-period required for adaptation.
Several studies have examined cardiac and metabolic changes following training lasting only a few days (Gibala et al. 2006; Goodman et al. 2005; Green et al. 1991, 1999). However, the vascular responses to short-term training have not yet been examined. The purpose of this study was to investigate the effects of a short-term, intensive endurance intervention on measures of central and peripheral arterial stiffness in healthy young males. Given evidence of differences in arterial stiffness (Otsuki et al. 2007) between healthy young males of various exercise capacities, we hypothesized that 6 days of intensive aerobic training would reduce central arterial stiffness. Second, we examined the effects of training on lower limb vascular conductance and autonomic nervous system function.
Methods
Seventeen subjects were recruited to the study; however, three were excluded because they did not meet the inclusion criteria. Thus, 14 males (25 ± 4 years) completed the study. All subjects participated in less than 2 h of low to moderate physical activity a week and were non-smokers, non-obese, normotensive, and had no prior history of cardiovascular, pulmonary, or other metabolic or musculo-skeletal diseases. All procedures were reviewed and approved by the University of Toronto Research Ethics Board, conforming to the Helsinki Declaration on the use of human subjects and informed written consent was obtained from all subjects prior to participation.
Subjects reported to the Cardiovascular Regulation Laboratory on eight separate sessions. The first visit consisted of baseline assessments of anthropometric variables, autonomic nervous system function, arterial stiffness, vascular conductance and exercise capacity, and a blood sample was obtained. The training program, which was initiated within 2 weeks of the pre-training assessment, required subjects to report to the laboratory on six consecutive days. Following the cessation of the training program, subjects were given 1 day off before returning for post-training assessments which mirrored the pre-training assessment. The timing of the assessments was dependent on subject availability; however, each subject was required to have their pre- and post-training assessments at the same time of day. Subjects were also asked to abstain from physical activity for at least 24 h, caffeine and alcohol consumption for 12 h and food consumption for 4 h prior to all assessments. Height (m) and weight (kg) were measured in exercise clothing with shoes removed and body mass index (BMI) was calculated.
Blood sampling
Duplicate microhematocrit determinations were made from resting blood samples taken pre- and post-training. Hematocrit readings were multiplied by 0.8736 to correct for trapped plasma and to convert venous hematocrit to whole body hematocrit. The percent change in plasma volume (PV) following training was calculated from the corrected hematocrit readings (van Beaumont et al. 1973). Two subjects declined the pre- and post-training blood sampling so changes in PV were calculated from 12 subjects.
Autonomic nervous system function
Heart rate variability (HRV) was measured as an index of autonomic nervous system control. All measurements were performed in accordance with the Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology (1996) in a quiet, temperature controlled room (22–26°C, 30–60% humidity) after 15 min of supine rest. Continuous 6 min supine and 6 min standing R–R intervals were recorded using heart rate monitoring (s810i model, Polar Electro Canada, Lachine, QC, Canada). Artifacts were corrected for by the Polar Precision Performance Software Version 4.01.029 (Polar, Kempele, Finland) by applying a filter power set to a minimum beat protection of 6 bpm. Five min sections of supine and standing data were selected and exported for analysis using HRV Analysis Software Version 1.1 (Biosignal Analysis and Medical Imaging Group, Kulplo, Finland), allowing for non-parametric and parametric spectral analyses. Frequency domain measures were determined by spectral analysis of the time course of R–R intervals using Fast Fourier transformation and included total spectral power (TP), low frequency (LF, 0.04–0.15 Hz) and high frequency (HF, 0.15–0.50 Hz). Frequency domain measures were transformed using the natural logarithm and are expressed in ms2. LF and HF are also expressed in normalized units (nu), calculated by dividing the LF and HF by TP–(very low frequency). Sympathovagal balance is indicated by the ratio of the LF to the HF (LF/HF) (Malliani 1999) with heart rate and the root mean square of the successive R–R interval differences (RMSSD), a measure of the short-term components of HRV, being the time domain measures reported. The intra-observer reliability coefficient for these determinations in our laboratory is r = 0.78.
Arterial stiffness
Triplicate measures of supine arterial blood pressure (BP) were obtained 1 min apart using an automated sphygmomanometer (Dinamap 1846 SX Monitor, Critikon Inc., Tampa, FL, USA) following 5 min of supine rest. Measures of arterial stiffness were assessed using a semi-automated device and proprietary software (SphygmoCor, AtCor Medical, Sydney, Australia). Briefly, arterial pressure waveforms were recorded by the application of a pencil-like probe (Millar Instruments, Houston, TX, USA) to the surface of the arterial site. An arterial pressure pulse is captured by a high-fidelity transducer and recorded for subsequent analysis using both spatial and temporal landmarks (Papaioannou et al. 2004). The arterial pressure waveform has two components: the incident wave generated by ventricular ejection, which travels distally, and the reflected wave emanating from peripheral points of resistance, traveling centrally. The shape of the incident and reflected waves are determined by the structural and functional characteristics of the vasculature (Nichols and O’Rourke 1998). Arterial stiffness was determined by analyzing the shape of the arterial pressure waveform using specific spatial landmarks. Aortic augmentation index (AI x ), a measure of systemic arterial stiffness, was determined from peripheral pressure waveforms collected from the right radial artery. The aortic pressure waveform was generated by calibrating the average peripheral waveform against the supine BP, then applying a validated transfer function (Nichols and O’Rourke 1998). AI x was then determined from the aortic waveform by dividing the augmentation pressure, defined as the difference between the peak pressure and peak flow, by the pulse pressure. In order to control for the influence of heart rates on AI x (Wilkinson et al. 2000), AI x data were normalized to a heart rate of 75 bpm (AI x @75 bpm) by proprietary software. Following the assessment of AI x , pulse wave velocity (PWV), which is defined as the speed of travel of the pulse between two sites along the arterial branch (O’Rourke et al. 2002), was calculated. The time delay between sampling sites was determined by concurrent measurements of QRS complexes using electrocardiography and arterial waveforms using applanation tonometry, while distance was measured with an anthropometric measuring tape using specific anatomical landmarks. For central PWV (CPWV), tonometry measures were taken at the right carotid and femoral arteries, and for peripheral PWV (PPWV), at the right femoral and posterior tibial arteries. The reliability and reproducibility of AI x and PWV have been demonstrated (Papaioannou et al. 2004; Salvi et al. 2008; Wilkinson et al. 1998). Unpublished data from our laboratory indicates a within-observer mean difference for AI x , CPWV and PPWV of 0.17 ± 4.31%, 0.02 ± 0.17, and 0.04 ± 0.21 m/s, respectively.
Blood flow
Resting and maximal flow-mediated blood flows (ml 100 ml−1 min−1) of the right calf were measured using venous occlusion strain-gauge plethysmography (O’Donnell et al. 2007). Subjects rested in a supine position with their right foot elevated 20° and secured in a weighted pedal so that their knee was slightly bent and their lower limb was parallel to the testing table. An indium–gallium strain gauge (Vasculab SPG16, Medasonics, Newark, CA, USA) was placed around the widest part of the belly of the calf muscle at approximately 10 g of tension. Calf blood flow was isolated during testing by inflating an exclusion cuff located proximal to the ankle, above systolic BP (180–200 mmHg). Venous occlusion was induced by rapid inflation of the occlusion cuff, located on the thigh proximal to the knee, to 60 mmHg for 7 s followed by rapid (<1 s) deflation for a period of 7 s with four sequential measurements. Following resting measures, local calf ischemia was induced for 5 min by inflating the occlusion cuff to 180 mmHg, immediately followed by local calf plantar flexion against a 10 kg mass using a pedal set at 50 per min. At volitional fatigue (failure to maintain cadence and/or intolerable pain) the occlusion cuff was released and maximal blood flow was measured as described. Concurrent beat to beat BP was recorded from the third digit of the right hand using a photoplethysmographic device (Finometer, Finapres Medical Systems BV, Amsterdam, The Netherlands). Both BP and blood flow data were analyzed using customized software (LabVIEW 7.1, Austin, TX, USA). Resting and maximal blood flows were derived from the maximal slope from the series of time-calf volume curves, with temporally aligned measures of systolic and diastolic BP used to determine mean arterial pressure (MAP) at each blood flow. Vascular conductance (ml 100 ml−1 min−1 mmHg−1) was calculated.
Exercise test
Graded exercise to exhaustion was performed on a cycle ergometer before and after training (Monark Ergomedic 828 E, Monark Exercise AB, Sweden). Following a brief warm up and familiarization with the procedure, subjects started cycling for 2 min at a resistance of 50 W, with pedaling frequency self-selected between 60 and 80 rotations per min. After the first 2 min, workload was increased by 20 W every min until voluntary exhaustion. Expired gas was analyzed using a semi-automated metabolic cart following a two-point calibration (HRTrak II Heart Rate Tracker, Equilibrated Bio Systems Inc, New York, NY, USA) and peak oxygen consumption (VO2peak) was determined from breath-by-breath samples averaged over 20 s.
Training program
A short-term training program was used (Goodman et al. 2005; Green et al. 1999). Exercise was performed in a temperature controlled room (22–26°C, 30–60% humidity). Briefly, subjects cycled for 2 h on six consecutive days at a power equivalent to 65% of their pre-training VO2peak. Subjects who were unable to perform continuous exercise at their given intensity were permitted to take short breaks (~5 min), however, they had to complete 2 h of exercise during each training session. Water was provided ad libitum and water intake (ml), heart rate (bpm), and rating of perceived exertion (6–20, Borg Scale) were recorded after every 30 min of exercise.
Statistical analysis
Statistical analyses were performed using SPSS for Windows (Version 12.0, Chicago, IL, USA). Data are presented as the mean ± SD, and were tested for distributions patterns using the Normal Q–Q and box plots. Pre- and post-training values that were normally distributed were compared using paired t tests. Skewed data were compared using Wilcoxon signed-rank tests. All comparisons were based on a 95% confidence limit with P < 0.05 considered statistically significant.
Results
Adherence to the supervised training sessions was >99% (167 of 168 h completed). By the sixth day of training there were reductions in the amount of breaks taken (22 ± 11 vs. 12 ± 6 min, P < 0.05) and ratings of perceived exertion (16 ± 1 vs. 15 ± 2, P < 0.05). There were no changes in height, body mass, BMI or BP. PV increased by 7.8% (P < 0.05). Following training, there was an increase in the peak power achieved (P < 0.001); however, VO2peak was unchanged (Table 1).
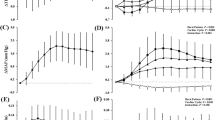
Pre- and post-training comparisons of arterial stiffness measures revealed no change in AI x @75 bpm (−9 ± 10 vs. −11 ± 11%); however, both CPWV (5.9 ± 0.8 vs. 5.4 ± 0.8 m/s) and PPWV (9.7 ± 0.8 vs. 8.9 ± 1.3 m/s) were reduced (Fig. 1). There were no changes between pre- and post-training measures of resting and maximal calf blood flow or vascular conductance (Table 2). No significant differences between pre- and post-training were observed in the frequency domain measures of HRV in both the standing and supine conditions (Table 3). RMSSD during the standing condition was significantly higher following the training program (Fig. 2).
Discussion
The purpose of the study was to identify the early adaptive responses of the body to exercise in order to understand the changes observed with long-term training. We used a 2 h training protocol which was modeled after other short-term training studies (Goodman et al. 2005; Green et al. 1999), and while it is not applicable to the general population, it was an amplified stimulus intended to induce physiological changes over a short period of time. The primary observation was a training-induced reduction in CPWV by 9% and PPWV by 8%. Additional findings include a PV expansion of 7.8%, and an increase in standing RMSSD.
Central arteries are more susceptible to arterial stiffening than peripheral arteries due to their higher content of collagen and elastin and chronic exposure to augmented arterial pressure (Nichols and O’Rourke 1998). Therefore, exercise intervention studies have focused on measures of central arterial stiffness because this component of vasculature is more likely to undergo change following training. The findings from this study are supported by Collier et al. (2008) who demonstrated similar reductions in CPWV (9.5%) and PPWV (8.5%) after 4 weeks of training in middle-aged males and females.
Attributing a sole mechanism to explain the improved CPWV and PPWV is difficult. Based on evidence from long-term training studies, there are several possible mechanisms that may be attributed to these changes including arterial remodeling, improvements in endothelial function and alterations in sympathetic tone. Vascular function is affected through remodeling, as seen by modifications in the collagen and elastin content with increasing age (Seals et al. 2006). While it is possible to attenuate these changes with endurance exercise training, it is unlikely to occur after 6 days. Increased arterial pressure and shear stress during acute exercise stimulates local release of growth factors that favor enhanced elasticity of the central vasculature (Prior et al. 2004). Increased aortic distensibility in rats was observed after 16 weeks of endurance exercise training, secondary to increased aortic elastin content and reduced calcium depositions (Matsuda et al. 1993). Conversely, structural changes to the artery independent of composition may have caused the reductions in PWV. Expansive arterial remodeling occurs from chronic increases in blood flow leading to a reduction in the thickness of the intimal and medial layers (Ben Driss et al. 1997). Increased vessel lumen diameter has been linked to decreased arterial stiffness (Nichols and O’Rourke 1998). Endurance exercise training increases aortic (Miyachi et al. 1998) and femoral (Hayashi et al. 2005; Miyachi et al. 2001) arterial lumens. While the reductions in CPWV and PPWV may be attributed to changes in vessel diameters, changes in arterial lumen diameter with endurance exercise likely occurs after prolonged training, rather than only 6 days.
Secondary to reductions in arterial stiffness, we examined changes in lower limb vascular conductance. Improvements in vascular function with exercise training have been attributed to improvements in endothelial function, which is facilitated by the vasodilator nitric oxide (NO) (Maiorana et al. 2003). In animals, the removal of the aortic endothelium has led to increases in aortic PWV (Boutouyrie et al. 1997). Inhibition of NO with l-N G-monomethyl arginine increases AI x and PWV (Wilkinson et al. 2002), whereas augmentation of endothelial function with administration of endothelium-dependent β2 adrenoceptor agonist albuterol decreases AI x and aortic PWV in healthy subjects (McEniery et al. 2006). Contrary to longer-term training studies in young (Goto et al. 2003) and old (DeSouza et al. 2000; Martin et al. 1990) males, we did not observe changes in maximal calf blood flow or vascular conductance. The lack of a change in endothelial function, as demonstrated by vascular conductance, may be attributed to a different time-course of change in NO bioavailability. Canine investigations suggest that improvements in NO-mediated dilation of coronary arteries occur after 7 days of treadmill exercise (Wang et al. 1993), with increases in endothelial NO synthase (eNOS) gene expression occurring after 10 days (Sessa et al. 1994). Increased eNOS gene expression with treadmill training has also been demonstrated in porcine pulmonary arteries after 7 days of exercise (Johnson et al. 2001). In humans, increases in plasma concentrations of NO have been demonstrated after 4 (Kingwell et al. 1997) and 8 (Maeda et al. 2001) weeks of endurance training. Conversely, endurance training has also reduced levels of the vasoconstrictor endothelin-1 after 8 (Maeda et al. 2001) and 12 (Maeda et al. 2003) weeks. Exercise training improves endothelial-dependent and -independent vasodilatation in individuals with chronic heart failure (Maiorana et al. 2000) and type 2 diabetics after 8 weeks of training. Our subjects had normal vascular function and maximal blood flow values comparable to healthy endurance trained males (Martin et al. 1991), thereby limiting the potential changes that a short-term training stimulus may have conferred.
Exercise training has been linked to a reduction in pressor stimuli from exercise and non-exercise sources that may act on various cardiovascular endpoints. We observed a modest but insignificant reduction in ischemic exercise MAP and increase in vascular conductance. More significant findings are more likely observed with longer-term training programs (O’Sullivan and Bell 2001). It is also possible that large-conduit arteries are more responsive to change in the short-term compared to ‘downstream’ resistance vessels, as demonstrated by Allen et al. (2003), who reported rapid changes in brachial artery diameter without improvements in hemodynamics after only 4 days of training. These results are similar to our finding of decreased PWVs and only minor changes in vascular conductance and blood flow.
The reduction in the pressor response during ischemic calf exercise could be indicative of a reduction in sympathetic tone. Long-term endurance exercise training can increase resting HRV and reduce sympathetic activity. Eight weeks of moderate to high (70–80% HRmax) aerobic exercise training increased HFnu power and decreased LFnu power and LF/HF ratio in healthy males, suggesting a shift in autonomic nervous system control toward parasympathetic dominance (Hautala et al. 2004). No changes in parasympathetic (ln HF, HFnu), sympathetic (ln LF, LFnu) or sympathovagal balance (LF/HF) were observed, which are attributed to the short length of the training program; however, an increase in standing RMSSD was manifested. Similar responses in young males are reported following 16 weeks of training (Melanson and Freedson 2001). The increase in RMSSD during standing infers an improved orthostatic tolerance. Therefore, this finding is unlikely to explain the reduction in CPWV and PPWV given that these measures were performed in the supine position; there was no correlation between the change in RMSSD and CPWV or PPWV. Acute PV expansion with saline infusion induces increases in HF and RMSSD, mediated through baroreceptor activation (Spinelli et al. 1999). Moderate exercise training increases PV and orthostatic tolerance and decreases baroreceptor sensitivity in healthy subjects (Mtinangi and Hainsworth 1999). Therefore, the improved orthostatic tolerance could be attributed to the PV expansion which is consistent with other short-term training studies (Goodman et al. 2005; Green et al. 1991). This expansion has been attributed to an increase in total body fluids through the retention of fluids within the vascular space, and results in post-training increases in body mass (Convertino 1991). We observed a modest gain in body mass (0.3 kg) that may be consistent with the 7.8% PV expansion, however, it may be PV expansion was specific to compartmental shift of fluids and proteins from extravascular into intravascular space (Convertino 1991). We could not identify a correlation between PV expansion and the increase in RMSSD; however, this may be due to the small sample size.
An intriguing observation was the absence of change in aortic stiffness (AI x ) despite reductions in both CPWV and PPWV. Augmentation pressure and pulse pressure were unchanged post-training resulting in no significant change in AI x @75 bpm. Given that long-term training studies have not examined AI x as an index of central arterial stiffness, it is difficult to compare the findings from this study to evidence from other training studies. In addition, comparisons to cross-sectional studies are limited. Our failure to detect changes in AI x @75 bpm is likely due to the young age of our subjects. This is supported by Tanaka et al. (2000) who found no significant differences in central arterial compliance (β-stiffness index) in young adults of varying physical activity levels.
Limitations
The subjects in our study acted as their own controls, similar to other short-term training studies (Collier et al. 2008; Goodman et al. 2005; Kakiyama et al. 2005). However, other training studies, which have used a control group did not observe changes in arterial stiffness (Casey et al. 2007; Kawano et al. 2006). We recruited healthy young males who reported minimal physical activity levels, yet none were completely sedentary. Consequently, more pronounced gains may have been demonstrated in a sedentary or older population where the likelihood of arterial stiffness is increased.
Post-training measures of VO2peak may have been compromised from training fatigue, which is attributed to muscle fatigue and energy depletion (Noakes 2000) and leads to reductions in performance at anaerobic threshold and maximal capacity (Lehmann et al. 1993). Despite an increase in work rate post-training, peak exercise heart rate was lower, suggesting our subjects failed to reach maximal effort. When extrapolating the VO2peak data to the peak heart rates observed during the pre-training exercise test, VO2peak increased significantly (42.8 ± 6.0 vs. 44.1 ± 5.5 ml kg−1 min−1). It is unlikely that measures of arterial stiffness, blood flow and HRV were affected by training fatigue or exercise habitation given that they were taken in the resting state.
Finally, the small sample size may have limited our ability to detect changes. The sample size selected for the study was based on the calculation for the primary outcome measure, CPWV. In order to detect changes in blood flow, vascular conductance or HRV, a larger sample size may be required since estimates of change were based upon long-term training studies, which may elicit greater changes than expected in our training model.
Conclusions
The study demonstrated 6 days of endurance exercise training elicits a decrease in central and peripheral arterial stiffness as assessed by PWV, which suggest that adaptations in arterial stiffness observed with long-term training programs can occur rapidly during an intensive endurance training program.
Abbreviations
- AI x :
-
Aortic augmentation index
- AI x @75 bpm:
-
Aortic augmentation index at 75 beats per minute
- BF:
-
Blood flow
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CPWV:
-
Central pulse wave velocity
- eNOS:
-
Endothelial nitric oxide synthase
- HF:
-
High frequency
- HRV:
-
Heart rate variability
- LF:
-
Low frequency
- LF/HF:
-
Sympathovagal balance
- ln:
-
Natural logarithm
- MAP:
-
Mean arterial pressure
- NO:
-
Nitric oxide
- nu:
-
Normalized units
- PPWV:
-
Peripheral pulse wave velocity
- PV:
-
Plasma volume
- PWV:
-
Pulse wave velocity
- RMSSD:
-
Root mean square of the successive R–R interval differences
- SD:
-
Standard deviation
- TP:
-
Total spectral power
- VC:
-
Vascular conductance
- VO2peak :
-
Peak oxygen consumption
References
Allen JD, Geaghan JP, Greenway F, Welsch MA (2003) Time course of improved flow-mediated dilation after short-term exercise training. Med Sci Sports Exerc 35:847–853
Ben Driss A, Benessiano J, Poitevin P, Levy BI, Michel JB (1997) Arterial expansive remodeling induced by high flow rates. Am J Physiol 272:851–858
Boutouyrie P, Bezie Y, Lacolley P, Challande P, Chamiot-Clerc P, Benetos A, de la Faverie JF, Safar M, Laurent S (1997) In vivo/in vitro comparison of rat abdominal aorta wall viscosity. Influence of endothelial function. Arterioscler Thromb Vasc Biol 17:1346–1355
Cameron J, Dart A (1994) Exercise training increases total systemic arterial compliance in humans. Am J Physiol 26:693–701
Casey DP, Beck DT, Braith RW (2007) Progressive resistance training without volume increases does not alter arterial stiffness and aortic wave reflection. Exp Biol Med 232:1228–1235
Collier SR, Kanaley JA, Carhart RJ, Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B (2008) Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens 22:678–686
Convertino VA (1991) Blood volume: its adaptation to endurance training. Med Sci Sports Exerc 23:1338–1348
DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR (2000) Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102:1351–1357
Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA (2006) Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575:901–911
Goodman JM, Liu PP, Green HJ (2005) Left ventricular adaptations following short-term endurance training. J Appl Physiol 98:454–460
Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I (2003) Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation 108:530–535
Green HJ, Coates G, Sutton JR, Jones S (1991) Early adaptations in gas exchange, cardiac function and haematology to prolonged exercise training in man. Eur J Appl Physiol 63:17–23
Green HJ, Grant S, Bombardier E, Ranney D (1999) Initial aerobic power does not alter muscle metabolic adaptations to short-term training. Am J Physiol Endocrinol Metab 277:39–48
Hautala AJ, Makikallio TH, Kiviniemi A, Laukkanen RT, Nissila S, Huikuri HV, Tulppo MP (2004) Heart rate dynamics after controlled training followed by a home-based exercise program. Eur J Appl Physiol 92:289–297
Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T (2005) Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol 55:235–239
Johnson LR, Rush JW, Turk JR, Price EM, Laughlin MH (2001) Short-term exercise training increases ACh-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol 90:1102–1110
Kakiyama T, Sugawara J, Murakami H, Maeda S, Kuno S, Matsuda M (2005) Effects of short-term endurance training on aortic distensibility in young males. Med Sci Sports Exerc 37:267–271
Kawano H, Tanaka H, Miyachi M (2006) Resistance training and arterial compliance: keeping the benefits while minimizing the stiffening. J Hypertens 24:1753–1759
Kingwell BA, Sherrard B, Jennings GL, Dart AM (1997) Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol 272:1070–1077
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A (2001) Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37:1236–1241
Lehmann M, Foster C, Keul J (1993) Overtraining in endurance athletes: a brief review. Med Sci Sports Exerc 25:854–862
Maeda S, Miyauchi T, Kakiyama T, Sugawara J, Iemitsu M, Irukayama-Tomobe Y, Murakami H, Kumagai Y, Kuno S, Matsuda M (2001) Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci 69:1005–1016
Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M (2003) Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol 95:336–341
Maiorana A, O’Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green D (2000) Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol Heart Circ Physiol 279:1999–2005
Maiorana A, O’Driscoll G, Taylor R, Green D (2003) Exercise and the nitric oxide vasodilator system. Sports Med 33:1013–1035
Malliani A (1999) The pattern of sympathovagal balance explored in the frequency domain. News Physiol Sci 14:111–117
Martin WH, Kohrt WM, Malley MT, Korte E, Stoltz S (1990) Exercise training enhances leg vasodilatory capacity of 65-yr-old men and women. J Appl Physiol 69:1804–1809
Martin WH, Ogawa T, Kohrt WM, Malley MT, Korte E, Kieffer PS, Schechtman KB (1991) Effects of aging, gender, and physical training on peripheral vascular function. Circulation 84:654–664
Matsuda M, Nosaka T, Sato M, Ohshima N (1993) Effects of physical exercise on the elasticity and elastic components of the rat aorta. Eur J Appl Physiol Occup Physiol 66:122–126
McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB (2006) Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 48:602–608
Melanson EL, Freedson PS (2001) The effect of endurance training on resting heart rate variability in sedentary adult males. Eur J Appl Physiol 85:442–449
Miyachi M, Iemitsu M, Okutsu M, Onodera S (1998) Effects of endurance training on the size and blood flow of the arterial conductance vessels in humans. Acta Physiol Scand 163:13–16
Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S (2001) Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol 90:2439–2444
Mtinangi BL, Hainsworth R (1999) Effects of moderate exercise training on plasma volume, baroreceptor sensitivity and orthostatic tolerance in healthy subjects. Exp Physiol 84:121–130
Nichols WW, Edwards DG (2001) Arterial elastance and wave reflection augmentation of systolic blood pressure: deleterious effects and implications for therapy. J Cardiovasc Pharmacol Ther 6:5–21
Nichols WW, O’Rourke MF (1998) McDonald’s blood flow in arteries: theoretic, experimental, and clinical principles. Oxford University Press, New York
Noakes TD (2000) Physiological models to understand exercise fatigue and the adaptations that predict or enhance athletic performance. Scand J Med Sci Sports 10:123–145
O’Donnell E, Harvey PJ, Goodman JM, De Souza MJ (2007) Long-term estrogen deficiency lowers regional blood flow, resting systolic blood pressure, and heart rate in exercising premenopausal women. Am J Physiol Endocrinol Metab 292:1401–1409
O’Rourke M, Staessen J, Vlachopoulos C, Duprez D, Plante G (2002) Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 15:426–444
O’Sullivan SE, Bell C (2001) Training reduces autonomic cardiovascular responses to both exercise-dependent and -independent stimuli in humans. Auton Neurosci 91:76–84
Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T (2007) Relationship between arterial stiffness and athletic training programs in young adult men. Am J Hypertens 20:967–973
Papaioannou T, Stamatelopoulos K, Gialafos E, Vlachopoulos C, Karatzis E, Nanas J, Lekakis J (2004) Monitoring of arterial stiffness indices by applanation tonometry and pulse wave analysis: reproducibility at low blood pressures. J Clin Monit Comput 18:137–144
Prior BM, Yang HT, Terjung RL (2004) What makes vessels grow with exercise training? J Appl Physiol 97:1119–1128
Salvi P, Magnani E, Valbusa F, Agnoletti D, Alecu C, Joly L, Benetos A (2008) Comparative study of methodologies for pulse wave velocity estimation. J Hum Hypertens 22:669–677
Seals DR, Moreau KL, Gates PE, Eskurza I (2006) Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol 41:501–507
Sessa WC, Pritchard K, Seyedi N, Want J, Hintze TH (1994) Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res 74:349–353
Spinelli L, Petretta M, Marciano F, Testa G, Rao MA, Volpe M, Bonaduce D (1999) Cardiac autonomic responses to volume overload in normal subjects and in patients with dilated cardiomyopathy. Am J Physiol 277:1361–1368
Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR (2000) Aging, habitual exercise, and dynamic arterial compliance. Circulation 102:1270–1275
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93:1043–1065
van Beaumont W, Stand JC, Petrofsky JS, Hipskind SG, Greenleaf JE (1973) Changes in total plasma content of electrolytes and proteins with maximal exercise. J Appl Physiol 34:102–106
Wang J, Wolin MS, Hintze TH (1993) Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res 73:829–838
Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockroft JR, Webb DJ (1998) Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 16:2079–2084
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ (2000) The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 525:263–270
Wilkinson IB, MacCallum H, Cockcroft JR, Webb DJ (2002) Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol 53:189–192
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Currie, K.D., Thomas, S.G. & Goodman, J.M. Effects of short-term endurance exercise training on vascular function in young males. Eur J Appl Physiol 107, 211–218 (2009). https://doi.org/10.1007/s00421-009-1116-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1116-4