Abstract
Aim of the study was to determine the influence of classic altitude training on hemoglobin mass (Hb-mass) in elite swimmers under the following aspects: (1) normal oscillation of Hb-mass at sea level; (2) time course of adaptation and de-adaptation; (3) sex influences; (4) influences of illness and injury; (5) interaction of Hb-mass and competition performance. Hb-mass of 45 top swimmers (male 24; female 21) was repeatedly measured (~6 times) over the course of 2 years using the optimized CO-rebreathing method. Twenty-five athletes trained between one and three times for 3–4 weeks at altitude training camps (ATCs) at 2,320 m (3 ATCs) and 1,360 m (1 ATC). Performance was determined by analyzing 726 competitions according to the German point system. The variation of Hb-mass without hypoxic influence was 3.0 % (m) and 2.7 % (f). At altitude, Hb-mass increased by 7.2 ± 3.3 % (p < 0.001; 2,320 m) and by 3.8 ± 3.4 % (p < 0.05; 1,360 m). The response at 2,320 m was not sex-related, and no increase was found in ill and injured athletes (n = 8). Hb-mass was found increased on day 13 and was still elevated 24 days after return (4.0 ± 2.7 %, p < 0.05). Hb-mass had only a small positive effect on swimming performance; an increase in performance was only observed 25–35 days after return from altitude. In conclusion, the altitude (2,320 m) effect on Hb-mass is still present 3 weeks after return, it decisively depends on the health status, but is not influenced by sex. In healthy subjects it exceeds by far the oscillation occurring at sea level. After return from altitude performance increases after a delay of 3 weeks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total hemoglobin mass (Hb-mass) is one key factor in endurance performance because it limits oxygen transport and thereby muscle tissue oxygen availability in elite athletes. Under normoxic conditions, a change in Hb-mass of 1 g is associated with a change in VO2max of approximately 4 ml/min (Schmidt and Prommer 2010). Therefore, one goal for elite endurance athletes is to optimize their Hb-mass, preferentially by altitude training, which is a legal form of performance enhancement. Recent studies (e.g. Garvican et al. 2010a; Heinicke et al. 2005; Levine and Sray-Gunderson 1997; Robertson et al. 2010; Schmidt and Prommer 2008) have reported significant increases in Hb-mass for classical altitude training as well as for live high-train low (LH-TL) protocols provided that the altitude (~2,300 m), duration of the camp (3–4 weeks), and daily hypoxic exposure (>14 h) exceeded the corresponding thresholds. However, all these studies focused on a few aspects of training and did not embed the altitude training measurements into the long-term training process. That is, it is not known if the statistically significant increases in Hb-mass after a single altitude training camp disappear within the normal biological variability that occurs during long-term training periods.
During altitude training, multiple possibly confounding factors may affect erythropoietic activity. Until now, no data have been available regarding sex-specific Hb-mass responses to altitude training. Theoretically, one might expect lower Hb-mass increases in female than in male athletes due to sex-specific respiratory reactions at altitude. The female hormones estrogen and progesterone augment the hypoxic ventilatory response and prevent the pronounced decreases in arterial pO2 and O2 saturation that occurs in men (Schoene et al. 1981). As a result, higher sO2 and lower Hb-mass are observed in untrained premenopausal women compared to postmenopausal women living permanently at 2,600 m (Cristancho et al. 2007).
Beside training volume (Garvican et al. 2010b) also the health status is assumed to modify the erythropoietic response in athletes. In general, in case of illness the production of pro-inflammatory cytokines, such as IL-1α, IL-1β and TNFα (Morceau et al. 2009), may suppress erythropoiesis which in long-term can be associated with anemia (Sawka et al. 2000). Illness and injury during altitude training may, therefore, suppress an adequate erythropoietic response to altitude.
In almost all of the previously conducted altitude training studies, Hb-mass was determined exclusively before and immediately after return from altitude. The time course of red cell production during LH-TL and natural altitude training protocols, respectively, was only described twice (Clark et al. 2009; Garvican et al. 2010a) showing a delayed response of approx. 1 week. After return from altitude only short-term data are available while the maintenance of Hb-mass over several weeks has not been sufficiently investigated.
Concerning the response to altitude inter-individual differences were subject of investigation. The intra-individual reproducibility of Hb-mass response, however, which may provide more precise information on the physiological mechanisms, has been rarely investigated. Until now, no convincing explanation for the individual response to altitude has been presented.
Although the main purpose of altitude training is a legal performance enhancement for competitions at sea level, the real effects of altitude are still under debate. The smallest worthwhile effect of altitude training for elite athletes has been calculated to be a 50 % increase of the variation of their maximum performance (Hopkins 2005). For elite swimmers, this variation was quantified to be 0.8 % (Trewin et al. 2004) indicating the relevant worthwhile improvement to be at least 0.4 %. Recently conducted meta-analyses and review articles (Bonetti and Hopkins 2009; Friedmann-Bette 2008) showed this level was exceeded by sub-elite athletes after at least 3 weeks of natural altitude training and LH-TL protocols. For elite athletes, however, enhancement of performance after natural altitude training could not be demonstrated. One confounding factor explaining this discrepancy may be the time after return from altitude when performance was determined. For natural altitude training, anecdotal information suggests that athletes perform well for a few days immediately after altitude, followed by a decrease in performance, before achieving an elevated performance level 2–3 weeks after return that may last for several weeks (see Millet et al. 2010). Scientific data are, however, rarely available.
In 2006, the German swimming federation planned a systematic preparation of its prospective team for the Olympic Games in Beijing, including a chain of six natural altitude training camps (ATC) within a period of 2 years; we monitored the team during this time. While participating at four of the altitude training camps and accompanying the team during their sea level training periods, we focused on the following altitude-related changes on Hb-mass and erythropoietic response:
-
1.
Erythropoietic response to altitude training in comparison to the normal oscillation of Hb-mass occurring at sea level;
-
2.
Sex-specific differences in Hb-mass response;
-
3.
Influence of illness and injury on the erythropoietic response;
-
4.
Kinetics of the Hb-mass adaptation during altitude exposure and of de-adaptation after return from altitude;
-
5.
Reproducibility of the altitude effects;
-
6.
Influence of Hb-mass on performance and the optimal timing for altitude training camps.
Because these topics were investigated in one group of elite athletes and all of them should be considered in the context of a long-term training period, our results are shown and discussed in this comprehensive paper.
Methods
Subjects
In preparation for the Olympic Games in Beijing, the German Swimming Federation built up a national Top Team including a total of 58 swimmers. 27 athletes finally participated at the Olympics in Beijing. In this study, we included the data from 21 female and 24 male athletes from the team of 58; 13 athletes were excluded because they performed less than two CO-rebreathing tests to measure Hb-mass. Except for two free-water swimmers the favorite distance was between 50 and 400 m corresponding to a swimming time between 22 s—3 min 42 s (males) and 24 s—4 min 10 s (females). All of the subjects signed a consent form; if a subject’s age was lower than 18 years, the parents provided written consent. The study was approved by the Ethics Committee of the Friedrich-Alexander-University, Erlangen-Nuremberg, Germany. The anthropometric data of the test subjects and their participation at altitude training camps (ATC) are presented in Table 1. Whilst it cannot be totally discounted, obvious blood manipulations probably did not occur because all of the athletes were subject to regular doping controls, and no irregularities in their hematological profiles, including their OFF-scores, were detected.
Study design
Within the 2-year preparation period for the Olympics, 282 Hb-mass measurements were performed at 20 different time points and places (Fig. 1). During the whole preparation period, six classical altitude training camps (ATC) occurred, and four of them were scientifically accompanied. Three of these camps took place in Monachil, Sierra Nevada, Spain, at an altitude of 2,320 m (barometric pressure ~570 mm Hg), and one camp took place in Pretoria, South Africa, at 1,360 m (~635 mm Hg). The training at Sierra Nevada was performed in indoor pools at constant ambient temperatures while in Pretoria an outdoor pool was used (ambient temperature ~22 °C). The duration of the camps was between 3 and 4 weeks. Thirty-one swimmers participated between 1 and 3 times at these four camps; the total number of stays at altitude was 47 (see Table 2). Most athletes (19 out of 26) participated at the first camp; thereafter, the number of participating athletes decreased considerably due to personal decision of the athletes (Table 2). Simultaneously with the second ATC, a matched control group (n = 11), some of whom (n = 5) had participated in the first ATC, performed a sea level training camp (~760 mm Hg, ambient temperature ~28 °C; STC) in Las Palmas, Gran Canaria, Spain.
Study design. Presented is the sequence of the training camps performed during the 2-year preparation period for the Olympic Games 2008. The altitude of the training camps was 2,320 m at Sierra Nevada, 2,100 m at Flagstaff, and 1,360 m at Pretoria. ATC indicates the number of the scientifically monitored altitude training camps. STC sea level training camp
During the training camps, the athletes were accompanied by their own trainers; therefore, training volume and intensity were different among individuals. During the four monitored altitude camps, eight swimmers (m: 4, f: 4) got sick, with gastro-enteral problems and infectious diseases, or suffered injuries (such as peripheral luxation and fractures) and thus had to stop or markedly reduce their training program for at least 1 week. They remained at the ATCs, and their data were separately analyzed in a subgroup. Swimmers who had ferritin levels below 40 μg/l (16 out of 31 participating at the ATCs) took oral iron supplements (f: 12, m: 4) (Ferro Sanol-duodenal®, Sanol GmbH, Monheim, Germany). Thereafter, no iron deficiency was detected in any of the athletes.
Determination of Hb-mass and blood sampling was performed at the Frankfurt Airport Hospital immediately before departing to the ATCs and approx. 8 h after leaving the ATCs at Sierra Nevada and 16 h after leaving the ATC at Pretoria. Additionally, at ATC3 Hb-mass was measured in seven swimmers every sixth day at altitude and at ATC1 + 2 in eight swimmers up to 33 days after returning to sea level. Further data were collected from eight swimmers 24 and 62 days after an altitude camp at 2,100 m (Flagstaff, USA) and from the whole group at several time points without relation to altitude training camps, e.g., at the world championships at Melbourne in 2007 and 1 week before leaving for Beijing in 2008.
Measurement of tHb-mass
Hb-mass was measured using the optimized CO-rebreathing method, as described by Schmidt and Prommer (2005) and modified by Gore et al. (2006) and Prommer and Schmidt (2007). Briefly, the subjects inhaled a bolus of carbon monoxide (CO) followed by pure oxygen and rebreathed in a closed system (spirometer, SpiCo®, Bayreuth, Germany) for 2 min. The amount of inhaled CO was calculated by body mass (1.0 ml CO/kg for males and 0.8 ml/kg for females). Before and after the rebreathing period (at 6 and 8 min) two capillaries with 85 μl of arterialized blood each were taken from an ear lobe for analyzing carboxyhemoglobin (COHb) using a CO-hemoximeter (OSM3, Radiometer, Denmark). COHb was measured in six replicates before and after the inhalation period. During the whole observation period, three different hemoximeters were used. All of them were regularly checked by custom-made standard blood samples (see Gough et al. 2011) and no significant differences were detected (referred to Hb-mass, mean difference between the analyzer 1 and 2 = 1.2 g, between analyzer 1 and 3 = 4.8 g). A CO-analyzer (Draeger®, Luebeck, Germany) was used to check the spirometer for any leaks during the rebreathing period. To calculate the amount of CO not taken up during the inhalation time and the amount exhaled after the test, the remaining CO in the system and the end-tidal CO concentration were determined. In our mobile laboratory, the reliability of this method, determined during this study by test–retests, was characterized by a typical error (TE) of 1.3 % in male athletes and 1.5 % in female athletes. The corresponding 95 % confidence limits (CL) were 0.1, 2.5 and 0.1, 2.9 %, respectively.
The Hb-mass data are expressed as absolute values or as a percentage change of the absolute values over time. In the discussion also body mass-related data were used for comparison with the literature.
Blood analyses
Venous blood samples (in total 14 ml) were drawn from a cubital vein before and after the ATCs and at most of the other examination dates. In total, 4 ml was drawn into an EDTA tube to determine hemoglobin concentration ([Hb]), hematocrit (Hct) and reticulocyte count using several hematological analyzers [Advia 120 and 2120 (Siemens, Germany), Cell-Dyn 3700 (Abbott, Illinois, USA), Sysmex XE 2100D and XT-2000i (Kobe, Japan)]. The identical analyzer was always used for the hematological determinations before and after each ATC. To detect any systematic influence of the blood analyzers, an analysis of variance was performed that did not yield significant differences among the machines. Due to technical reasons, the samples obtained after ATC-3 could not be analyzed.
The OFF-score was calculated according to Gore et al. (2003) using the following formula (1):
where Hb is the hemoglobin concentration in g/L and ret is the percentage of reticulocytes.
In total, 10 ml of the venous blood was centrifuged, and the serum was used for measuring the concentrations of ferritin (immunoturbidimetry, Advia 2400, Siemens Healthcare Diagnostics GmbH, Germany; coefficient of variation (CV) 4 %), erythropoietin (EPO) (Immulite, Siemens Healthcare Diagnostics GmbH, Germany; CV 7 %), and the soluble transferrin receptor (sTFR) (Modular E, Roche Deutschland Holding GmbH, Germany; CV 4 %).
Swimming performance
All of the competition results obtained within the time period of 2 weeks before or after determining Hb-mass were included in the analysis of possible effects of altitude, changes in Hb-mass, and illness/injury on performance. To compare the results of different disciplines and swimming distances, the competition point system of the German Swimming Federation was applied. Using this system, the world record existing at the time of the most recent Olympic Games is set to 1,000, and the actual competition performance is calculated according to formula (2):
where wr = the world record valid at the most recent Olympic Games (in this study: Athens 2004), and t = competition time. Thus, a competition time below the world record is coded by >1,000 points, and a time slower than the record by <1,000 points.
During the whole period, the swimmers had to wear swimsuits of identical brand. Therefore, systematic influences on competition time due to changing suits can be excluded.
Statistics
Data are represented as the mean ± standard deviation (SD). For analysis, the Statistical Package for the Social Sciences for Windows (version 18, SPSS Inc., Chicago, IL) was used. For the calculation of mean changes during two or more training camps the average value of a swimmer was used if he/she participated more than once at these camps (e.g. Table 3). Paired t tests for equal variance were applied to compare results before, during, and after an altitude training camp as well as when comparing the data from identical subjects after ATC1 and the sea level training camp. In case more than 2 means were compared, a Bonferroni correction was used (see e.g. Figs. 4, 6).
Differences between groups, e.g., ATC2 versus sea level training camp, were assessed with an unpaired t test for equal variance. In cases for which the data did not fit a normal distribution (EPO concentration), the Wilcoxon test was used. Pearson’s correlation coefficient and standard error of estimate (SEE) was calculated to check the reproducibility of the changes in Hb-mass and serum [EPO] at repeated altitude training camps. Linear regression analysis was performed to evaluate a possible effect of changes in serum [EPO] at day 2 at altitude on Hb-mass changes during the ATCs.
To quantify the variation of Hb-mass with and without hypoxic influence the coefficient of variation (CV), the methodological noise (TE) and the biological effect were determined.
To take into account the number of Hb-mass measurements of an individual athlete, the CV was weighted according to the following formula (3):
where Varintra-ind is the intra-individual variance and DFind is the individual degree of freedom (number of measurements − 1).
To separate the biological effect (biological variance, BV) from the methodological noise (TE; TE = standard deviation of the difference in test-retest measurements divided by √2), which both account for the CV the following formula (4) was applied:
To determine any influences of the potential confounding factors sex, age, swimming distance, time after return from altitude, Hb-mass, and illness or injury on performance, an analysis of covariance (mixed model, heterogeneous autoregressive with main effects) was applied. Performance was defined as the point difference between the monitored competition from the mean of the points from the whole observation period obtained for the same discipline and distance in 25 or 50 m lanes. Sex, swimming distance (≤100 m, >100 m), return from altitude (0–14 days, 15–24 days, 25–35 days), and health status (no problem, ill ≥1 week, or injured ≥1 week) were set as factors, while age and Hb-mass were considered as covariates.
Results
Hb-mass at sea level
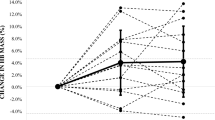
The variation of the Hb-mass at sea level over the 2-year period was 3.0 % in male and 2.7 % in female swimmers when altitude effects (until 45 days after return) and effects of illness/injury were excluded. The biological variation (BV), e.g. due to different training periods, was calculated to be 2.7 % in males and 2.2 % in females. Figure 2 presents the normal variation over 2 years and illustrates that these data constitute a valid baseline for quantifying and judging the altitude effect on Hb-mass.
Individual oscillation in tHb-mass over a period of 2 years. Each point presents the percentage difference of Hb-mass from the individual mean value obtained during a 2-year observation period without hypoxic influences (indicated as 100 %-line). Sea level values are indicated by a filled circle, altitude values acquired immediately after return are indicated by an open triangle (2,320 m) or an open square (1,360 m). Each number depicted on the x axis represents one swimmer
Hb-mass at altitude
Total hemoglobin mass was increased after the altitude training camps at Sierra Nevada by 7.2 ± 3.3 %, which represented the mean increase of all 3 ATCs (the range was between 6.5 ± 4.5 % for ATC1 and 8.6 ± 2.6 % for ATC3). Hb-mass increased after the ATC at Pretoria (ATC4) by 3.8 ± 3.4 %, which was significantly different from the increase at Sierra Nevada (p < 0.001, for mean absolute values, see Table 3). The altitude effects were very similar when evaluated in a subgroup (n = 5) of athletes who participated at ATC1 and 3 months later at a sea level training camp (STC, see Fig. 3) or when compared between the participants of ATC2 (n = 10) and the matched control group (STC; n = 11; see Fig. 3).
Changes in Hb-mass after altitude (ATC) and sea level (STC) training camps. I: Comparison of the ATC1 (2,320 m) and STC effects in the same athletes (n = 5). II: Comparison of the ATC2 (2,320 m) and STC effects in matched altitude (n = 10) and control (n = 11) groups. III: Comparison between healthy (H) and sick or injured (S) subjects at 2,320 m (H: n = 29, S: n = 7) and at 1,360 m (H: n = 10, S: n = 1). The data are represented as the mean (±SD) percentage difference from sea level baseline values. Significant differences between the presented groups: *p < 0.05, ***p < 0.001
The CVs calculated for the change in Hb-mass during the altitude training camps were considerably higher at 2,320 m than at sea level and did not significantly differ between males and females (4.9 % in males, 6.2 % in females). The BV was 4.7 and 6.0 % in males and females. After the camps at 1,360 m the CVs were lower than at 2,320 m ranging between 4.5 % (males) and 2.5 % (females) and the BV was 4.3 and 2.1 %.
The altitude effects, therefore, obviously exceeded the normal oscillation at sea level which is presented in Fig. 2.
Blood parameters
[Hb] and Hct tended to increase after the ATCs at 2,320 m; however, the increase was only significant for [Hb] in the male group. Except reticulocytes the other markers as ferritin, serum concentrations of sTFR, and of EPO suggest accelerated erythropoiesis at 2,320 m. Also the higher MCHC after the ATCs at 2,320 m indicate a younger erythrocyte population. [EPO] showed the expected changes over time and no sex-related differences were observed (Fig. 4). Despite a slight increase in Hb-mass after the ATC at 1,360 m, no significant differences were detected for the other hematological parameters determined immediately after return from Pretoria (Table 3).
Sex differences
At 2,320 m, the increase of the absolute Hb-mass after the ATCs tended to be higher in the male than in the female swimmers; this finding is obviously due to their higher baseline values (see Table 3). The percentage change did not differ between the groups (men: 7.1 ± 2.1 %; women: 8.5 ± 3.9 %), indicating a similar reaction to altitude in men and women (Fig. 5). In contrast, at 1,360 m, the percentage increase was significantly weakened in the female group (men: 6.0 ± 3.2 %; women: 2.3 ± 2.9 %, difference: p ≤ 0.05).
Individual changes in Hb-mass of healthy male and female athletes as well as of injured and sick athletes during a training camp at 2,320 m. Demonstrated are the percentage differences from sea level baseline values determined immediately before leaving to altitude. In addition, the mean differences ± SD are shown for each group. Significant difference between the healthy groups and the sick/injured group: ***p < 0.001
Reproducibility of changes in [EPO] and Hb-mass
Eight out of the 19 athletes from ATC1 also participated at ATC2. The increase in [EPO] at day 2 was highly reproducible, which is indicated by the highly significant correlation coefficient of r = 0.945 (p < 0.001, SEE = 1.2). In contrast, there was only a very weak trend toward a reproducible Hb-mass response at the different ATCs (r = 0.379, p = 0.160, SEE = 19.4, n = 14). Linear regression analysis did not yield any relationship between the increase in serum [EPO] at day 2 and the change in Hb-mass after return from altitude (r = 0.05, p = 0.82, SEE 30.9, n = 21).
Illness/injury
In total, eight swimmers (7 swimmers at 2,320 m, 1 swimmer at 1,360 m; see Table 2) were not able to participate at all of the altitude training sessions because of sickness or injuries. Although they stayed at altitude for the same time as the healthy athletes, only a slight or no increase in Hb-mass occurred in the sick/injured athletes (see Figs. 3, 5).
Time course of adaptation and de-adaptation
In the course of the third ATC (2,320 m), Hb-mass was measured before, at day 6, 13, 19, and immediately after the end of the ATC. There were no changes during the first 6 days; thereafter, Hb-mass increased by approximately 0.5 % per day until the end of the ATC. Very different responses were observed among the individual athletes (Fig. 6).
Individual changes in Hb-mass during a 3-week long altitude training camp at 2,320 m (ATC3). The solid squares indicate the mean values of the healthy subjects (male: n = 4, female: n = 2). The open squares represent the data of an injured athlete. The data shown are percentage differences from sea level baseline values. Significant differences from baseline values: *p < 0.05, **p < 0.01
24.3 ± 6.0 days after ATC1 and ATC3, 46.5 ± 34.6 % of the altitude-related gain in Hb-mass was lost and the Hb-mass value was still 4.1 ± 2.9 % above baseline (p < 0.01, Fig. 7). Very similar effects were found in eight swimmers 24 days after return from Flagstaff (2,100 m). In these athletes Hb-mass was increased by 4.0 ± 2.8 % compared to the values at day 62.
Individual changes in tHb-mass after ATC 1 and 3 at Sierra Nevada (SN 2,320 m) and after an ATC at Flagstaff (FS 2,100 m). Hb-mass was determined before, immediately after, and 19, 26, or 33 days after return from Sierra Nevada (open circles) as well as 24 and 62 days after return from Flagstaff (solid circles). The baseline values determined immediately before the ATCs at Sierra Nevada and the values determined 62 days after return from the ATC at Flagstaff are both set as 100 %. This presentation is used because both of these values similarly correspond to the mean values of the 2-year observation period of the respective athletes (before Sierra Nevada: 98.1 ± 2.1 %, 62 days after Flagstaff: 98.7 ± 1.5 %) and, therefore, represent valid baseline values
Performance
Out of the influencing factors tested during the whole observation period, Hb-mass, health status (illness as well as injury), and time after return from altitude had significant effects on competition performance (Table 4). A change in Hb-mass by 1 % changed performance by 1.8 points. Participation at an ATC tended to decrease performance by 11 points until 14 days after return, had no effect the following 10 days, and significantly improved performance by 23 points 25–35 days after return. Illness reduced swimming performance by 25 points and injury by 16 points. Swimming distance (p = 0.16), sex (p = 0.86), and age (p = 0.23) did not interfere with performance. Combining the effects of swimming distance with Hb-mass or time after return from altitude did not reveal any significant influence on performance.
Discussion
The particular characteristic of the present study is the examination of altitude training effects on Hb-mass in comparison with the normal Hb-mass oscillation in normoxia for a time period of 2 years. Additionally, detailed information on confounding influences on the effect of altitude on Hb-mass, i.e., sex, illness and injury, are provided as well as the time course of acclimatization and re-acclimatization of Hb-mass during and after altitude exposure. Finally, data on performance after return from altitude are presented.
Altitude training effects on Hb-mass over the course of a 2-year training period
The mean body mass-related Hb-mass in normoxia was 12.7 g kg−1 in the male and 10.7 g kg−1 in the female athletes, which corresponds to the data for swimmers described in the literature (Friedmann et al. 2005; Heinicke et al. 2001) and ranges between the values for sedentary subjects [females ~9 g kg−1 and males ~10 g kg−1 and elite endurance athletes such as runners, cyclists, and biathletes (females ~13 g kg−1 and males ~15 g kg−1)] (Garvican et al. 2010b; Gore et al. 1997; Heinicke et al. 2001, 2005 Prommer and Schmidt 2009). The reason for not reaching the high values observed in other endurance disciplines may be the supine posture combined with the stay in water during the training hours. Both effects remarkably reduce the venous pooling of blood in the legs leading to a blood shift to the thorax, to an increased diastolic filling of the ventricles, and to a higher stroke volume. Swimmers, therefore, need a lower blood volume to fill the active circulation. Their central volume load during swimming is counter-regulated by an increased diuresis which subsequently leads to a reduced training-related expansion of blood volume and possibly also of Hb-mass (Böning et al. 1988).
For the first time, this study also describes the variability in Hb-mass in elite swimmers over the course of up to two seasons and thereby provides the baseline data necessary to evaluate the hematological changes occurring at altitude. When analyzing data obtained exclusively without hypoxic influences, the weighted CVs of 2.7 % (females) and 3.0 % (males), correspond well to data from longitudinal studies describing a CV of 3.3 % in elite female cyclists (Garvican et al. 2010b) and 2.2 % in male and female leisure athletes (Prommer et al. 2008) over the course of one training season. And they are even slightly smaller than the results of Eastwood et al. (2012) observed over a whole year in elite endurance athletes (3.4 % males, 4.0 % females). This small variation is partly due to the methodological noise, which is characterized by a typical error of 1.3 and 1.5 % in males and females, and to biological effects between 2.7 % (males) and 2.2 % (females). Training influences, being approximately 1 % during one season in elite athletes (Garvican et al. 2010b) are probably the main reasons.
When using the long-term mean value from normoxia as a reference, Hb-mass was increased by 5.9 and 2.8 % after return from 2,320 to 1,360 m, respectively (for individual data see Fig. 2). These data, therefore, show that altitude training at 2,320 m induces an objective increase in Hb-mass compared to the normal oscillation occurring under normoxic conditions.
The immediate altitude effect becomes obvious when analyzing the pre and post-altitude data, which showed mean Hb-mass increases after the 3 ATCs at 2,320 m of between 6.5 and 8.6 %. When comparing the altitude data from ATC1 to ATC2 with those of the sea level control group (STC), the altitude effect was 7.4 % when the identical athletes were used in a fully controlled crossover trial and 6.9 % when a matched group was used in a controlled crossover trial. These effects are in the same range as described by Friedmann et al. (2005) for elite junior swimmers. They are, however, considerably higher than the changes observed in cyclists and runners after natural ATCs or LH-TL protocols (>14 h/day of hypoxia) (Clark et al. 2009; Garvican et al. 2010a; Robertson et al. 2010). One reason for the different response may be the lower baseline Hb-mass in swimmers before starting the altitude training, which may allow higher increases before the maximum individual limit is achieved. Our results are in line with most of the hitherto conducted altitude training studies. They are, however, in a clear contrast to the recently published data of Siebenmann et al. (2012) showing no change in a group of cyclists after a 4-week LH-TL protocol with >16 h in hypoxia per day.
The slight but significant increase in Hb-mass of the male swimmers after the ATC at 1,360 m seems to be in contrast to the prevalent scientific opinion suggesting that the erythropoietic threshold is above 1,600 m (Weil et al. 1968). In a recent publication, however, Frese and Friedmann-Bette (2010) demonstrated a very similar increase in Hb-mass (+5.1 %) after repetitive training camps at 1,500 and 1,360 m, while no significant change was observed after a single camp. Interestingly, the male swimmers and the two female swimmers who showed an increase in Hb-mass above 4 % regularly participated at the previous altitude training camps which let us assume a similar adaptation mechanism as described by Frese and Friedmann-Bette (2010).
Individual responses and reproducibility
The individual response of Hb-mass to the hypoxic influence was very heterogeneous, ranging between −2 and +13 % (see Fig. 5). In accordance with Friedmann et al. (2005), but in contrast to Chapman et al. (1998) the latter showing a close positive relationship between changes in Hb-mass after return from altitude to plasma EPO concentration on the second day at altitude—we did not find any difference of the plasma [EPO] in responders and non-responders to altitude. In our study, the initial EPO response to altitude after 2 days was highly reproducible at two different ATCs (r = 0.905, p < 0.01), which coincides with data from Friedmann et al. (2005) showing similar individual EPO reactions in artificial and natural hypoxia. However, in our study, there was no detectable relationship between changes in plasma [EPO] at any time at altitude and Hb-mass after return.
In this study, we present data on the individual reproducibility of the Hb-mass response at repeated natural altitude training camps. A previous study (Robertson et al. 2010) has shown a reproducible increase of the mean Hb-mass in a group of runners after 2- and 3-week LH-TL protocols, but this finding was not confirmed for individual athletes. Also in our study, we found a reproducible mean response and only a small trend toward similar individual reactions (p = 0.16), i.e. the altitude response varies due to inter-individual as well as intra-individual conditions. The physiological reason for the intra-individual scattering may be different baseline conditions due to different training states or health conditions. However, when comparing the absolute Hb-mass values of each athlete immediately after return from the altitude training camps, there was only small individual scattering (CV = 2.1 %), which is slightly above the methodological noise of the CO-rebreathing method (TE ~ 1.4 %) and presents a biological variation of 1.6 %. Therefore, we may assume that 3–4 weeks of altitude increases Hb-mass up to an individual limit (Gore et al. 1998) and that the prevailing erythropoietic response may vary depending on the specific individual baseline conditions.
Sex-specific effects
In contrast to our expectation, we did not find any significant differences in the percentage increase in Hb-mass between men and women at 2,320 m (men: 7.1 ± 2.1 %, women: 8.5 ± 3.9 %). Also plasma EPO levels showed nearly identical time courses at altitude for both sexes (see Fig. 4). One possible explanation for the relatively high female hypoxic response may be the disturbance of the menstrual cycle due to reduced steroid hormone production that is frequently observed in elite endurance athletes (De Souza 2003). Interestingly, the two female swimmers showing the highest increase in Hb-mass (>10 %) reported irregular menstrual cycles before and/or during the altitude training camp. At 1,360 m, the female athletes did not react with the same magnitude as the male swimmers. While the significance of this result is limited due to the low number of subjects (males n = 4, females n = 6), it hints at the result we initially expected.
Illness/injury
In this study, we also demonstrate a lack of erythropoietic response in athletes who are sick or injured during altitude training. For sea level conditions, we recently showed a decrease in Hb-mass in elite endurance athletes by ~40 g after more than 2 weeks of severe inflammation, viral infection, and injury (Schmidt et al. 2011). A dramatic drop in Hb-mass after acute injury has also been described by (Schumacher et al. 2008) in a case report. We assume this suppression to be mediated by a lower responsiveness of erythropoietic precursor cells and/or by lower renal EPO production due to the activation of pro-inflammatory cytokines (Morceau et al. 2009).
The fact that Hb-mass did not decrease in ill and injured athletes during the ATCs suggests that erythropoietic stimulation mediated by altitude exposure compensates for the inhibitory effects occurring at sea level.
Time course of adaptation and de-adaptation
In agreement with the studies of Clark et al. (2009) and Garvican et al. (2010a), we did not observe an increase in Hb-mass within the first week at altitude, which is due to the maturing time (5–7 days) of the red cell precursor cells within the bone marrow tissue (Lewis 1989). The first significant increase by ~5 % after 13 days also fits well with the results of Garvican et al. (2010a), showing a gain in Hb-mass of 3 % after 11 days at altitude. In our study, the mean weekly expansion of Hb-mass by 2.8 % clearly exceeds the values of Clark et al. (2009) (1 %) and Garvican et al. (2010a) (1.4 %), which may be due to the different athlete groups investigated in these studies and to the different hypoxic protocols. The absence of a plateau after 3 weeks further indicates that a complete adaptation of the erythropoietic system had not yet been achieved. However, as demonstrated by the 11 % higher Hb-mass values in elite athletes native to 2,600 m compared to sea level athletes (Schmidt et al. 2002), only small additional effects may be expected when the time at altitude is extended. We also did not find any difference in the Hb-mass response when athletes stayed for 3 or for 4 weeks at altitude, which is probably caused by the different inter- and intra-individual hypoxic reactions. From the data presented here, we may conclude that 3–4 weeks may be sufficient to exploit most of the erythropoietic potential of altitude training. This assumption has, however, to be confirmed by longer studies of e.g. 6–8 weeks duration.
After return from altitude, hemoglobin concentration decreases within some days which is at least partly due to a fast plasma volume expansion (Böning et al. 1997). One also might expect a rapid decrease in Hb-mass due to decreased serum [EPO], which in turn may lead to neocytolytic processes, as described by (Rice et al. 2001) after the rapid descent of subjects native to high altitude (4,500 m) to sea level. When elite Kenyan runners native to ~2,100 m, however, descended to sea level, no change in Hb-mass occurred within the first 2 weeks, followed by a continuous drop by 6 % until day 33 (Prommer et al. 2010). After return from altitude training camps or after finishing LH-TL protocols, baseline values were similarly achieved within 4–6 weeks (Ashack et al. 1985; Brugniaux et al. 2006). More closely monitored studies (Clark et al. 2009) did not observe any decrease in Hb-mass within 1 week while Garvican et al. (2010a) described a neocytolytic-like drop by 1.5 % within 2 days and no further decrease in the following 9 days after return from altitude. In our study, 3 weeks after return from ATCs at 2,320 m ~50 % of the gained Hb-mass was still present, which agrees perfectly with the time course observed in the Kenyan runners and closes the gap that still existed in our knowledge about Hb-mass after the return of sea level athletes from altitude training camps. Summarizing all available studies, we can predict a small drop in Hb-mass immediately after the return from altitude, an elevated plateau for the following 10 days, a sustained gain of 50 % after ~3 weeks, and values that return to baseline after ~5 weeks.
Swimming performance
Our results agree with the anecdotal reports on an immediate decrease in performance after return from altitude and a delayed performance peak after several weeks by showing a small trend toward lower performance until 14 days, unchanged performance at 14–24 days, and a significant increase in performance of 23 points between 25 and 35 days after return (see Table 4). Our results also confirm data of Gough et al. (2011) demonstrating a significant drop in competition performance by 1–2 % on days 1 and 7 after 3-week long natural and LH-TL altitude training protocols, despite a 4 % increase in Hb-mass values. In their study, 14 and 28 days later, baseline performance was achieved.
The reasons for the decrease in performance during the first 2 weeks after return are not yet evaluated and may be a lower buffer capacity due to lower bicarbonate and non-bicarbonate buffer systems (Böning et al. 2001). Also the re-adaptation of several endocrinological systems, as e.g. of aldosterone and EPO, may play a role.
The increase of 23 points corresponds to an improvement in swimming time for “freestyle men” (baseline value = 1,000 points or 47.84 s) of 0.37 s or 0.8 %, which is above the smallest worthwhile performance enhancement for elite athletes (Bonetti and Hopkins 2009; Hopkins 2005; Trewin et al. 2004). This result, however, has to be cautiously interpreted because it derives from only 17 competitions of 4 athletes during the 24–35 day period, and a very high individual response to altitude has to be assumed. Interestingly, our data coincide with unpublished results from our group demonstrating the best performance of elite swimmers (+2.8 %, n = 4) in a 200 m step test 3–5 weeks after a 3-week altitude training camp at 2,310 m (Mexico City). In both studies, the number of athletes was low, but the similar results obtained with two completely different methods support our conclusion of best performance 3–5 weeks after return from altitude.
Hb-mass exerted a small but significant influence on competition performance over the whole study duration, i.e. including also periods without altitude influences. As indicated in Table 4, the change of 1 % in Hb-mass, i.e., ~11 g in a male swimmer, increases performance by 1.8 points. The mean increase of 6.5 % immediately after the ATCs should, therefore, improve performance by 11.7 points corresponding to a 0.4 % increase in swimming speed (see the example above). The physiological mechanisms leading to slightly worse performance immediately after return despite elevated Hb-mass cannot be explained by this study. However, after 3–4 weeks, 50 % of the total gain in Hb-mass is still present and may contribute to peak performance at this time. The underlying mechanism may be that buffer capacity and hormonal status have been normalized and the higher Hb-mass enables a higher training volume and intensity as well as a reduced time for recovery after the training sessions.
Conclusions
Monitoring Hb-mass in elite swimmers over a period of 2 years shows low oscillations when hypoxic influences are excluded. i.e. elite athletes cannot increase Hb-mass by sea level training. Participation at natural altitude training camps at ~2,300 m for 3–4 weeks clearly elevates the mean Hb-mass above the normal oscillation at sea level, although the results show variable responses. The erythropoietic activity at altitude seems to depend on baseline fitness conditions and Hb-mass increases up to an individual limit. No difference between male and female athletes is detected; altitude training is, therefore, suitable for both sexes. Because illness and injury blunt the erythropoietic response poor health status may be one important reason for athletes non-responding to altitude.
Competition performance tends to be slightly reduced (−0.4 %) immediately after return from altitude and is best (+0.8 %) 3–5 weeks thereafter. Over the whole season, Hb-mass is positively related to performance, but its role after return from altitude remains unclear. We, therefore, recommend performing regular natural altitude training camps as part of a long-term training program and well-timed ATCs scheduled 3–5 weeks before important competitions.
References
Ashack R, Farber MO, Weinberger M, Robertson G, Fineberg NS, Manfredi F (1985) Renal and hormonal responses to acute hypoxia in normal individuals. J Lab Clin Med 106:12–16
Bonetti DL, Hopkins WG (2009) Sea-level exercise performance following adaptation to hypoxia: a meta-analysis. Sports Med 39:107–127
Böning D, Mrugalla M, Maassen N, Busse M, Wagner TOF (1988) Exercise versus immersion: antagonistic effects on water and electrolyte metabolism during swimming. Eur J Appl Physiol 57:248–253
Böning D, Maassen N, Jochum F, Steinacker J, Halder A, Thomas A, Schmidt W, Noé G, Kubanek B (1997) After-effects of a high altitude expedition on blood. Int J Sports Med 18:179–185
Böning D, Maassen N, Thomas A, Steinacker JM (2001) Extracellular pH defense against lactic acid in normoxia and hypoxia before and after a Himalayan expedition. Eur J Appl Physiol 84:78–86
Brugniaux JV, Schmitt P, Robach P, Nicolet G, Fouillot J-P, Moutereau S, Lasne F, Pialoux V, Saas P, Chorvot J, Cornolo J, Olsen NV, Richalet J-P (2006) 18 days of “living high, training low” stimulate erythropoiesis and enhance aerobic performance in elite middle-distance runners. J Appl Physiol 100:203–211
Chapman RF, Stray-Gunderson J, Levine BD (1998) Individual variation in response to altitude training. J Appl Physiol 85:1448–1456
Clark SA, Quod MJ, Clark MA, Martin DT, Saunders PU, Gore CJ (2009) Time course of haemoglobin mass during 21 days live high: train low simulated altitude. Eur J Appl Physiol 106:399–406
Cristancho E, Reyes O, Serrato M, Mora MM, Rojas JA, Robinson Y, Boning D (2007) Arterial oxygen saturation and hemoglobin mass in postmenopausal untrained and trained altitude residents. High Alt Med Biol 8:296–306
De Souza MJ (2003) Menstrual disturbances in athletes: a focus on luteal phase defects. Med Sci Sports Exerc 35:1553–1563
Eastwood A, Sharpe K, Bourdon PC, Woolford SM, Saunders PU, Robertson EY, Clark SA, Gore CJ (2012) Within subject variation in hemoglobin mass in elite athletes. Med Sci Sports Exerc 44(4):725–732
Frese F, Friedmann-Bette B (2010) Effects of repetitive training at low altitude on erythropoiesis in 400 and 800 m runners. Int J Sports Med 31:382–388
Friedmann B, Frese F, Menold E, Kauper F, Jost J, Bärtsch P (2005) Individual variation in the erythropoietic response to altitude training in elite junior swimmers. Br J Sports Med 39:148–153
Friedmann-Bette B (2008) Classical altitude training. Scand J Med Sci Sports 18:11–20
Garvican L, Martin D, Quod M, Stephens B, Sassi A, Gore C (2010a) Time course of the hemoglobin mass response to natural altitude training in elite endurance cyclists. Scand J Med Sci Sports 22:95–103
Garvican LA, Martin DT, McDonald W, Gore CJ (2010b) Seasonal variation of haemoglobin mass in internationally competitive female road cyclists. Eur J Appl Physiol 109:221–231
Gore CJ, Hahn AG, Burge CM, Telford RD (1997) VO2max and haemoglobin mass of trained athletes during high intensity training. Int J Sports Med 18:477–482
Gore CJ, Craig N, Hahn A, Rice A, Bourdon P, Lawrence S, Waish C, Stanef T, Barnes P, Parisotto R, Martin D, Pyne D (1998) Altitude training at 2,690 m does not increase total haemoglobin mass or sea level VO2max in world champion track cyclist. J Sci Med Sport 1:156–170
Gore CJ, Parisotto R, Ashenden MJ, Stray-Gundersen J, Sharpe K, Hopkins W, Emslie KR, Howe C, Trout GJ, Kazlauskas R, Hahn AG (2003) Second-generation blood tests to detect erythropoietin abuse by athletes. Haematologica 88:333–344
Gore CJ, Bourdon PC, Woolford SM, Ostler LM, Eastwood A, Scroop GC (2006) Time and sample site dependency of the optimized CO-rebreathing method. Med Sci Sports Exerc 38:1187–1193
Gough CE, Sharpe K, Ashenden MJ, Anson JM, Saunders PU, Garvican LA, Bonetti DL, Gore CJ, Prommer N (2011) Quality control technique to reduce the variability of longitudinal measurement of hemoglobin mass. Scand J Med Sci Sports 21:365–371
Heinicke K, Wolfahrt B, Winchenbach P, Biermann B, Schmid A, Huber G, Friedmann B, Schmidt W (2001) Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med 22:504–512
Heinicke K, Heinicke I, Schmidt W, Wolfarth B (2005) A three-week traditional altitude training increases hemoglobin mass and red cell volume in elite biathlon athletes. Int J Sports Med 26:350–355
Hopkins WG (2005) Competitive performance of elite track-and-field athletes: variability and smallest worthwhile enhancements. Sportscience 9:17–20
Levine BD, Sray-Gunderson J (1997) “Living high-training low”: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol 83:102–112
Lewis SM (1989) Erythropoiesis. In: Hoffbrand AV, Lewis SM (eds) Postgraduate hematology, 3rd edn. Heinemann, London, pp 1–25
Millet GP, Roels B, Schmitt L, Woorons X, Richalet JP (2010) Combining hypoxic methods for peak performance. Sports Med 40:1–25
Morceau F, Dicato M, Diederich M (2009) Pro-inflammatory cytokine-mediated anemia: regarding molecular mechanisms of erythropoiesis. Mediators Inflamm, Article ID405016, p 11. doi:10.1155/2009/405016
Prommer N, Schmidt W (2007) Loss of CO from the intravascular bed and its impact on the optimised CO-rebreathing method. Eur J Appl Physiol 100:383–391
Prommer N, Schmidt W (2009) Hämoglobinmenge und Sport. Dtsch Z Sportmed 60:293–294
Prommer N, Sottas PE, Schoch C, Schumacher YO, Schmidt W (2008) Total hemoglobin mass-a new parameter to detect blood doping? Med Sci Sports Exerc 40:2112–2118
Prommer N, Thoma S, Quecke L, Gutekunst T, Völzke C, Wachsmuth N, Niess A, Schmidt W (2010) Total hemoglobin mass and blood volume of elite Kenyan runners. Med Sci Sports Exerc 42:791–797
Rice L, Ruitz W, Driscoll T, Whitley CE, Tapia R, Hachey DL, Gonzales GF, Alfrey CP (2001) Neocytolysis on descent from altitude: a newly recognized mechanism from the control of red cell mass. Ann Intern Med 134:652–656
Robertson EY, Saunders PU, Pyne DB, Aughey RJ, Anson JM, Gore CJ (2010) Reproducibility of performance changes to simulated live high/train low altitude. Med Sci Sports Exerc 42:394–401
Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ (2000) Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc 32:332–348
Schmidt W, Heinicke K, Rojas J, Gomez JM, Serrato M, Mora M, Wolfarth B, Schmid A, Keul J (2002) Blood volume and hemoglobin mass in endurance athletes from moderate altitude. Med Sci Sports Exerc 34:1934–1940
Schmidt W, Prommer N (2005) The optimised CO-rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol 95:486–495
Schmidt W, Prommer N (2008) Effects of various training modalities on blood volume. Scan J Med Sci Sports 18:57–69
Schmidt W, Prommer N (2010) Impact of alterations in total hemoglobin mass on VO2max. Exerc Sport Sci Rev 38:68–75
Schmidt W, Völzke C, Wachsmuth N, Wolfarth B, Schmidt-Trucksäss A, Steinacker J, Treff G, Gundersen J, Eastwood A, Prommer N (2011) Variation of hemoglobin mass in elite Endurance athletes. Med Sci Sports Exerc 43(5 Supplement):437
Schoene RB, Robertson HT, Pierson DJ, Peterson AP (1981) Respiratory drives and exercise in menstrual cycles of athletic and nonathletic women. J Appl Phys 50:1300–1305
Schumacher YO, Ahlgrim C, Ruthardt S, Pottgiesser T (2008) Hemoglobin mass in an elite endurance athlete before, during, and after injury-related immobility. Clin J Sport Med 18:172–173
Siebenmann C, Robach P, Jacobs RA, Rasmussen P, Nordsborg N, Diaz V, Christ A, Olsen NV, Maggiorini M, Lundby C (2012) “Live high-train low” using normobaric hypoxia: a double-blinded, placebo-controlled study. J Appl Physiol 112:106–117
Trewin CB, Hopkins WG, Pyne DB (2004) Relationship between world-ranking and Olympic performance of swimmers. J Sports Sci 22:339–345
Weil JV, Jamieson G, Brown DW, Grover RF, Balchum OJ, Murray JF (1968) The red cell mass—arterial oxygen relationship in normal man. J Clin Investig 47:1627–1639
Acknowledgments
The project was financially supported by the World-Anti-Doping-Agency (WADA, grant no. 05A5FS) and the German Federal Institute of Sports Sciences (BISp, grant no. IIA1-070309/08). We thank all of the swimmers and their trainers for their excellent compliance. We especially acknowledge the cooperation with Ørjan Madsen, the head coach and technical director of the German swimming federation. We would also like to thank the administration and the staff of the Frankfurt Airport Hospital for providing their laboratory as well as the medical head of the Olympic Centre Heidelberg Prof. Dr. Friedmann-Bette, and of the IAT Leipzig, Dr. Berbalk.
Conflict of interest
No conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Guido Ferretti.
Rights and permissions
About this article
Cite this article
Wachsmuth, N.B., Völzke, C., Prommer, N. et al. The effects of classic altitude training on hemoglobin mass in swimmers. Eur J Appl Physiol 113, 1199–1211 (2013). https://doi.org/10.1007/s00421-012-2536-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2536-0