Abstract
The purpose of the present study was to investigate whether increased tendon-aponeurosis stiffness and contractile strength of the triceps surae (TS) muscle-tendon units induced by resistance training would affect running economy. Therefore, an exercise group (EG, n = 13) performed a 14-week exercise program, while the control group (CG, n = 13) did not change their training. Maximum isometric voluntary contractile strength and TS tendon-aponeurosis stiffness, running kinematics and fascicle length of the gastrocnemius medialis (GM) muscle during running were analyzed. Furthermore, running economy was determined by measuring the rate of oxygen consumption at two running velocities (3.0, 3.5 ms−1). The intervention resulted in a ∼7 % increase in maximum plantarflexion muscle strength and a ∼16 % increase in TS tendon-aponeurosis stiffness. The EG showed a significant ∼4 % reduction in the rate of oxygen consumption and energy cost, indicating a significant increase in running economy, while the CG showed no changes. Neither kinematics nor fascicle length and elongation of the series-elastic element (SEE) during running were affected by the intervention. The unaffected SEE elongation of the GM during the stance phase of running, in spite of a higher tendon-aponeurosis stiffness, is indicative of greater energy storage and return and a redistribution of muscular output within the lower extremities while running after the intervention, which might explain the improved running economy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Running economy is accepted as an important determinant of performance in long-distance running (Saunders et al. 2004a; Foster and Lucia 2007). For elite distance runners, who have a similar maximum rate of oxygen consumption (\({\dot{V}{\rm O}}_{\rm 2,max}\)), running economy may be considered as a better predictor of performance than \({\dot{V}{\rm O}}_{\rm 2,max}\) (Costill et al. 1973; Morgan et al. 1989a). To quantify running economy, a standard approach is to measure the steady state rate of oxygen consumption (\({\dot{V}{\rm O}}_2\)) per body weight during running at a given constant submaximal velocity (Morgan et al. 1989b; Daniels 1985) or as energy cost of running expressed as the metabolic energy cost per body weight and per distance traveled (di Prampero et al. 1986; Margaria et al. 1963; Fletcher et al. 2009; di Prampero et al. 1993).
The inter-individual variation (coefficient of variance) in running economy has been reported to be about 8 and 10 % in highly trained runners (10 km race time 33.8 ± 1.98 min) and moderately trained runners (10 km race time 43.7 ± 3.47 min), respectively (Pereira and Freedson 1997). Similar variations were reported by di Prampero et al. (1986) and Heise and Martin (2001) for recreational middle- and long-distance runners (7.4 and 9.5 %) and by Saunders et al. (2004b) among national and international level middle- and long-distance runners (5.6–7.2 %). It is suggested that the contractile and series-elastic properties related to muscle force production and energy storage and release of the locomotor muscle-tendon units (MTUs) have the potential to partly explain the reported inter-individual differences in running economy (Martin and Morgan 1992; Arampatzis et al. 2006). Arampatzis et al. (2006) found that more economical runners show a greater plantarflexor muscle strength and greater tendon-aponeurosis stiffness of the triceps surae (TS) MTU. More recently, Fletcher et al. (2010) found a relationship between TS tendon-aponeurosis stiffness and running economy and thus confirmed the results from Arampatzis et al. (2006). If tendon-aponeurosis stiffness affects the metabolic energy cost of the locomotor muscles during running, it could be due to the energy storage and recovery in the series-elastic element (SEE). A great energy storage and release would reduce the work done by the contractile element during the propulsion phase (Roberts et al. 1997; Alexander and Bennet-Clark 1977). It may be argued that for a given force, a more compliant SEE is more favorable for elastic energy storage and that, therefore, a stiffer tendon and aponeurosis would increase the metabolic energy cost during running. However, studies investigating the fascicle behavior during submaximal running report a continuous shortening of the gastrocnemius medialis (GM) fascicles during stance phase (Ishikawa et al. 2007; Lichtwark et al. 2007), while the SEE and the whole MTU are lengthened during the first half of stance. This implies that muscular work as well as potential and kinetic energy of the human body are stored within the SEE during the first phase of stance. For a given tendon force and length of the MTU, i.e., for a given joint angular configuration, a more compliant tendon and aponeurosis would result in an increased fascicle shortening and thus an increase in muscular work. This muscular work would be less efficient due to a higher fascicle shortening velocity (Hill 1938; Houdijk et al. 2006; Woledge et al. 1985). Furthermore, assuming a similar hysteresis, the absolute amount of energy loss would be higher in a more compliant tendon and aponeurosis. Therefore, it is supposed that a stiffer TS tendon and aponeurosis would be advantageous for the efficiency of the GM muscular work and energy loss due to the viscosity of the SEE.
It is well accepted that tendons are able to remodel their mechanical and morphological properties in response to mechanical loading (Woo et al. 1980; Hayashi 1996; Kubo et al. 2002; Reeves et al. 2003). It was shown that dynamic and isometric heavy resistance training as well as plyometric training (e.g. Kubo et al. 2002; Arampatzis et al. 2007a; Foure et al. 2010) increased both tendon stiffness and contractile muscle strength. In addition, previous studies showed that concurrent endurance and resistance training have the potential to improve running economy (e.g. Paavolainen et al. 1999; Spurrs et al. 2003; Johnson et al. 1997; Millet et al. 2002). It was supposed that these improvements in running economy were mainly due to better neuromuscular characteristics. For example, an increase in the contractile specific force would require a lower activated muscle volume to generate the same muscle force and thus would contribute to a more economical force generation during running. Spurrs et al. (2003) argued that an increase in the lower leg musculotendinous stiffness after 6 weeks of plyometric training led to the improvement in running economy through enhanced utilisation of stored energy during running.
Although some studies report that resistance training and plyometric training increase running economy (e.g. Paavolainen et al. 1999; Spurrs et al. 2003; Johnson et al. 1997; Millet et al. 2002), there is no information in the literature regarding the effect of an exercise-induced increase in triceps surae tendon-aponeurosis stiffness on running economy. Therefore, the purpose of the present study was to investigate whether increased tendon-aponeurosis stiffness and contractile strength of the TS MTU induced by a resistance training similar to that proposed by Arampatzis et al. (2007b) affect running economy. It was hypothesized that an increased tendon stiffness and contractile strength of the TS MTU would lead to advantageous conditions for force production within the lower extremities and thus would affect the metabolic energy cost during running. Furthermore, we hypothesized that one of those mechanisms is a reduced shortening velocity of the GM muscle during running due to the stiffer tendon and aponeurosis.
Methods
Subjects
Twenty-six healthy recreational long-distance runners were participated in the study after giving informed consent to the experimental procedure, complying with the rules of the local scientific board. All subjects performed regular endurance running training for at least three times a week. The training volume ranged from 30 to 120 km/week. None of the subjects had a history of neuromuscular or musculoskeletal impairments at the time of the study that could affect their running technique. Thirteen participants (height 180 ± 6 cm, body mass 76 ± 7 kg, age 27 ± 5 years, training volume 66 ± 29 km/week) were recruited for the exercise group (EG). The remaining 13 participants (height 178 ± 7 cm, mass 75 ± 9 kg, age 25 ± 3 years, training volume 62 ± 31 km/week) formed the control group (CG). The EG added a resistance training intervention to their usual endurance training for 14 weeks, while the CG did not change their training habits during this period. The subjects were tested before and after the training period at three different testing sessions to determine (1) mechanical properties of the MTU, (2) kinematics and fascicle behavior of the GM muscle during running and (3) the \({\dot{V}{\rm O}}_2\) for a given velocity. All measurements were performed on the right leg. The CG only performed the test for running economy, because it was previously shown that tendon-aponeurosis stiffness in young adults remained unchanged during a period of 14 weeks without any intervention (Arampatzis et al. 2007a), and that endurance running exercise is not a sufficient stimulus to increase TS tendon-aponeurosis stiffness (Arampatzis et al. 2007b; Hansen et al. 2003).
Exercise protocol
For 14 weeks, the EG performed five sets of four repetitive (3 s loading, 3 s relaxation) isometric ankle plantarflexion contractions four times a week with the ankle joint 5° dorsiflexed, the knee joint fully extended and the hip joint 40° flexed for both legs. Repetitive isometric ankle plantarflexion contractions were used to induce cyclic strain on the TS tendon and aponeurosis. According to our earlier intervention protocol (Arampatzis et al. 2007a), we used an ankle plantarflexion moment at 90 % of the maximally achieved moment during a maximum voluntary contraction (MVC), which was adjusted every week.
Mechanical properties of the muscle-tendon unit
The contractile strength of the TS muscles and the TS tendon-aponeurosis stiffness were determined by means of four maximal isometric voluntary ankle plantarflexion contractions with at least 3-min rest between the contractions. The subjects were seated with the knee joint fully extended at 0°, the hip joint flexed at 40° and the ankle joint fixed in a dorsiflexed position at either −15°, −10° or −5° to determine the maximal voluntary torque. The fourth MVC was performed to determine the tendon-aponeurosis elongation. The ankle joint was fixed in the anatomical neutral position (0°) and the subjects were asked to increase the torque gradually within 3 s. The methods used to determine contractile strength of the TS muscles and the tendon-aponeurosis elongation are only briefly explained as a detailed description has already been published (Arampatzis et al. 2005a, b).
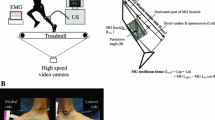
The measured moments from the dynamometer (Biodex-System 3, Biodex Medical Systems. Inc., New York, USA) were corrected by considering gravitational effects and the co-contraction of the tibialis anterior muscle (Mademli et al. 2004). Furthermore, to account for the inevitable misalignment between the rotational axis of the dynamometer and ankle joint axis, the resultant joint moments were calculated using inverse dynamics (Arampatzis et al. 2005a). Achilles tendon forces were estimated by dividing the corrected resultant joint moment by the moment arm of the Achilles tendon. The length of the moment arms was estimated using the data provided by Maganaris et al. (2000). The tendon-aponeurosis elongation during contraction was determined by visualizing the GM muscle fascicles and its distal aponeurosis using ultrasonography (UST-579T, Aloka SSD 4000, Tokyo, Japan) during the gradually increasing contraction. The ultrasound images were recorded by a video recorder with a frame rate of 50 Hz (half images). For analysis, the displacement of a point at the distal aponeurosis was calculated in every half ultrasound image using a video analysis software (SIMI Motion 7.0, SIMI Reality Motion Systems GmbH, Unterschleissheim, Germany). During the MVC, it is not possible to completely prevent joint angular rotation at the ankle joint despite using external fixations (Muramatsu et al. 2001; Magnusson et al. 2001). Therefore, the displacement of the analyzed point at the aponeurosis during an inactive (passive) condition at the same ankle angle position as observed during the MVC (Arampatzis et al. 2005b) was determined and subtracted from the elongation measured during the MVC. The TS tendon-aponeurosis stiffness was calculated as the ratio of the increase in the calculated tendon force and the increase in the tendon-aponeurosis elongation from 50 to 100 % of the maximum tendon force. Tendon-aponeurosis resting length of the GM was determined at rest with the knee in full extension and the ankle joint plantarflexed at 20°, because in this specific position, de Monte et al. (2006) reported the existence of slackness in the inactive GM MTU. To analyze changes in muscle architecture, fascicle length (l 0,fl) and pennation angle (θ 0) were determined using ultrasonography in a relaxed position with the knee fully extended and the foot in the anatomical neutral position. Furthermore, this measure of fascicle length was used as a reference to normalize the MTU length, SEE length and GM fascicle length during running.
Running economy
Running economy was determined at steady state during submaximal running velocities on a treadmill (pulsar, h/p/cosmos sports & medical GmbH, Traunstein, Germany) and expressed as both the steady state rate of oxygen consumption (\({\dot{V}{\rm O}}_2\)) per body weight and the metabolic energy cost of running per body weight and distance traveled (energy cost), which was calculated from the rate of oxygen consumption, the respiratory exchange ratio and the associated energy equivalent (Lusk 1924).
After a 2-min warm-up period (2.5 ms−1), the subjects ran at 3.0 and 3.5 ms−1 in that order, for 15 min separated by a 10-min rest period. Blood samples were taken from the earlobe directly before and after finishing each velocity test to determine blood lactate concentration. \({\dot{V}{\rm O}}_2\) was measured during the 15-min period using a breath-by-breath portable Spirometer (Metamax 3B, Cortex, Germany). The Spirometer was calibrated before each session by means of a two-point calibration using environment air and a gas mixture (4.5 % CO2, 0 % O2, balance N2). The volume sensor was calibrated with a manual 2-l syringe. For each velocity, average values of \({\dot{V}{\rm O}}_2\) and the respiratory exchange ratio were determined between the 10th and 14th minute of running to describe running economy (Fig. 1). As proposed by Rossiter et al. (2000), occasional breath values were omitted from the analysis, using an exclusion criterion of greater than four standard deviations about the local mean. The measured values for \({\dot{V}{\rm O}}_2\) are expressed relative to body mass. In order to reduce the test–retest variability (Saunders et al. 2004a; Morgan et al. 1991), the type of shoes worn, time of day for testing and the training activity during the last 72 h before testing were the same for the test and retest situation for each subject but could differ between subjects. No food intake was allowed during the last 3 h before testing.
Running kinematics and fascicle behavior
Sagittal plane lower limb joint angle kinematics and fascicle behavior of the GM muscle were determined at the right leg during stance for both running velocities (3.0 and 3.5 ms−1). A 5-min familiarization period preceded the data acquisition at each velocity. Five spherical retro-reflective markers were attached either to the skin or to the shoe of the right leg (greater trochanter, the lateral femoral condyle, lateral malleolus, most posterior point of the calcaneus and the 5th metatarsal head) and recorded using a Motion Capture System (VICON motion systems, Oxford, UK) with 6 cameras operating at 200 Hz. Touchdown was determined using a one dimensional accelerometer (1000 Hz) attached to the lateral rearfoot. Take-off was determined using a high speed camera (A602fc, Basler AG, Ahrensburg, Germany) from posterior in frontal plane view operating at 100 Hz.
B-mode ultrasonography was used to measure fascicle length during stance as described by Aggeloussis et al. (2010). A 7.5 MHz linear array ultrasound probe with 60 mm scanning width (UST-579T, Aloka SSD 4000, Tokyo, Japan) was fixed at about the middle of the GM muscle belly of the right leg using a lightweight self-made fixation, in a way that enabled the visualization of both the deep and superficial aponeurosis throughout the stance phase and was secured with hook-and-loop straps and an elastic bandage. Ultrasonographic images were recorded on a video tape at 50 Hz (half images) and synchronized with the kinematic data. In the recorded images, two points on each aponeurosis and two points on one fascicle were manually digitized with a video analysis software (SIMI Motion 7.0, SIMI Reality Motion Systems GmbH, Unterschleissheim, Germany). Assuming that the fascicular trajectory is linear, fascicle length was defined as the distance between the insertions of the fascicle into the superficial and deep aponeurosis. If the fascicle exceeded the ultrasound image, the length of the missing portion was estimated by linear extrapolation.
A recent study (Aggeloussis et al. 2010) demonstrated that fascicle length shows a good reproducibility for successive measurements, both within the same day and between 2 days throughout the walking cycle. That study revealed that 2–6 trials were needed to determine fascicle length reliably. Therefore, six successive stance phases were analyzed in the present study. GM muscle fascicle shortening and average shortening velocity were determined during the stance phase. The length of the entire GM MTU (l MTU) was calculated with the regression equation provided by Hawkins and Hull (1990) using knee and ankle angles as independent variables and shank length for individual scaling. As proposed by Fukunaga et al. (2001), a MTU model was used to estimate the length of the SEE during running assuming that SEE length (l SEE) is equal to \(l_{\rm MTU}-l_{\rm fl} \cdot \cos(\theta), \) where l fl is the fascicle length and θ the pennation angle. This definition of the SEE includes all series elastic structures within the MTU (e.g. series-elastic structures within the muscle, proximal and distal tendon and aponeurosis). The pennation angle was defined as the angle of insertion of the muscle fascicle into the deep aponeurosis and was also determined from the ultrasonographic images acquired during running. To account for individual differences, the MTU length, SEE length, fascicle length and the corresponding velocities are presented normalized to the reference fascicle length (l 0,fl) determined at rest with the knee in full extension and the ankle in the neutral anatomical position (0°).
Statistics
An analysis of variance (ANOVA) for repeated measures with time of testing (before and after the training) and running velocity as within-subject factors and the training intervention (exercise group and control group) as the between-subject factor was used to identify potential effects on \({\dot{V}{\rm O}}_2\) and the blood lactate concentration by examining the group-by-time interactions. In case of a significant group-by-time interaction, a Bonferroni post hoc test was conducted. Paired t tests were used to determine the training-related changes in the other variables. For the time-dependent measures, i.e., joint kinematics and fascicle behavior, the comparisons between pre- and post-training measures were performed every 10 % of the stance phase (i.e., at 11 time points). For the tendon-aponeurosis strain–force relationship, the comparison was performed every 200 N of tendon force. The results are presented as mean ± standard deviation (SD) in the text and tables and as mean ± standard error of mean (SEM) in the figures. For all statistical analysis, α = 0.05 was accepted as the level of statistical significance.
Results
Muscle-tendon unit properties
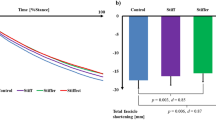
After the 14-week training intervention, the maximum voluntary ankle plantarflexion joint moment showed a significant increase of about ∼7 % (p = 0.004) (Table 1), whereas the tendon-aponeurosis strain for a given tendon force (every 200 N) beyond 1,400 N was significantly lower (p < 0.05) than the pre-training strain values (Fig. 2). Moreover, the maximum strain was also significantly (p = 0.003) lower after the intervention (Table 1). Thus, the tendon-aponeurosis stiffness showed a significant increase (p < 0.0001) of ∼16 % (Table 1).
Tendon-aponeurosis strain of the gastrocnemius medialis at every 200 N of the calculated Achilles tendon force during the maximum voluntary plantarflexion isometric contraction determined before (pre) and after (post) the training intervention. Values are mean ± SEM. *Significantly different from pre-training values (p < 0.05)
Metabolic parameters
The body mass of neither the EG (pre-training: 76 ± 7 kg, post-training: 76 ± 8 kg) nor the CG (pre-training: 75 ± 9 kg, post-training: 76 ± 9 kg) changed after the 14-week intervention. The metabolic and respiratory parameters determined during running on the treadmill are presented in Table 2. Significant group-by-time interactions were found for \({\dot V {\rm O}}_2\) (F = 5.2, p = 0.03) and the energy cost (F = 5.4, p = 0.03).
The post hoc test revealed a significant reduction in \({\dot V{\rm O}}_2\) (p = 0.02) and in energy cost (p = 0.02) for the EG while the CG did not show any significant (p ≥ 0.98) changes. Thus, during submaximal running, the EG showed a reduction of ∼4 % in \({\dot V{\rm O}}_2\) and energy cost after the training intervention.
Running kinematics and fascicle behavior
Figure 3 shows the ankle and knee joint angles and the corresponding angular velocities in the sagittal plane of the right leg before and after the training intervention during the stance phase of running at 3.0 and 3.5 ms−1. None of the investigated angular parameters (Fig. 3; Table 3) were significantly different between pre- and post-training values. Also, all temporal parameters such as contact time, swing time and stride frequency were unchanged (Table 3). The average GM muscle fascicle lengths during stance for all subjects before and after the training intervention are shown in Fig. 4 in comparison to the calculated GM MTU length and the SEE length. Since joint angles did not differ significantly, the MTU length also showed no significant difference (Fig. 4). Similarly, no significant differences can be found in the time course of fascicle length and SEE length during the stance phase of running. The calculated parameters, fascicle shortening, average fascicle shortening velocity and maximum fascicle shortening velocity, presented in Table 4, show no significant differences.
Average values of the ankle and knee joint angles (top) and angular velocities (bottom) during the stance phase of running before (pre) and after (post) the training intervention for both running velocities (left 3.0 ms−1; right 3.5 ms−1). The horizontal axis is normalized to the stance phase, where 0 % corresponds to touchdown and 100 % to take-off. For the ankle joint angle, negative values correspond to a dorsiflexed position and positive values to a plantarflexed position. Values are mean ± SEM
Average values of the gastrocnemius medialis fascicle length (middle) in comparison to the entire length of the muscle-tendon unit (MTU) (top) and the series-elastic element (SEE) (bottom) during the stance phase of running before (pre) and after (post) the training intervention for both running velocities (left 3.0 ms−1; right 3.5 ms−1). The horizontal axis is normalized to the stance phase, where 0 % corresponds to touchdown and 100 % to take-off. MTU length, fascicle length and SEE length are normalized to the reference fascicle length (l 0,fl) determined at rest with the knee in full extension and the ankle at 0°. Values are means ± SEM
Discussion
The aim of the present study was to investigate whether increased tendon-aponeurosis stiffness and contractile strength at the TS MTU induced by resistance training (Arampatzis et al. 2007b) affect running economy. After the 14-week intervention, a significant increase of ∼7 % in the maximum plantarflexion muscle strength and a significant increase of ∼16 % in the TS tendon-aponeurosis stiffness were observed. The EG subjects showed a significant reduction (∼4 %) in \({\dot V{\rm O}}_2\) and energy cost during submaximal running, indicating an enhanced running economy after the intervention, while the CG showed no changes in both the \({\dot V{\rm O}}_2\) and energy cost after 14 weeks. Thus, our main hypothesis, that an increase in muscle strength and tendon-aponeurosis stiffness at the TS MTU would improve running economy, was confirmed.
The observed improvement in running economy of ∼4 % is higher than the smallest worthwhile change reported by Saunders et al. (2004a) and can therefore be considered to be of practical significance. It has been suggested by di Prampero et al. (1993) and Capelli (1999) that a 2.5 and 5.0 % decrease in the energy cost of running explains an improvement in long-distance running performance time of about 2 and 4 %, respectively. Further, Spurrs et al. (2003) and Paavolainen et al. (1999) observed a 3 % increase in running performance time after a 4–8 % improvement in running economy.
Considering the results of the present study together with the results from Arampatzis et al. (2006) and Fletcher et al. (2010), it is supposed that the stiffer tendon and aponeurosis and the greater plantarflexion muscle strength at the TS MTU have the potential to lead to a better economy of force and work production within the lower extremities during running. Neither the contact time, swing time, stride frequency nor the examined parameters describing ankle and knee joint kinematics showed significant differences before and after the intervention. This is in line with the results of the earlier study by Arampatzis et al. (2006) and with the studies by Kyröläinen et al. (2001) and Williams and Cavanagh (1987) examining kinematic parameters of runners with different running economy.
It was hypothesized that the stiffer TS tendon and aponeurosis induced by the intervention would lead to a reduced excursion of the SEE during the stance phase of running. For a given tendon force and length of the MTU, i.e., for a given joint angular configuration, the smaller excursion of the SEE was assumed to reduce fascicle shortening and thus shortening velocity, which would enable force production at a lower metabolic energy cost mainly due to the force–velocity relationship (Hill 1938). However, this hypothesis must be rejected because we could not find a reduction in the amplitude of shortening or shortening velocity of the GM muscle fascicles.
The fact that GM fascicle length remained unaffected by the stiffer tendon and aponeurosis, while the ankle and knee joint angles were not affected (i.e., same MTU length), leads to the elongation of the SEE also remaining unchanged (Fig. 4). However, since the tendon-aponeurosis stiffness increased by about 16 % after the intervention, a greater force must be exerted by the TS muscle group to reach the same elongation of the SEE during running. Based on the calculated elongation of the SEE during running (Fig. 4) and the measured force–strain relationship of the GM tendon-aponeurosis under isometric conditions (Fig. 2), we estimated that the tendon force after the intervention should be about 23–27 % higher compared to the pre-exercise condition at the same running speed, which would lead to a greater energy storage of similar magnitude.
As described by Biewener et al. (2004), one possible explanation for a change in energy cost may be a change in the effective mechanical advantage (EMA) within the lower limb that alters the force produced by the leg extensor muscles. The higher tendon forces exerted by the plantarflexors imply that the external ankle joint moment has increased to the same amount. Because running speed was kept constant and no differences in swing time, stride frequency and contact time were observed, it is assumed that the ground reaction force (GRF) also remained unchanged. Thus, for the same running kinematics and a similar GRF, an increase in the external ankle joint moment is most likely be achieved by a lengthening of the moment arm of the GRF at the ankle joint due to an anterior shift of the point of force application (PFA). However, an anterior shift of the PFA would also result in a shorter moment arm of the GRF at the knee joint and a longer moment arm at the hip joint, implying a smaller EMA for the ankle and hip joint but a greater EMA for the knee joint (Biewener et al. 2004). The lower EMA at the ankle and hip joints would result in an increased muscle force of the plantarflexors and hip extensors during the stance phase of running at a given speed, while the greater EMA at the knee joint would result in a reduced force of the knee extensor muscles. Compared to the knee extensor muscles, the human plantarflexor muscles have relatively short fascicles (Wickiewicz et al. 1983) and are metabolically less costly than long-fibred muscles to generate the same force (Biewener and Roberts 2000). Furthermore, the plantarflexor muscles have long tendons favorable for elastic energy storage and recovery (Alexander 2002; Ker et al. 1987). The lower EMA at the hip joint would result in higher forces of the hip extensors, but the external flexion moment of the GRF only contributes to the resultant hip joint moment during the first ∼25 % of the stance phase and can be affected by a change in the moment arm. During the remaining ∼75 % of the stance phase, the contribution of the moment due to inertia of limb segments is most important (Krabbe et al. 1997). In addition, forces generated by the knee extensor are generally higher than those generated by the hip extensors during running at the investigated submaximal running velocities (Dorn et al. 2012). Therefore, we suggest that a change of the EMA within the lower limb leading to a redistribution of the muscular output within the lower extremities might partly explain the observed reduction in \({\dot V{\rm O}}_2.\)
The combined adaptation of tendon stiffness and TS muscle strength seems to be necessary to maintain a high economy of force generation within the TS muscle. The average fascicle shortening velocity was below 1.3 fascicle lengths per second. Considering that the maximal shortening velocity of human fast and slow fibers is comprised between 8 and 14 fiber length per second (Zajac 1989; Epstein and Herzog 1998), the observed fascicle shortening velocity during running is favorable to generate forces economically. An increase in muscle strength without a simultaneous increase in tendon stiffness would have probably resulted in a faster fascicle shortening velocity. Using a model of the MTU, Lichtwark and Wilson (2008) described a similar interrelation between the ankle joint moment and tendon stiffness during running. These authors found that a 20 % increase in the ankle joint moment during running would require a 16–23 % increase in tendon stiffness to maintain a high efficiency.
However, our measurement of running economy was performed on a treadmill and no GRFs were available. Therefore, ankle and knee joint moments during running were not directly determined. The assumption that the force exerted by the triceps surae muscle group is higher after the training intervention during running is only based on the fact that fascicle length of the gastrocnemius medialis muscle has remained unchanged despite an increase in tendon stiffness. Despite the constant running speed and the lack of difference in swing time, stride frequency and contact time, training-induced changes in the rate of GRF development may have occurred as described by Cormie et al. (2010) and may have also contributed to changes in energy cost of running. Further research is necessary to investigate the effect of an increased plantarflexion strength and tendon-aponeurosis stiffness on the GRF, the position of the PFA and the external moment arms and EMAs of the ankle, knee and hip joints.
Limitations
Daily variation of running economy could have an impact on the results. Morgan et al. (1991) found that the aerobic demand of running in trained but non-elite subjects would be expected to vary between ±1.32 and ±2.64 % in trials that were controlled for treadmill running experience, time of day, footwear and training. Similar results were obtained later for well-trained or elite-distance runners (Morgan et al. 1994; Saunders et al. 2004b). Daily variation should produce high and low values by chance. However, for the slow running velocity 12 of 13 EG subjects and for the fast running velocity 10 of 13 EG subjects showed an improvement in running economy while the changes for CG were small and showed no systematic pattern.
The elongation of the SEE during running predicted with the model proposed by Fukunaga et al. (2001) is consistent with previously reported data (Ishikawa et al. 2007; Lichtwark et al. 2007). However, maximum elongation of the SEE during running (∼34–38 mm) as well as the corresponding strain (∼8.4–9.4 %) exceed the maximum elongation (15.2/16.4 mm) and maximum strain (5.3/5.7 %) of the tendon and aponeurosis determined during the isometric contractions. This is a common problem that has been discussed previously (Lichtwark and Wilson 2005; Lichtwark et al. 2007). The used model is a substantial simplification of the complex three dimensional geometry of the MTU that might explain these discrepancies. In addition, the SEE includes all series-elastic structures within the MTU (e.g., series-elastic structures within the muscle, proximal and distal tendon and aponeurosis), while the maximum strain determined during the isometric contraction is mainly the result from the elongation of the distal tendon and aponeurosis. A lower stiffness of the other series-elastic structures combined in the SEE may also explain the high strain values.
Conclusion
In conclusion, the present study shows that the increase in tendon stiffness and muscular contractile strength of the TS MTU resulting from an exercise intervention has the potential to enhance running economy. The observed improvement of ∼4 % is likely to be of practical significance (Saunders et al. 2004a). The enhanced running economy found after increasing TS tendon stiffness and contractile strength indicates that force generation during running became more economical within the lower extremities. A higher energy storage and release in the SEE of the triceps surae and a redistribution of the muscular output within the lower extremities might explain the improved running economy.
Abbreviations
- C :
-
Energy cost
- CG:
-
Control group
- EG:
-
Exercise group
- EMA:
-
Effective mechanical advantage
- GM:
-
Gastrocnemius medialis
- GRF:
-
Ground reaction force
- l fl :
-
Fascicle length
- l 0,fl :
-
Reference fascicle length
- l MTU :
-
Length of the muscle-tendon unit
- l SEE :
-
Length of the series-elastic element
- MTU:
-
Muscle-tendon unit
- MVC:
-
Maximum voluntary contraction
- PFA:
-
Point of force application
- RMS:
-
Root mean square
- SEM:
-
Standard error of mean
- SEE:
-
Series elastic element
- SD:
-
Standard deviation
- TS:
-
Triceps surae
- θ :
-
Pennation angle
- θ 0 :
-
Reference pennation angle
- \(\overline{v}_{\rm fl}\) :
-
Average fascicle shortening velocity
- v max, fl :
-
Maximum fascicle shortening velocity
- \({\dot{V}{\rm O}}_{\rm 2,max}\) :
-
Rate of oxygen consumption
References
Aggeloussis N, Giannakou E, Albracht K, Arampatzis A (2010) Reproducibility of fascicle length and pennation angle of gastrocnemius medialis in human gait in vivo. Gait Posture 31(1):73–77
Alexander RM (2002) Tendon elasticity and muscle function. Comp Biochem Physiol A Mol Integr Physiol 133(4):1001–1011
Alexander RM, Bennet-Clark HC (1977) Storage of elastic strain energy in muscle and other tissues. Nat Biotechnol 265(5590):114–117
Arampatzis A, Morey-Klapsing G, Karamanidis K, DeMonte G, Stafilidis S, Brüggemann GP (2005a) Differences between measured and resultant joint moments during isometric contractions at the ankle joint. J Biomech 38(4):885–892
Arampatzis A, Stafilidis S, DeMonte G, Karamanidis K, Morey-Klapsing G, Brüggemann GP (2005b) Strain and elongation of the human gastrocnemius tendon and aponeurosis during maximal plantarflexion effort. J Biomech 38(4):833–841
Arampatzis A, De Monte G, Karamanidis K, Morey-Klapsing G, Stafilidis S, Brüggemann GP (2006) Influence of the muscle-tendon unit’s mechanical and morphological properties on running economy. J Exp Biol 209(Pt 17):3345–3357
Arampatzis A, Karamanidis K, Albracht K (2007a) Adaptational responses of the human achilles tendon by modulation of the applied cyclic strain magnitude. J Exp Biol 210(Pt 15):2743–2753
Arampatzis A, Karamanidis K, Morey-Klapsing G, Monte GD, Stafilidis S (2007b) Mechanical properties of the triceps surae tendon and aponeurosis in relation to intensity of sport activity. J Biomech 40(9):1946–1952
Biewener AA, Roberts TJ (2000) Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc Sport Sci Rev 28(3):99–107
Biewener AA, Farley CT, Roberts TJ, Temaner M (2004) Muscle mechanical advantage of human walking and running: implications for energy cost. J Appl Physiol 97(6):2266–2274
Capelli C (1999) Physiological determinants of best performances in human locomotion. Eur J Appl Physiol Occup Physiol 80(4):298–307
Cormie P, McGuigan MR, Newton RU (2010) Influence of strength on magnitude and mechanisms of adaptation to power training. Med Sci Sports Exerc 42(8):1566–1581
Costill DL, Thomason H, Roberts E (1973) Fractional utilization of the aerobic capacity during distance running. Med Sci Sports 5(4):248–252
Daniels JT (1985) A physiologist’s view of running economy. Med Sci Sports Exerc 17(3):332–338
de Monte G, Arampatzis A, Stogiannari C, Karamanidis K (2006) In vivo motion transmission in the inactive gastrocnemius medialis muscle-tendon unit during ankle and knee joint rotation. J Electromyogr Kinesiol 16(5):413–422
di Prampero PE, Atchou G, J C Brückner JC, C Moia C (1986) The energetics of endurance running. Eur J Appl Physiol Occup Physiol 55(3):259–266
di Prampero PE, Capelli C, Pagliaro P, Antonutto G, Girardis M, Zamparo P, Soule RG (1993) Energetics of best performances in middle-distance running. J Appl Physiol 74(5):2318–2324
Dorn TW, Schache AG, Pandy MG (2012) Muscular strategy shift in human running: dependence of running speed on hip and ankle muscle performance. J Exp Biol 215(Pt 11):1944–1956
Epstein M, Herzog W (1998) Theoretical models of skeletal muscle: biological and mathematical considerations. Wiley Chichester, UK
Fletcher JR, Esau SP, Macintosh BR (2009) Economy of running: beyond the measurement of oxygen uptake. J Appl Physiol 107(6):1918–1922
Fletcher JR, Esau SP, MacIntosh BR (2010) Changes in tendon stiffness and running economy in highly trained distance runners. Eur J Appl Physiol 110(5):1037–1046
Foster C, Lucia A (2007) Running economy : the forgotten factor in elite performance. Sports Med 37(4–5):316–319
Foure A, Nordez A, Cornu C (2010) Plyometric training effects on achilles tendon stiffness and dissipative properties. J Appl Physiol 109(3):849–854
Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris C (2001) In vivo behaviour of human muscle tendon during walking. Proc Biol Sci 268(1464):229–233
Hansen P, Aagaard P, Kjaer M, Larsson B, Magnusson SP (2003) Effect of habitual running on human achilles tendon load-deformation properties and cross-sectional area. J Appl Physiol 95(6):2375–2380
Hawkins D, Hull ML (1990) A method for determining lower extremity muscle-tendon lengths during flexion/extension movements. J Biomech 23(5):487–494
Hayashi K (1996) Biomechanical studies of the remodeling of knee joint tendons and ligaments. J Biomech 29(6):707–716
Heise GD, Martin PE (2001) Are variations in running economy in humans associated with ground reaction force characteristics? Eur J Appl Physiol 84(5):438–442
Hill A (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci 1:136–195
Houdijk H, Bobbert MF, de Haan A (2006) Evaluation of a Hill based muscle model for the energy cost and efficiency of muscular contraction. J Biomech 39(3):536–543
Ishikawa M, Pakaslahti J, Komi P (2007) Medial gastrocnemius muscle behavior during human running and walking. Gait Posture 25(3):380–384
Johnson RE, Quinn T, Kertzer R, Vroman N (1997) Strength training in female distance runners: impact on running economy. J Strength Cond Res 11(4):224
Ker RF, Bennett MB, Bibby SR, Kester RC, Alexander RM (1987) The spring in the arch of the human foot. Nat Biotechnol 325(7000):147–149
Krabbe B, Farkas R, Baumann W (1997) Influence of inertia on intersegment moments of the lower extremity joints. J Biomech 30(5):517–519
Kubo K, Kanehisa H, Fukunaga T (2002) Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J Physiol 538(Pt 1):219–226
Kyröläinen H, Belli A, Komi PV (2001) Biomechanical factors affecting running economy. Med Sci Sports Exerc 33(8):1330–1337
Lichtwark GA, Wilson AM (2005) In vivo mechanical properties of the human achilles tendon during one-legged hopping. J Exp Biol 208(Pt 24):4715–4725
Lichtwark GA, Wilson AM (2008) Optimal muscle fascicle length and tendon stiffness for maximising gastrocnemius efficiency during human walking and running. J Theor Biol 252(4):662–673
Lichtwark GA, Bougoulias K, Wilson AM (2007) Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J Biomech 40(1):157–164
Lusk G (1924) Animal calorimetry: analysis of the oxidation of mixtures of carbohydrate and fat. J Biol Chem 59(1):41
Mademli L, Arampatzis A, Morey-Klapsing G, Brüggemann GP (2004) Effect of ankle joint position and electrode placement on the estimation of the antagonistic moment during maximal plantarflexion. J Electromyogr Kinesiol 14(5):591–597
Maganaris CN, Baltzopoulos V, Sargeant AJ (2000) In vivo measurement-based estimations of the human achilles tendon moment arm. Eur J Appl Physiol 83(4–5):363–369
Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M (2001) Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol 531(Pt 1):277–288
Margaria R, Cerretelli P, Aghemo P, Sassi G (1963) Energy cost of running. J Appl Physiol 18:367–370
Martin PE, Morgan DW (1992) Biomechanical considerations for economical walking and running. Med Sci Sports Exerc 24(4):467–474
Millet GP, Jaouen B, Borrani F, Candau R (2002) Effects of concurrent endurance and strength training on running economy and .vo(2) kinetics. Med Sci Sports Exerc 34(8):1351–1359
Morgan DW, Baldini FD, Martin PE, Kohrt WM (1989a) Ten kilometer performance and predicted velocity at vo2max among well-trained male runners. Med Sci Sports Exerc 21(1):78–83
Morgan DW, Martin PE, Krahenbuhl GS (1989b) Factors affecting running economy. Sports Med 7(5):310–330
Morgan DW, Martin PE, Krahenbuhl GS, Baldini FD (1991) Variability in running economy and mechanics among trained male runners. Med Sci Sports Exerc 23(3):378–383
Morgan DW, Craib MW, Krahenbuhl GS, Woodall K, Jordan S, Filarski K, Burleson C, Williams T (1994) Daily variability in running economy among well-trained male and female distance runners. Res Q Exerc Sport 65(1):72–77
Muramatsu T, Muraoka T, Takeshita D, Kawakami Y, Hirano Y, Fukunaga T (2001) Mechanical properties of tendon and aponeurosis of human gastrocnemius muscle in vivo. J Appl Physiol 90(5):1671–1678
Paavolainen L, Häkkinen K, Hämäläinen I, Nummela A, Rusko H (1999) Explosive-strength training improves 5-km running time by improving running economy and muscle power. J Appl Physiol 86(5):1527–1533
Pereira MA, Freedson PS (1997) Intraindividual variation of running economy in highly trained and moderately trained males. Int J Sports Med 18(2):118–124
Reeves ND, Maganaris CN, Narici MV (2003) Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548(Pt 3):971–981
Roberts TJ, Marsh RL, Weyand PG, Taylor CR (1997) Muscular force in running turkeys: the economy of minimizing work. Sci Agric 275(5303):1113–1115
Rossiter HB, Howe FA, Ward SA, Kowalchuk JM, Griffiths JR, Whipp BJ (2000) Intersample fluctuations in phosphocreatine concentration determined by 31p-magnetic resonance spectroscopy and parameter estimation of metabolic responses to exercise in humans. J Physiol 528(Pt 2):359–369
Saunders PU, Pyne DB, Telford RD, Hawley JA (2004a) Factors affecting running economy in trained distance runners. Sports Med 34(7):465–485
Saunders PU, Pyne DB, Telford RD, Hawley JA (2004b) Reliability and variability of running economy in elite distance runners. Med Sci Sports Exerc 36(11):1972–1976
Spurrs RW, Murphy AJ, Watsford ML (2003) The effect of plyometric training on distance running performance. Eur J Appl Physiol 89(1):1–7
Wickiewicz TL, Roy RR, Powell PL, Edgerton VR (1983) Muscle architecture of the human lower limb. Clin Orthop Relat Res 1(179):275–283
Williams KR, Cavanagh PR (1987) Relationship between distance running mechanics, running economy, and performance. J Appl Physiol 63(3):1236–1245
Woledge RC, Curtin NA, Homsher E (1985) Energetic aspects of muscle contraction. Monogr Physiol Soc 41:1–357
Woo SL, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC, Garfin SR, Akeson WH (1980) The biomechanical properties of swine tendon—long term effects of exercise on the digital extensors. Connect Tissue Res 7(3):177–183
Zajac FE (1989) Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17(4):359–411
Acknowledgments
This research has been supported by The Federal Institute of Sport Science (BISp), Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jean-René Lacour.
Rights and permissions
About this article
Cite this article
Albracht, K., Arampatzis, A. Exercise-induced changes in triceps surae tendon stiffness and muscle strength affect running economy in humans. Eur J Appl Physiol 113, 1605–1615 (2013). https://doi.org/10.1007/s00421-012-2585-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2585-4