Abstract
A routine method to determine total haemoglobin mass (tHb) in clinical practice and sports medicine is non-existent. Radioactive tracers or other dilution procedures like the common CO-rebreathing method (Proccom) are impractical, the latter in particular because of the relatively long time of respiration. According to the multicompartment model of Bruce and Bruce (J Appl Physiol 95:1235–1247, 2003) the respiration time can be considerably reduced by inhaling a CO-bolus instead of the commonly used gas mixture. The aim of this study was to evaluate this theoretical concept in practice. The kinetics of the HbCO formation were compared in arterialised blood sampled from an hyperaemic earlobe after inhaling a CO-bolus (Procnew) for 2 min and a CO–O2 mixture (Proccom) for ∼10 min. The reliability of Procnew was checked in three consecutive tests, and phlebotomy was used to determine the validity. VO2max was determined with and without previous application of Procnew and the half-time of HbCO was registered also in arterialised blood after resting quietly and after the VO2max test. Procnew yielded virtual identical tHb values compared to Proccom when HbCO determined 5 min after starting CO-rebreathing was used for calculation. The typical error of Procnew was 1.7%, corresponding to a limit of agreement (95%) of 3.3%. The loss of 95 g (19) haemoglobin was detected with an accuracy of 9 g (12). After application of Procnew VO2max was reduced by 3.0% (3.7) (P=0.022) and half-time was lowered from 132 min (77) to 89 min (23) after the VO2max test. Inhaling a CO-bolus markedly simplifies the CO-rebreathing method without reducing validity and reliability and can be used for routine determination of tHb for various indications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In clinical practice as well as in the field of sports medicine the routine determination of total haemoglobin mass and blood volume is valuable (Barker 1998). Beside the radioactive methods, i.e. radioactive labelling by 51Cr or other radioactive markers, which are considered to be the gold standard (International Committee for Standardization in Haematology 1980), less harmful dilution techniques have been developed. These are methods using Evans blue (Gibson and Evans 1937), hydroxy-ethyl-starch (Tschaikowsky et al. 1997), indocyanine green (He et al. 1998) or carbon monoxide (CO) as markers (Douglas et al. 1912; Arnold et al. 1921). The CO-rebreathing method was revived by Fogh-Andersen et al. (1990) and Thomsen et al. (1991) and is till now frequently used in sports medicine to determine the effects of training and altitude exposure (Gore et al. 1997; Ashenden et al. 1999; Heinicke et al. 2001; Böning et al. 2001). Since that time, however, the method was subjected to various improvements and refinements. The major aim of these improvements was to obtain precise results as was achieved by Burge and Skinner (1995) and Hütler et al. (2000). A close relationship to the 51Cr method (r=0.98; Thomsen et al. 1991), a high sensitivity [changes in the range of 1.5% in total Hb-mass (tHb-mass); Burge and Skinner 1995], and a low test–retest variability (between 0.8 and 2.5%; Burge and Skinner 1995; Heinicke et al. 2001) were demonstrated.
The practical demand on a method to determine tHb-mass routinely, however, includes further aspects. Beside the overriding need for accuracy and reliability it must not be harmful or dangerous and should have minimal effects on physical performance and well being. Ideally it should be inexpensive, easy to handle in the laboratory, minimally invasive and of low inconvenience for the patient or athlete.
The principle of the CO-rebreathing method used up to now is to inspire an O2–CO gas mixture for about 10–15 min. From the difference in carboxyhaemoglobin concentration (HbCO) before and after rebreathing, the volume of CO and the binding capacity of Hb for CO (1.39 ml g−1), tHb-mass can be derived. Although the precision of this common procedure (Proccom) is high, the disadvantages are the relatively long time of respiration (10 min, Burge and Skinner 1995; 15 min, Heinicke et al. 2001); 2 × 10 min, Gore et al. 1997) and the count of blood samples (in most studies approximately eight), which are in the majority of cases taken by a venous catheter (e.g. Burge and Skinner 1995; Friedmann et al. 1999; Heinicke et al. 2001). This procedure is inconvenient for the subject and makes it impractical to apply this method routinely to a high number of patients or athletes.
To minimise these disadvantages, we modified the breathing procedure based on the multicompartment model of Bruce and Bruce (2003). They proposed that the time of CO inhalation can be reduced to approximately 2 min by administration of a CO-bolus leading to a faster uptake and thereby distribution of CO within the cardiovascular system. Accordingly, we hypothesised that a 2 min bolus application of CO, hereafter referred to as the new procedure (Procnew), would obtain the same tHb-mass as Proccom without reducing precision. This would lead to a marked acceleration and simplification making the method suitable for routine application in sports’ medical and clinical practice. To evaluate the applicability of the Procnew, especially to endurance athletes, the effects of the CO-rebreathing procedure on physical performance and the half-time of HbCO in blood were also tested.
Methods
To compare the new procedure (Procnew) with the commonly used CO-rebreathing procedure (Proccom) and to quantify its reproducibility, tHb-mass was measured in 11 subjects four times (study I). In a second study the validity of the method was tested in five subjects by determination of tHb-mass before and after phlebotomy of 550 ml blood. A third study was carried out to evaluate the influence of the application of the Procnew on VO2max and to determine the HbCO half-time (t 1/2) in blood. The studies were approved by the Human Ethics Committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany.
All subjects (Table 1) were moderately trained students. They gave their written consent after they were informed about the studies and the corresponding risks. None of the subjects participated in more than one study.
Study I
Four tests were performed: one using the Proccom and three using the Procnew. The Proccom was carried out according to Heinicke et al. (2001) and Hütler et al. (2000). Briefly, after 15 min in a sitting position (to allow plasma volume to stabilise) the subjects were connected to a Krogh-spirometer (Student Spirometer, ZAK, Germany) filled with a mixture of O2 (9.6 l, of which 4.6 l was the volume of the connecting tubes) and CO (1.0 ml kg−1 for trained males, 0.8 ml kg−1 for untrained males, 0.7 ml kg−1 for trained females and 0.6 ml kg−1 for untrained females). Arterialised blood samples were taken from an earlobe via a capillary and analysed immediately (ABL 520, Radiometer, Denmark) twice before and every 2 min during the respiration of the gas mixture until reaching a plateau of HbCO. The CO volume remaining in the spirometer system was calculated after disconnecting the subject by determining spirometer volume and CO concentration.
Beginning 5 days after this reference test (Proccom) all subjects performed three further tests using the Procnew with a mean time lag of 5 days between consecutive tests. Two of the Procnew procedures were identical (test-1 and test-2). The third Procnew test (test-3) was performed 10 min after a 1 min pre-inspiration of 15 ml CO to minimise CO loss from Hb to myoglobin (Mb) during the test as was assumed by Burge and Skinner (1995).
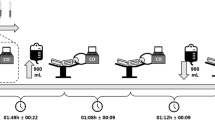
For the Procnew a new spirometer was developed (Fig. 1) to permit inhalation of the whole CO-bolus with the first breath. The procedure was as following: after connecting the subjects to the spirometer via the mouth piece H (Fig. 1) they completely exhaled to residual volume via their nose, which was closed immediately thereafter with a nose-clip. The subject was instructed to inhale deeply as the individualised CO dose was administered via the pre-filled 100 ml syringe D (Fig. 1). At the same time, the valve C (Fig. 1) between the subject and the oxygen reservoir I [a 3 l anaesthetic bag pre-filled with pure oxygen via A and B (Fig. 1)] was opened. By this means all the CO was inhaled in the first part of the breath and was distributed within the alveoli. Given the high affinity of CO for Hb (Douglas et al. 1912), it therefore can be expected that a large part of CO diffused into the blood during the first seconds. To further support the diffusion process the subject held their breath for 10 s after the first inspiration, after which they continued normal respiration from the spirometer for 1 min 50 s. Soda lime (∼10 g) was added (F, Fig. 1) to absorb CO2. To verify that no gas escaped during the rebreathing procedure a portable CO analyser (Fluke CO-220, Germany) with parts-per-million sensitivity was placed beside the mouth piece and nose-clip. The subject was disconnected after exhaling to residual volume to nearly fully inflate the anaesthetic bag (I, Fig. 1), which was closed thereafter by valve C (Fig. 1). This full exhalation is necessary to quantify the volume of CO, which was not taken up by the body. We therefore estimated the residual volume (1.5 l in men, 1.2 l in women), determined the remaining gas volume in the spirometer and measured the CO concentration (with the CO analyser mentioned above) in these compartments. To also quantify the CO volume that was exhaled after disconnecting from the spirometer until the last blood sample (the timing of which is described in the next paragraph), the end-tidal CO concentration was measured at the time of the last blood sample and multiplied by the alveolar ventilation (estimated to be 5 l min−1).
Arterialised blood samples were taken from an earlobe twice before, every minute after starting the rebreathing period until minute 7 as well as on minutes 9, 15 and 20. In order to compare the Procnew with the Proccom, total haemoglobin mass was calculated for every time point of blood sampling:
-
K = current barometric pressure × 760−1 × [1 + (0.003661 × current temperature)]
-
MCO = COadm − (COsystem+lung (after disconnection) + COexhaled (after disconnection))
-
COadm = CO volume administered into the system
-
COsystem+lung (after disconnection) = CO concentration in spirometer × (spirometer volume + lung residual volume)
-
COexhaled (after disconnection) = end-tidal CO concentration × alveolar ventilation × time
-
-
ΔHbCO% = difference between basal HbCO and HbCO in the blood samples after CO administration
-
1.39 = Hüfners number (ml CO × g Hb−1) (e.g. Gorelov 2004)
Study II
To test the validity of the Procnew tHb-mass was determined 1 day before and 2 h after a phlebotomy of 550 ml in four men and one woman (for subject characteristics, see Table 1). The phlebotomy was a normal blood donation and was conducted by the staff of the German Red Cross. In order to calculate the reduction in tHb-mass (550 ml × [Hb]), [Hb] was measured in duplicate (ABL 520, Radiometer, Denmark) in an antecubital venous blood sample taken after 15 min of quiet rest immediately before the blood donation.
Study III
To test the applicability of the Procnew on athletes 13 recreationally trained male (n=8) and female (n=5) subjects participated in this study. Within 2 weeks they performed two VO2max tests on a cycle ergometer (Excalibur, Lode, Germany): one without previous treatment and one immediately after completing the Procnew. The exercise test started with 100 W (males) or 50 W (females) for 3 min, thereafter increasing the workload by 17 W every minute until subjective exhaustion. Oxygen consumption and ventilation data were obtained by a closed spirometry system (MetaMax, Biophysik, Germany).
In order to determine the HbCO t 1/2 after the Procnew and the influence of exercise on this parameter, the fraction of HbCO was measured under the following two conditions: (1) resting quietly after completing the Procnew and (2) after a VO2max test which commenced 30 min after the Procnew. Arterialised blood samples were taken immediately after and every hour during the subsequent 6 h from a hyperemised earlobe. t 1/2 was determined graphically in terms of calculating the time span which is required to half ΔHbCO% obtained from measurements before and 5 min after CO-rebreathing.
Statistics
The reliability of the method was tested by a test–retest correlation analysis and quantified by the calculation of the typical error of measurements (Hopkins 2000). The typical error (TE) is the standard deviation of the difference scores of n series of measurements divided by √ n. The limits of agreement are calculated by multiplying the typical error by 1.96 (95% confidence), 2.58 (99%) and 3.01 (99.9%). To visualise the differences between test and retest the Bland–Altman plot was used (Bland and Altman 1986). For the comparison of the results of the VO2max tests with and without previous CO-rebreathing the paired student’s test was applied.
Results
Study I
The increase in HbCO (ΔHbCO%) when using the Procnew showed a different time course compared with the Proccom as predicted by Bruce and Bruce (2003). ΔHbCO% increased slowly during the Proccom reaching a virtual plateau after 9.5 min (2.3) (Fig. 2). In contrast ΔHbCO% peaked in the first minute for Procnew, dropped markedly until the fourth minute and decreased slightly but continuously thereafter. tHb-mass calculated for every minute for Procnew was identical in the fifth minute compared with the values of the Proccom in minute 9.5. At minutes 4 and 6 the calculated tHb-mass values deviate from the plateau value obtained by the Proccom by only −1.5% (1.7) and +1.7% (2.0), respectively. It therefore follows that one blood sample taken in the fifth minute after beginning the rebreathing period for Procnew is sufficient to determine tHb-mass. However, in order to minimise errors the mean value of minutes 4 and 6 and not the single minute 5 value was used for the calculation of tHb-mass. The raw data for initial HbCO%, ΔHbCO% as well as the CO volumes not bound to haemoglobin after the fifth minute are presented in Table 2.
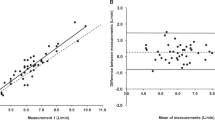
Figure 3 shows the differences in tHb-mass between the tests performed with the Procnew and the Proccom in the Bland–Altman plot. The mean differences and standard deviations of the Proccom were −0.4% (2.1) (test-1), 1.7% (2.3) (test-2) and −0.1% (1.7) (test-3). When comparing the test-1 with test-2 and test-3 (Fig. 4) we found a close relationship with r 2=0.989 (test-1 vs. test-2) and r 2=0.988 (test-1 vs. test-3). The mean difference between test-1 and test-2 was −2.0% (2.7) and between test-1 and test-3 0.3% (2.9). This resulted in a typical error and 95% confidence limits (CL) of 1.9% (CL 3.7) and 2.0% (CL 3.9) when comparing test-1 vs. test-2 and test-1 vs. test-3, respectively.
When both comparisons were included in the calculation of the typical error it decreased to a mean value of 1.7% and 95% CL of 3.3%. Thus, relative to the tHb-mass of 790 g (mean value of test-1) a change of more than 26 g exceeds the limits of agreement with a significance of P<0.05. Calculating the limits of agreement for significance of P<0.01 and P<0.001 the difference in haemoglobin mass has to be greater than 35 and 41 g, respectively.
Study II
tHb-mass measured immediately after the blood donation decreased similarly in all subjects (Table 3) being 95 g (19) lower than the reference value determined the day before. The calculated decrease in tHb-mass was 85 g (8) and the mean difference between calculated and measured values was 9 g (12).
Study III
VO2max ranged between 47.0 and 62.9 ml kg−1 min−1 in men and 44.0 and 49.0 ml kg−1 min−1 in women. After application of the Procnew VO2max decreased by 3.0% (3.7) (P<0.05) from 51.0 to 49.5 ml kg−1 min−1 in the whole group. For individual data see Fig. 5. Maximum ventilation increased after the Procnew from 138.4 l min−1 (43.9) to 146.0 l min−1 (45.7) (P<0.05) leading to a higher respiratory equivalent [from 37.5 (6.2) to 41.3 (6.3), P<0.01]. HbCO t 1/2 was 132 min (27) after the Procnew and was reduced to 89 min (23) (P<0.001) after the maximum bout of exercise (Fig. 6).
Discussion
Our key result is that breathing a bolus of CO for 2 min (Procnew) yields the same tHb-mass as rebreathing a mixture of O2 and CO for 10 min (Proccom), when blood is sampled 5 min (mean of 4 and 6 min) after rebreathing commenced. Our independent empirical data support the multicompartment model of CO uptake and distribution of Bruce and Bruce (2003). In our hands, reliability of Procnew (TE = 1.7%) is similar to that previously reported for Proccom (TE = 1.8%; Heinicke et al. 2001), and the validity of Procnew is proved by the close agreement between the measured and calculated losses of tHb-mass via 550 ml phlebotomy. Key benefits of Procnew compared with Proccom are the reduction in CO-rebreathing time by 80% and the minimally invasive procedure. We conclude that this method can be used routinely with both patients and athletes.
Factors influencing the precision of the method
The main influence on HbCO and therefore on the calculation of tHb-mass derives from the time point of blood sampling. Factors influencing HbCO over time are: first the procedure of administering CO, i.e. inhaling a CO-bolus or a large (about 10 l) CO–O2 gas mixture influencing the CO uptake by the blood and the distribution of CO within the cardiovascular system; second the type of blood used for analysis, i.e. arterial or venous blood; third the diffusion of CO from blood to extravascular compartments, i.e. especially to Mb and to a lesser extent to muscular cytochromes; and fourth the exhalation of CO when the subject is disconnected from the spirometer. Each of these is discussed below.
Type of CO administration and type of blood sampling
Comparing the effect of inhaling a CO-bolus for 1 min with the effect of inhaling the same amount of CO in a larger gas mixture (consisting of CO and O2) for 5 min, Bruce and Bruce (2003), calculating with the data of Tikuisis et al. (1987), showed a faster increase in HbCO after bolus application. The HbCO curves obtained under both breathing procedures, however, converged to the identical plateau about 7 min after commencing CO-rebreathing. In case of inspiring a CO-bolus a further difference in time course becomes obvious for arterial and mixed venous blood (Bruce and Bruce 2003). In arterial blood HbCO is predicted to increase within some seconds and decrease the following 2–3 min until reaching an approximate plateau. In contrast, in mixed venous blood HbCO is modelled to increase continuously until converging to the same plateau as in arterial blood after ∼3 min.
These observations obtained theoretically from the multicompartment model of Bruce and Bruce (2003) are confirmed by our results when applying the Procnew. As shown in study I, we also found a rapid increase in arterialised HbCO in the first minute decreasing thereafter until reaching a virtual plateau after 4–6 min (Fig. 2). At this time HbCO is equally distributed within the circulation as reported by Kisch et al. (1995), who described that, at rest, mixing of a substance in blood administered by a bolus is completed after 80–150 s. Calculating tHb-mass of minutes 4 and 6 of Procnew shows virtually identical values as derived from the 9.5 min plateau value of the Proccom. We therefore conclude that the Procnew using only 2 min of CO-rebreathing provides the same results as the Proccom, which needs at least 10 min of respiration (Burge and Skinner 1995; Hütler et al. 2000; Heinicke et al. 2001). A further advantage is the low invasive character, taking only four blood samples from an earlobe instead of multiple blood samples from an antecubital vein.
CO-diffusion to extravascular compartments
A further influence on the accuracy of the determination of tHb-mass can be prevented by considering a possible loss of CO within the body. The mechanism of CO leakage to extravascular space is mostly due to the binding to Mb and to a negligible part to cytochromes (CC) as the ratio of CO-binding sites is: 1.0 (Hb) : 0.14 (Mb) : 0.003 (CC) (Shimazu et al. 2000). According to Möller and Sylvén (1981) a 70 kg man would be expected to have 135 g of Mb suggesting that muscle could be a significant storage site for CO. Earlier Drabkin (1951) estimated that Mb comprises only 3.8% of the total Hb. Godin and Shephard (1972) calculated the diffusion coefficient of CO to Mb to be 0.1 ml CO s−1 mmHg−1. Assuming an increase in HbCO content by 5%, the increase in pCO in blood is 0.02 mmHg [Joels and Pugh 1958; i.e. that within 300 s (mean time of minutes 4 and 6) after starting the CO-rebreathing of 60 ml CO, 0.6 ml CO is bound to Mb]. This minor loss of CO is also described in the multicompartment model of Bruce and Bruce (2003).
The somewhat negative attitude towards the CO-rebreathing method due to the loss of CO to Mb (Sawka et al. 2000) is refuted by various authors, such as Richardson et al. (2002), provoking rather high HbCO concentrations (20%) without finding a measurable increase in MbCO. Likewise our results (study I) show no discernible effects in ΔHbCO when comparing the test with prebreathing 15 ml of CO (test-3) to that without prebreathing (test-1). An impact on the calculation of tHb-mass by this procedure was not detected likely reflecting insensitivity in our measurement of HbCO on a CO-oximeter. However, it is inappropriate to pre-inhale larger amounts of CO as the diffusion of CO from blood to Mb is very slow (less than 1 ml after 10 min when ΔHbCO is 9%) and a constant saturation of Mb cannot be reached within the short time (4–10 min) until the first blood sampling (Bruce and Bruce 2003). To compensate for the small error by CO leakage mentioned above one should include a correction factor into the calculation of tHb-mass. It consists of muscle mass (about 41 and 33% of body mass in non-overweight men and women, respectively; Lee et al. 2000), pCO (Joels and Pugh 1958) and the diffusion coefficient for CO (Godin and Shephard 1972). We calculated that in case of an increase of HbCO by 5% a relative constant leakage of 1% of the inhaled CO volume occurred, which would correspond to 1% overestimation in tHb-mass. We therefore recommend to correct the CO volume bound to Hb by −1%. In case of investigating athletes with very high muscle mass (e.g. body builders), however, an individual factor should be used.
Specification of CO volume within the circulation
To take into consideration that the administered CO has not completely diffused into the blood after 2 min, the CO remaining in the spirometer and the lung was determined (see Methods and Table 2). Due to different respiratory patterns, this value varies individually. In terms of accuracy it is therefore necessary to correct the administered CO volume. In contrast to Burge and Skinner (1995) using a fix correction factor of 2.2% we determined the remaining value for each subject in the range between 0.9 and 2.6% of the administered CO volume.
In the Proccom the subject remains connected to the spirometer until the last blood sample is taken. In the Procnew they are already disconnected after 2 min of respiration and blood samples are taken at minutes 4 and 6, i.e. the amount of CO which is exhaled between disconnecting the subject and blood sampling has to be taken into account. The volume of CO exhaled between minutes 2 and 5 had to be subtracted from the initially applied volume. An individual measurement of the alveolar ventilation (V A) is not necessary to increase the precision of the method as the subjects usually show normal ventilation after being disconnected from the spirometer. Only in case of extreme hyperventilation is the determination of individual alveolar ventilation recommended.
Calculation of the maximum error due to equipment and procedure
To evaluate the maximum error in tHb-mass the following worst-case calculation was performed for a representative subject possessing 790 g of tHb-mass and inhaling 60 ml of CO.
The main impact on the accuracy of the result is the exactness of HbCO measurement. In our experiments the CO-oximeter ABL 520 was used. The resolution and the reliability of this equipment is 0.1% (Barnett and Wilson 1998), so that the maximum error before and after CO inhalation may accumulate to 0.2% in HbCO corresponding to a mismeasurement of 32 g Hb. This error is further augmented when the following factors are miscalculated: (a) a 40% higher Mb content maximally results in an additional leakage of about 0.24 ml CO and therefore leads to an overestimation in tHb-mass by 3 g; (b) miscalculating the volume in the spirometer by 200 ml (at a CO concentration of 250 ppm) results in an error of 1.0 g Hb; (c) a variation in V A by 3 l min−1 instead of the assumed 5 l min−1 leads to a deviation of tHb-mass by 4 g. Adding up all possible mismeasurements mentioned above yields a maximum error of 40 g, i.e. 5.1%. As this error, however, is mainly due to the CO-oximeter, it can be decreased by a factor of ∼2 by measuring HbCO in duplicate samples before CO inhalation and twice after the respiration period, as reflected by the low typical error (∼2%) in test–retest experiments that we achieved.
Reliability and validity of the method
The reliability of the Procnew (typical error = 1.7%) is in accordance with the CV values obtained by the Proccom (2.5%, Thomsen et al. 1991; 3.0% in venous and 3.3% in arterial blood, Hütler et al. 2000; and 2.5%, Heinicke et al. 2001). The lower CV of 0.8% described by Burge and Skinner (1995) is due to the calculation of CV from the average of the SDs for each subject. A similar typical error obtained with the Procnew was also determined for the Proccom by Gore et al. (1997) as 1.9% and Ashenden et al. (1999) as 2.3%. In contrast radio-labelling of red blood cells which is accepted as the gold standard is characterised by CV values between 4 and 5% (Remington and Baker 1961; Mollison 1967). We therefore conclude that the Procnew is as reliable as the Proccom and the radioactive methods although the Procnew is markedly shortened, minimally invasive and avoids the handling problems associated with radioactive tracers.
The phlebotomy results (mean error of 9 g) confirm that Procnew has adequate sensitivity to detect small changes in tHb-mass.
Impact of the CO-rebreathing method on VO2max
The application of the CO-rebreathing method in the field of sports requires the knowledge of its impact on performance. According to various investigations (Pirnay et al. 1971; Vogel and Gleser 1972; Ekblom and Huot 1972) VO2max decreases linearly with increasing HbCO concentration. In contrast to these authors, Horvath et al. (1975) failed to demonstrate a decrease in VO2max below a variation in HbCO by 4.3%. In our study the increase in HbCO by 4.9% was associated with a decrease in VO2max by 3.0% (3.7). This reduction is lower than that described by Klausen et al. (1983) describing a 7% decrease when HbCO was increased by 5.3%, but fits well with the results of Hirsch et al. (1985) demonstrating a decrease in VO2max by 4% after increasing HbCO by 4.8% due to smoking. The physiological mechanisms leading to the decline in VO2max are the lower O2 transport capacity of the blood acting like inspiratory hypoxia (Richardson et al. 2002) and the slightly left-shifted oxygen dissociation curve impairing O2 uptake by the muscle cell (Hogan et al. 1990). For practical application we therefore conclude that the CO-rebreathing test should not be performed shortly before competition but there are no contraindications for its use after a competition or within training and recovery periods.
HbCO half-time
To determine an adequate time lapse between the application of the CO-rebreathing test and a competition of an athlete the HbCO t 1/2 and its complete disappearance from circulation were evaluated. In the literature the decrease in HbCO is described with large variations. The relatively homogenous HbCO t 1/2 found in this study [132 min (27)] is in accordance with the data presented by Weaver et al. (2001) describing t 1/2 of 131 min (133). Slightly higher t 1/2 data were published by Peterson and Stewart (1970) demonstrating a mean of 320 min with a large scattering between 128 and 409 min. According to Shimazu et al. (2000) a biphasic decrease in HbCO has to be considered for a correct calculation of t 1/2; the initial rapid decrease is due to the distribution of CO within the body and is followed by a slower phase in which CO is excreted by ventilation, i.e. the first phase should not be included into the calculation. In our study the initial value for calculating t 1/2 was obtained 5 min after beginning CO-rebreathing when mixing time of CO in the blood was complete and further CO distribution within the body can be disregarded.
Generally HbCO t 1/2 is mostly influenced by V A and alveolar pO2 (Peterson and Stewart 1970). We therefore measured t 1/2 after a bout of heavy exercise and observed a decrease of 43 min (25). This mechanism can be explained by the increased exercise- and post-exercise-related ventilation (V Emax = 146 l min−1) as was also demonstrated by Hauck (1989). In contrast to the exercise effects, Togores et al. (2000) showed a higher HbCO t 1/2 in patients with obstructive pulmonary disease, which was due to lower effective ventilation. Considering that a short bout of exercise (25 min) decreases t 1/2 by more than 40%, we may speculate that longer lasting exercise exerts much higher effects on HbCO degradation. Without exercise, the basal HbCO concentration was nearly reached after 6 h. We therefore suggest that the Procnew can also be used at days of competition considering a time lapse of 12 h.
Conclusion
In this study we report that 2 min of rebreathing a CO-bolus attains reliable (typical error = 1.7%) and valid results (predict 550 ml blood loss to within 9 g Hb) for tHb-mass compared with the commonly used 10 min or longer lasting CO-rebreathing procedures. The theoretical maximum error of this 2 min method is also ∼2.5%. We recommend correcting the CO volume bound to Hb by −1% to account for CO efflux to Mb and 12 h lag between measurement and athletic competition. By means of a custom-made spirometer the new procedure can be easily performed without the problems of handling radio-labelled red cells and with similar reliability to the 51Cr method of assessing red cell volume. The new minimally invasive procedure with only four blood samples from an earlobe and the short rebreathing time of 2 min results in a routinely applicable method with almost no inconvenience for the patient or athlete.
References
Arnold HR, Carrier EB, Smith HP, Whipple GH (1921) Blood volume studies. Am J Physiol 56:313–327
Ashenden MJ, Gore CJ, Dobson GP, Hahn AG (1999) Live high, train low does not change the total haemoglobin mass of male endurance athletes sleeping at a simulated altitude of 3000 m for 23 nights. Eur J Appl Physiol 80:479–484
Barker SJ (1998) Blood volume measurement. Anesthesiology 89:1310–1312
Barnett K, Wilson JF (1998) Quantitation of carboxyhaemoglobin in blood: external quality assessment of techniques. Br J Biomed Sci 55:123–126
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 8476:307–310
Böning D, Rojas J, Serrato M, Ulloa C, Coy L, Mora M, Hütler M (2001) Haemoglobin mass and peak oxygen uptake in untrained and trained residents of moderate altitude. Int J Sports Med 22:1–7
Bruce EN, Bruce MC (2003) A multicompartment model of carboxyhaemoglobin and carboxymyoglobin responses to inhalation of carbon monoxide. J Appl Physiol 95:1235–1247
Burge CM, Skinner SL (1995) Determination of haemoglobin mass and blood volume with CO: evaluation and application of a method. J Appl Physiol 79:623–631
Douglas CG, Haldane JS, Haldane JBS (1912) The laws of combination of haemoglobin with carbon monoxide and oxygen. J Physiol 44:275–304
Drabkin DL (1951) Metabolism of the hemin chromoproteins. Physiol Rev 31:345–431
Ekblom B, Huot R (1972) Response to submaximal and maximal exercise at different levels of carboxyhaemoglobin. Acta Physiol Scand 86:474–482
Fogh-Andersen N, Thomsen JK, Foldager N, Siggaard-Andersen O (1990) pH effect on the COHb absorption spectrum: importance for calibration of the OSM3 and measurement of circulating haemoglobin and blood volume. Scand J Clin Lab Invest 50:247–252
Friedmann B, Jost J, Rating T, Weller E, Werle E, Eckhardt K-U, Bärtsch P, Mairläurl H (1999) Effects of iron supplement on total body haemoglobin during endurance training at moderate altitude. Int J Sports Med 20:78–85
Gibson JG, Evans WA Jr (1937) Clinical studies of the blood volume. I. Clinical application of a method employing the azo dye “Evans Blue” and the spectrophotometer. J Clin Invest 16:301–316
Godin G, Shephard RJ (1972) On the course of carbon monoxide uptake and release. Respiration 29:317–329
Gore CJ, Hahn AG, Burge CM, Telford RD (1997) VO2max and haemoglobin mass of trained athletes during high intensity training. Int J Sports Med 18:477–482
Gorelov V (2004) Theoretical value of Hüfner’s count. Anesthesia 59:97
Hauck H (1989) Parameters influencing carbon monoxide kinetics. Exp Pathol 37:170–176
He Y-L, Tanigami H, Ueyama H, Mashimo T, Yoshiya I (1998) Measurment of blood volume using indocyanine green measured with pulse-spectrophotometry: its reproducibility and reliability. Crit Care Med 26:1446–1451
Heinicke K, Wolfahrt B, Winchenbach P, Biermann B, Schmid A, Huber G, Friedmann B, Schmidt W (2001) Blood volume and haemoglobin mass in elite athletes of different disciplines. Int J Sports Med 22:504–512
Hirsch GL, Sue DY, Wasserman K, Robinson TE, Hansen JE (1985) Immediate effects of cigarette smoking on cardiorespiratory responses to exercise. J Appl Physiol 58:1975–1981
Hogan MC, Bebout DE, Gray AT, Wagner PD, West JB, Haab PE (1990) Muscle maximal O2 uptake at constant O2 delivery with and without CO in the blood. J Appl Physiol 69:830–836
Hopkins WG (2000) Measures of reliability in sports medicine and science. Sports Med 30:1–15
Horvath SM, Raven PB, Dahms TE, Gray DJ (1975) Maximal aerobic capacity at different levels of caboxyhaemoglobin. J Appl Physiol 38:300–303
Hütler M, Beneke R, Böning D (2000) Determination of circulating haemoglobin mass and related quantities by using capillary blood. Med Sci Sports Exerc 32:1024–1027
International Committee for Standardization in Haematology (1980) Recommended methods for measurement of red-cell and plasma volume: International Committee for Standardization in Haematology. J Nucl Med 21:793–800
Joels N, Pugh LGCE (1958) The carbon monoxide dissociation curve of human blood. J Physiol 142:63–77
Kisch H, Leucht S, Lichtwarck-Schoff M, Pfeiffer UJ (1995) Accuracy and reproducibility of the measurement of actively circulating blood volume with an integrated fiberoptic monitoring system. Crit Care Med 23:885–893
Klausen K, Andersen C, Nandrup S (1983) Acute effects of cigarette smoking and inhalation of carbon monoxide during maximal excercise. Eur J Appl Physiol 51:371–379
Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB (2000) Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr 72:796–803
Möller P, Sylvén C (1981) Myoglobin in human skeletal muscle. Scand J Clin Lab Invest 41:479–482
Mollison PL (1967) Blood volume. In: Mollison PL (ed) Blood transfusion in clinical medicine. Blackwell, Oxford, pp 115–150
Peterson JE, Stewart RD (1970) Absorption and elimination of carbon monoxide by inactive young men. Arch Environ Health 21:165–171
Pirnay F, Dujardin J, Deroann R, Petit JM (1971) Muscular exercise during intoxication by carbon monoxide. J Appl Physiol 31:573–575
Remington JW, Baker CH (1961) Evaluation of blood volume measurment techniques. Circ Res 9:60–68
Richardson RS, Noyszewski EA, Saltin B, Gonzáles-Alonso J (2002) Effect of mild carboxy-haemoglobin on exercising skeletal muscle: intravascular and intracellular evidence. Am J Physiol Regul Integr Comp Physiol 283:1131–1139
Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ (2000) Blood volume: importance and adaptions to exercise training, environmental stresses, and trauma /sickness. Med Sci Sports Exerc 32:332–348
Shimazu T, Ikeuchi H, Sugomoto H, Goodwin CW, Mason AD, Pruitt BA (2000) Half-life of blood carboxyhaemoglobin after short-term and long-term exposure to carbon monoxide. J Trauma Inj Infect Crit Care 49:126–131
Thomsen JK, Fogh-Andersen N, Bülow K, Devantier A (1991) Blood and plasma volumes determined by carbon monoxide gas, 99mTc-labelled erythrocytes, 125 I-albumin and the T 1824 technique. Scand J Clin Lab Invest 51:185–190
Tikuisis P, Buick F, Kane DM (1987) Percent carboxyhaemoglobin in resting humans exposed repeatedly to 1,500 and 7,500 ppm CO. J Appl Physiol 63:820–827
Togores B, Bosch M, Agustí AGN (2000) The measurement of exhaled carbon monoxide is influenced by airflow obstruction. Eur Respir J 15:177–180
Tschaikowsky K, Meisner M, Durst R, Rügheimer E (1997) Blood volume determination using hydroxyethyl starch: a rapid and a simple intravenous injection method. Crit Care Med 25:599–606
Vogel JA, Gleser MA (1972) Effect of carbon monoxide on oxygen transport during exercise. J Appl Phys 32:234–239
Weaver LK, Howe S, Hopkins R, Chan KJ (2001) Carboxyhaemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest 117:801–808
Acknowledgements
The project was supported by the German Federal Institute of Sports Sciences (BISp,# VF 0407/03/42). The authors wish to thank Mrs. H. Gaisser, Mr. A. Beller, Mr. A. Schmeiduch and Mr. M. Gebert for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, W., Prommer, N. The optimised CO-rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol 95, 486–495 (2005). https://doi.org/10.1007/s00421-005-0050-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-005-0050-3