Abstract
It has been suggested that circulating hormones and cytokines are important in the adaptive response to low-load resistance training (LLRT) with blood flow restriction (BFR); however, their response following this type of training in older men is unclear. Seven healthy older men (age 71.0 ± 6.5 year, height 1.77 ± 0.05 m, body mass 80.0 ± 7.5 kg; mean ± SD) performed five sets of unilateral LLRT knee extensions (20 % 1-RM) of both limbs, with or without BFR in a counterbalanced order. For the BFR condition, a pressure cuff was applied on the upper thigh and inflated to ~110 mmHg. Venous blood samples were taken at rest and 30-, 60- and 120-min post-exercise and measured for plasma concentrations of growth hormone (GH), insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), cortisol and interleukin-6 (IL-6). GH increased (P < 0.05) from rest to 30-min post-exercise and was greater (P < 0.05) during LLRT with BFR than without. VEGF was significantly (P < 0.05) elevated from resting levels at 30-, 60- and 120-min post-exercise following LLRT with BFR with no change seen following LLRT without BFR. IL-6 increased (P < 0.05) from 30- to 60-min post-exercise and remained elevated at 120-min post-exercise in both conditions. Cortisol and IGF-1 were unaffected following exercise. In conclusion, a single bout of LLRT with BFR increases the circulating concentrations of GH and VEGF in older men and may explain the skeletal muscle and peripheral vascular adaptations observed following training with BFR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ageing process is associated with a reduction in strength (Lindle et al. 1997; Skelton et al. 1994) and muscle blood flow (Dinenno et al. 1999; Proctor and Parker 2006), both of which contribute to the decline in physical function. It is well established that the decline in strength and blood flow can be attenuated or halted by regular physical activity such as resistance training. A large number of studies have demonstrated gains in maximal muscle strength in older people following resistance training, with increases in 1-repetition maximum (1-RM) ranging from 23 to 134 % (Frontera et al. 1988; Harridge et al. 1999; Reeves et al. 2004), which is associated with an improved ability to perform functional tasks, such as chair rising, stair climbing and walking speed, over a wide age range (60–90 years; Judge et al. 1993; Beyer et al. 2007; Caserotti et al. 2008). As well as the positive effects on skeletal muscle strength, resistance training can influence the age-associated decline in limb blood flow. Resistance training has been shown to increase both basal limb blood flow and vascular conductance in older people (Anton et al. 2006). Indeed, older men who have engaged in resistance training do not exhibit the age-related decrease in limb blood flow as seen in non-trained age-matched controls (Miyachi et al. 2005).
One model of resistance-type exercise that is gaining popularity is that of low-load resistance training (LLRT) with blood flow restriction (BFR). This technique has been shown to induce gains in strength (Burgomaster et al. 2003; Moore et al. 2004; Patterson and Ferguson 2010) and muscle size (Takarada et al. 2002; Laurentino et al. 2008), as well as peripheral vascular adaptations (Evans et al. 2010, Patterson and Ferguson 2010) in young men and women (under 35 years of age). Similar observations have also been made in older women (Over 60 years of age; Patterson and Ferguson 2011), thus demonstrating its potential applicability to the older population.
Despite these observations, it is still not clear what the potential mechanisms of LLRT with BFR are, especially in relation to older people. Evidence suggests a role for circulating hormones and cytokines in the adaptation process to conventional resistance training (Gavin et al. 2007a; Izquierdo et al. 2009) and recent evidence from LLRT with BFR suggests similar adaptive responses (Abe et al. 2005; Reeves et al. 2006; Takano et al. 2005; Takarada et al. 2000a. For example, following LLRT with BFR there are acute elevations in circulating hormones and cytokines such as GH (Reeves et al. 2006; Takano et al. 2005; Takarada et al. 2000a), IGF-1 (Abe et al. 2005; Takano et al. 2005) and VEGF (Takano et al. 2005). Additionally, skeletal muscle IL-6 protein expression does not change following LLRT with BFR (Fry et al. 2010), however systemic IL-6 is acutely elevated (Takarada et al. 2000a), whilst cortisol is elevated post-exercise (Fujita et al. 2007). These acute changes may give an indication of the strength, hypertrophy and blood flow changes that are associated with LLRT with BFR.
Therefore, the aim of this study was to investigate the mechanisms of LLRT with BFR by examining the effect of an acute bout of LLRT with and without BFR on plasma concentrations of cortisol, GH, IGF-1, IL-6 and VEGF in older men. It was hypothesised that LLRT with BFR would provide a sufficient stimulus to increase anabolic (GH and IGF-1), anti-catabolic (cortisol), inflammatory (IL-6) and angiogenic (VEGF) hormones and cytokines in response to an acute bout of exercise.
Methods
Participants
Seven older males volunteered to participate in the study (age 71.0 ± 6.5 years, height 1.77 ± 0.05 m, body mass 80.0 ± 7.5 kg; BMI 25.6 ± 3.0 kg/m2; mean ± SD) and were selected according to the criteria of Greig et al. (1994) for ‘healthy’ older participants. These criteria ensured individuals were not on any medication throughout the study. All participants were habitually physically active (assessed during pre-screening by asking participants to detail the type, frequency, and volume of exercise performed per week), and, performed regular physical activity such as walking, jogging or gardening (two or three times per week, 30 min at a time), but none specifically performed resistance-exercise training. The participants were fully informed of the purposes, risks and discomfort associated with the experiment before providing written, informed consent. The sample size for this study was based on previous data from Takano et al. (2005) and was powered (≥0.80) to detect significant increases in GH, VEGF, IGF-1, IL-6 and cortisol at P < 0.05, with a sample size of at least six participants required. This study conformed to current local guidelines and the Declaration of Helsinki and was approved by Loughborough University Ethics Advisory Committee.
1-RM testing and familiarisation
At least 7 days prior to conducting the study unilateral 1-RM knee extension of both legs was assessed on a leg extension machine (Ortus Fitness, Valencia, Spain). Participants began with a warm-up of two sets of 10 repetitions at 5 kg, each separated by 2 min. Thereafter, the load was set at 80 % of the predicted 1-RM (Baechle and Earle 2008). Following each successful lift, the load was increased by ~5 % until the subject failed to lift the load through the entire range of motion. A test was only considered valid when the participant used proper form and completed the entire lift in a controlled, unassisted manner. Approximately 2–3 min of rest was allowed between each attempt to ensure full recovery. After it was judged that 1-RM had been achieved and a sufficient rest period had been adhered to, each participant had the load increased one last time to ensure that they could not lift any more weight. On average, each participant needed five attempts to reach 1-RM. Participants were then familiarised to the nature of LLRT with BFR by performing the resistance exercise protocol as described below. This also provided an indication of the number of repetitions that could be performed in the BFR condition which would be replicated in the non-restricted condition and allow a counterbalanced order in the main experimental protocol. This ultimately allowed the work done during the BFR and control trials to be matched.
Experimental protocol
Participants arrived at the laboratory in the morning (7–9 am) following an overnight fast and having abstained from caffeine, alcohol or strenuous exercise for the 24-h period prior to testing. With the participant resting in a semi-supine position, a cannula (21 G) was inserted into the antecubital vein for blood sampling. The cannula was kept patent throughout the trial by regular flushing with 0.9 % NaCl saline solution. Following a 30-min rest period, a blood sample was taken. Participants then performed five sets of unilateral knee extension exercise at 20 % 1-RM with 30-s rest between each set. Immediately after the fifth set, participants repeated the exercise with the contralateral leg. In the experimental condition, blood flow was partially occluded with an inflatable cuff (SC12D-13 cm width, Hokanson Inc, Bellevue, WA) which was placed around the most proximal potion of the thigh. The cuff pressure (110 mmHg) was maintained throughout the duration of exercise (8–10 min), including rest periods. The number of repetitions was pre-determined from the habituation session with each set performed to fatigue. The participant then remained in the seated position and further blood samples were taken 30-, 60- and 120-min post-exercise. In the control condition, participants performed the identical exercise protocol (number of sets and repetitions) as during the occlusion trial, except the cuff was not inflated and no pressure was applied to the legs. Participants completed the two trials in a counterbalanced order with 7 and 10 days between trials.
Blood sampling and analysis
All blood samples were drawn into 2 × 10 ml vacutainers (K+EDTA, BD Biosciences, San Diego, USA). Blood samples for plasma were placed on ice for approximately 30 min, before centrifugation at 4,000 rpm for 10 min at 4 °C. The plasma was then frozen and stored at −80 °C until further analysis. Haematocrit was determined from whole-blood in triplicate, using the microcapillary technique (Hawksley Ltd, Lancing, UK). Haemoglobin concentration was measured in duplicate using a commercially available kit (Randox, Co Antrim, UK). Plasma volume changes were estimated using the method described by Dill and Costill (1974) and presented data are corrected for any changes in plasma volume from rest.
Plasma IGF-1 and GH concentration were measured in duplicate by ELISA (Quantikine Immunoassay; R&D Systems, Inc., Minneapolis, MN, USA). The mean intra- and inter-assay coefficient of variation was 3.0 and 8.0 % for IGF-1 and 2.4 and 6.9 % for GH. Plasma IL-6 was determined via a non-commercial ELISA as previously described (Leggate et al. 2010). Briefly, plates were coated with anti-human IL-6 monoclonal capture antibody (OptEIA, BD Biosciences, Oxford, UK) diluted 1:250 in 0.1 M sodium carbonate. The following day, the plates were washed and blocked with 5 % bovine serum albumin (BSA; Probumin, Millipore, Illinois, USA) in Tris buffered saline (TBS). The plates were incubated for 1 h at room temperature after which they were washed and samples or standards were added to the wells. Samples were diluted 1:5 in TBS with 10 % foetal calf serum. After 2 h, plates were washed and 100 μL IL-6 detection antibody (OptEIA, BD Biosciences, Oxford, UK) diluted 1:250 in TBS-T with 1 % BSA was added per well. Plates were incubated for further 1 h before being washed. The enzyme streptavidin alkaline phosphatase was diluted 1:2,000 in TBS with 1 % BSA and 100 μL was added per well. Plates were then incubated for 45 min. After washing, an ELISA amplification system was used (Invitrogen, Paisley, UK). The reaction was stopped with 10 % sulphuric acid, and the absorbance of the wells was read at 490 nm with a correction of 690 nm (Varioskan Flash, Thermo Scientific, Vantaa, Finland). Samples were analysed in duplicate with an inter-assay coefficient of 7.4 %. This assay measures total IL-6 content and does not distinguish between free and receptor-bound IL-6. Plasma VEGF was determined in duplicate by ELISA (Bendermedsystems, Vienna, Austria). The mean intra- and inter-assay coefficient of variation was 6.2 and 4.3 %, respectively. Plasma cortisol was measured in duplicate by ELISA (DRG Instruments, Germany). The mean intra- and inter-assay coefficient of variation was 5.6 and 6.6 %, respectively.
Statistical analysis
Statistical analysis was carried out using SPSS software (SPSS 15). Data were analysed by a repeated measures two-way (Trial (2) × Time (4)) ANOVA. Post hoc analysis was performed with paired t tests with Bonferonni corrections. IL-6 and VEGF data were log-transformed prior to the ANOVA as they failed to meet the assumptions of normal distribution. Statistical significance was accepted as P < 0.05. Data are presented as mean ± SD.
Results
The experimental and control trials were matched for the number of repetitions performed which were: first set 30 ± 10, second set 9 ± 3, third set 6 ± 3, fourth set 5 ± 2, fifth set 4 ± 2. BFR continued for a total duration of 564 ± 55 s.
Plasma GH concentration (Fig. 1) demonstrated a significant time effect (P < 0.001), trial effect (P < 0.01) and time × trial interaction (P < 0.01). Following LLRT with BFR, GH increased (P < 0.05) from rest to 30-min post-exercise and was greater (P < 0.05) when compared to LLRT without BFR at this time point. GH concentration following LLRT with BFR returned to baseline levels after 60 min.
There were no changes in plasma IGF-1 concentration (Fig. 2) at any time point following exercise in either condition.
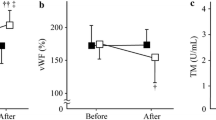
Plasma VEGF concentration (Fig. 3) demonstrated a significant time effect (P < 0.05), trial effect (P < 0.05) and time × trial interaction (P < 0.01). Following LLRT with BFR, VEGF increased (P < 0.05) and was significantly elevated at all time points compared to resting VEGF. Plasma VEGF was higher (P < 0.05) at 60 and 120 min following LLRT with BFR compared to LLRT without BFR.
Plasma IL-6 concentration (Fig. 4) demonstrated a significant time effect (P < 0.01), but no trial (P > 0.05) or group × trial interaction (P > 0.05). In both the conditions, IL-6 increased (P < 0.05) from 30- to 60-min post-exercise and remained elevated at 120-min post-exercise.
Plasma cortisol concentration (Fig. 5) demonstrated a significant trial effect (P < 0.05) and time × trial interaction (P < 0.05); however, Bonferroni-corrected post hoc tests did not locate any differences.
Discussion
This study has demonstrated that in older men LLRT with BFR increased plasma GH and VEGF when compared to LLRT without BFR. It has also been demonstrated that LLRT with and without BFR increased circulating IL-6.
The increase in plasma VEGF following LLRT with BFR in older men is in agreement with the findings of others who demonstrated elevated levels of VEGF following similar exercise in younger men (Takano et al. 2005). It is likely that the reduced oxygen tension that occurs during LLRT with BFR (Manini and Clark 2009) plays a role in the increase in VEGF concentration. Furthermore, it is well established that VEGF is critical in the stimulation of angiogenesis and arteriogenesis (Prior et al. 2004; Zhang et al. 2010). It has been suggested that exercise-induced increases in capillarisation would be similar irrespective of age, as both young and older men have been shown to increase both capillary contacts and capillary to fibre ratio following a period of endurance training (Gavin et al. 2007b). The increase in VEGF following LLRT with BFR would, therefore, support the recent findings of increased calf filtration capacity, an indirect measure of capillarisation (Brown et al. 2001; Gamble et al. 2000), following 4 weeks of ischaemic exercise training in young men (Evans et al. 2010). Whether an increase in capillarisation occurs in older people in response to this type of training has yet to be established; however, vascular adaptations, including an enhanced reactive hyperemic blood flow in young females and older male and females (Patterson and Ferguson 2010, 2011), arterial remodeling in young men (Hunt et al. 2012) and artery compliance in older men and women (Ozaki et al. 2011) have been observed suggests it is a distinct possibility and may, therefore, be a useful method to offset the age-associated decline in muscle blood flow (Dinenno et al. 1999; Proctor and Parker 2006).
There are numerous studies reporting that an acute bout of LLRT with BFR can increase circulating GH in young adults (Pierce et al. 2006; Reeves et al. 2006; Takarada et al. 2000a; Takano et al. 2005). Takarada et al. (2000a) reported a 290-fold increase in GH following five sets of bilateral knee extension with BFR at 20 % 1-RM. This is much higher than seen in the work by Pierce et al. (2006) who reported a ninefold increase in serum GH following knee extensions with BFR. Both these studies in younger individuals show greater responses than seen in this study on older men, which represented only a 3.3-fold increase. The differences may be due to the lower secretion of GH in older men following acute exercise, when compared to younger individuals (Kraemer et al. 1998) as a result of a blunted pituitary response to resistance exercise as happens with any GH challenge test in the elderly (Kern et al. 1996; Pyka et al. 1994). Another reason for the different responses may be due to the occlusion pressure applied to the exercising limb. In this study, BFR was maintained using a pressure of 110 mmHg compared to 214 (Takarada et al. 2000b) and 280 mmHg (Pierce et al. 2006) used previously. The greater pressure leads to a greater stimulation of sensory nerves such as type III and IV afferents in response to an increased acidic environment (Fujita et al. 2007; Takano et al. 2005) within the muscle, thus causing a greater GH response.
GH is secreted from the anterior pituitary gland in a pulsatile manner; however, the physiological role of exercise-induced GH is unknown. One proposed function is to stimulate the release of IGF-1 from skeletal muscle and the liver (Harridge 2003). In this study, plasma IGF-1 did not change following an acute bout of LLRT with BFR in older men, which is in agreement with other studies following acute bouts of LLRT with BFR (Abe et al. 2005; Fujita et al. 2007). However, there is contradictory evidence to suggest that LLRT with BFR may have an influence on circulating IGF-1 as two studies have demonstrated significant increases in circulating IGF-1 following exercise in young men (Abe et al. 2005; Takano et al. 2005). It is possible that the acute increases in GH seen in this study may have not had an immediate impact on circulating IGF-1 because the response of IGF-1 to GH pulses is delayed by 3–9 h and peak IGF-1 may not be reached until 16–28-h post-exercise (Kraemer and Ratamess 2005), implying that the measurement times for IGF-1 may have been taken too early. However, whether IGF-1 plays a role in hypertrophy at all has been questioned, with evidence suggesting that in response to acute loading IGF-1 mRNA increases over a period of days (Adams and Haddad 1996; Hameed et al. 2003), compared to an increase in mTOR activation within a few hours of exercise (O’Neil et al. 2009).
The elevation in IL-6 in this study following LLRT with and without BFR is similar to Takarada et al. (2000a) who found an increase in IL-6 in young men, following LLRT with BFR, although they did not see an increase during LLRT alone. The mechanism behind the increase in IL-6 is unclear as this type of exercise is unlikely to have resulted in glycogen depletion, which is considered to be an important stimulus for IL-6 release from the skeletal muscle during exercise (Steensberg et al. 2000). Acute bouts of LLRT with BFR have been reported to result in mild exercise-induced muscle damage and sarcolemmal permeability (Umbel et al. 2009; Wernbom et al. 2012). This would suggest an increased inflammatory response following acute exercise and may explain the findings by Takarada et al. (2000a). The timing of blood sampling in this study do not allow for a comparison to those studies that have demonstrated skeletal muscle damage in their participants as their sampling continued for 168 h in some cases (Wernbom et al. 2012). It is possible, however, that the slight increase in IL-6 in both the conditions in this study may have continued to a greater extent following LLRT with BFR. Evidence also suggests that IL-6 may play a role in enhancing plasma cortisol levels in humans (Steensberg et al. 2003). Cortisol is a glucocorticoid secreted by the adrenal cortex and is involved with increasing glyconeogenesis and promoting fat mobilisation and utilisation (Duclos et al. 2003). It is also described as the primary catabolic hormone as it increases protein degradation and decreases protein synthesis (Hammarqvist et al. 1994; McNurlan et al. 1996). In this study, cortisol levels increased to a greater extent following LLRT with BFR, however IL-6 may not have played a role as it increased following both the conditions. This is similar to previous work showing elevations in cortisol in older men following LLRT with BFR, but no increase with LLRT alone (Fry et al. 2010). It is also unlikely that the increase in cortisol observed following LLRT with BFR would lead to a decrease in protein synthesis as previous literature has demonstrated an elevation in muscle protein synthesis levels following LLRT with BFR in young (Fujita et al. 2007) and older men (Fry et al. 2010).
The present findings need to be considered in respect to some limitations. The small sample size of the study (n = 7) could have reduced the power to detect significant differences between trials and, therefore, limit the generalisation of the present findings to all older men. However, the observed power for significant changes was over 0.9 in all cases and similar sample sizes have previously been used when investigating hormone responses to LLRT with BFR in young men (Fujita et al. 2007; Reeves et al. 2006). Second, the increase in IL-6 following exercise is minimal; however, the increase from pre-exercise to 120-min post-exercise is more than double the coefficient of variation of the measurement, thus seems a legitimate increase.
In conclusion, a single bout of LLRT with BFR increased circulating levels of GH and VEGF in older men. Plasma cortisol levels also increased when compared to LLRT alone; however, post hoc tests could not reveal where differences lay. There was no change in IGF-1 following LLRT with BFR, and IL-6 increased post-exercise irrespective of the trial. It is possible that the acute responses observed, specifically increased GH and VEGF, may play a role in adaptations to the vascular system and within skeletal muscle (hypertrophy) following longer term LLRT with BFR.
References
Abe T, Yasuda T, Midorikawa T, Sata Y, Kearns CF, Inoue K, Koizumi K, Ishii N (2005) Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int J Kaatsu Train Res 1:6–12
Adams GR, Haddad F (1996) The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol 81:2509–2516
Anton MM, Cortez-Cooper MY, DeVan AE, Neidre DB, Cook JN, Tanaka H (2006) Resistance training increases basal limb blood flow and vascular conductance in ageing humans. J Appl Physiol 101:1351–1355
Baechle TR, Earle RW (2008) Essentials of strength training and conditioning. Human Kinetics, Champaign
Beyer N, Simonsen L, Bülow J, Lorenzen T, Jensen DV, Larsen L, Rasmussen U, Rennie M, Kjaer M (2007) Old women with a recent fall history show improved muscle strength and function sustained for six months after finishing training. Ageing Clin Exp Res 19:300–309
Brown MD, Jeal S, Bryant J, Gamble J (2001) Modifications of microvascular filtration capacity in human limbs by training and electrical stimulation. Acta Physiol Scand 173:359–368
Burgomaster KA, Moore DR, Schofield LM, Phillips SM, Sale DG, Gibala MJ (2003) Resistance training with vascular occlusion: metabolic adaptations in human muscle. Med Sci Sports Exer 35:1203–1208
Caserotti P, Aagaard P, Larsen JB, Puggaard L (2008) Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports 18:773–782
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Dinenno FA, Jones PP, Seals DR, Tanaka H (1999) Limb blood flow and vascular conductance are reduced with age in healthy humans. Circulation 100:164–170
Duclos M, Gouarne C, Bonnemaison D (2003) Acute and chronic effects of exercise on tissue sensitivity to glucocorticoids. J Appl Physiol 94:869–875
Evans C, Vance S, Brown M (2010) Short-term resistance training with blood flow restriction enhances microvascular filtration capacity of human calf muscles. J Sports Sci 28:999–1007
Frontera WR, Meredith CN, O’Reilly CP, Knuttgen HG, Evans WJ (1988) Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 64:1038–1044
Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB (2010) Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol 108:1199–1209
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB (2007) Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 103:903–910
Gamble J, Bethell D, Day NPJ, Loc P, Phu NH, Gartside IB, Farrar JF, White NJ (2000) Age-related changes in microvascular permeability: a significant factor in the susceptibility of children to shock? Clin Sci 98:211–216
Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC (2007a) Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol 191:139–146
Gavin TP, Ruster RS, Carrithers JA, Zwetsloot KA, Kraus RM, Evans CA, Knapp DJ, Drew JL, McCartney JS, Garry JP, Hickner RC (2007b) No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol 585:231–239
Greig CA, Young A, Skelton DA, Pippet E, Butler FM, Mahmud SM (1994) Exercise studies with elderly volunteers. Age Ageing 23:185–189
Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SDR (2003) Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547:247–254
Hammarqvist F, von der Decken A, Vinnars E, Wernerman J (1994) Stress hormone and amino acid infusion in healthy volunteers: short-term effects on protein synthesis and amino acid metabolism in skeletal muscle. Metabolism 43:1158–1163
Harridge SD (2003) Ageing and local growth factors in muscle. Scand J Med Sci Sports 13:34–39
Harridge SDR, Kryger A, Stensgaard A (1999) Knee extensor strength, activation and size in very elderly people following strength training. Muscle Nerve 22:831–839
Hunt JE, Walton LA, Ferguson RA (2012) Brachial artery modifications to blood flow restricted handgrip training and detraining. J Appl Physiol 112:956–961
Izquierdo M, Ibañez J, Calbet JA, Navarro-Amezqueta I, González-Izal M, Idoate F, Häkkinen K, Kraemer WJ, Palacios-Sarrasqueta M, Almar M, Gorostiaga EM (2009) Cytokine and hormone response to resistance training. Eur J Appl Physiol 107:397–409
Judge JO, Underwood M, Gennosa T (1993) Exercise to improve gait velocity in older persons. Arch Phys Med Rehab 74:400–406
Kern W, Dodt C, Born J, Fehm HL (1996) Changes in cortisol and growth hormone secretion during nocturnal sleep in the course of ageing. J Gerontol Ser A Biol Sci 51:3–9
Kraemer WJ, Ratamess NA (2005) Hormonal responses and adaptations to resistance exercise and training. Sports Med 35:339–361
Kraemer WJ, Häkkinen K, Newton RU, McCormick M, Nindl BC, Volek JS, Gotshalk LA, Fleck SJ, Campbell WW, Gordon SE, Farrell PA, Evans WJ (1998) Acute hormonal responses to heavy resistance exercise in younger and older men. Eur J Appl Physiol 77:206–211
Laurentino G, Ugrinowitsch C, Aihara AY, Fernandes AR, Parcell AC, Ricard M, Tricoli V (2008) Effects of strength training and vascular occlusion. Int J Sports Med 29:664–667
Leggate M, Nowell M, Jones S, Nimmo M (2010) The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaper 15:827–833
Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF (1997) Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol 83:1581–1587
Manini TM, Clark BC (2009) Blood flow restricted exercise and skeletal muscle health. Exerc Sports Sci Rev 37:78–85
McNurlan MA, Sandgren A, Hunter K, Essén P, Garlick PJ, Wernerman J (1996) Protein synthesis rates of skeletal muscle, lymphocytes, and albumin with stress hormone infusion in healthy man. Metabolism 45:1388–1394
Miyachi M, Tanaka H, Kawano H, Okajima M, Tabata I (2005) Lack of age-related decreases in basal whole leg blood flow in resistance-trained men. J Appl Physiol 99:1384–1390
Moore DR, Burgomaster KA, Schofield LM, Gibala MJ, Sale DG, Phillips SM (2004) Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur J Appl Physiol 92:399–406
O’Neil TK, Duffy LR, Frey JW, Hornberger TA (2009) The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol 587:3691–3701
Ozaki H, Miyachi M, Nakajima T, Abe T (2011) Effects of 10 weeks walk training with leg blood flow reduction on carotid arterial compliance and muscle size in the elderly adults. Angiology 62:81–86
Patterson SD, Ferguson RA (2010) Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur J Appl Physiol 108:1025–1033
Patterson SD, Ferguson RA (2011) Enhancing strength and postocclusive blood flow in older people with training with blood flow restriction. J Aging Phys Act 19:201–213
Pierce JR, Clark BC, Ploutz-Snyder LL, Kanaley JA (2006) Growth hormone and muscle function responses to skeletal muscle ischemia. J Appl Physiol 101:1588–1595
Prior BM, Yang HT, Terjung RL (2004) What makes vessels grow with exercise training? J Appl Physiol 97:1119–1128
Proctor DN, Parker BA (2006) Vasodilation and vascular control in contracting muscle of the ageing human. Microcirculation 13:315–327
Pyka G, Lindenberger E, Charette SL, Markus R (1994) Muscle strength and fibre adaptations to a year-long resistance training program in elderly men and women. J Gerentol 49:22–27
Reeves ND, Narici MV, Maganaris CN (2004) In vivo human muscle structure and function: adaptations to resistance training in old age. Exp Physiol 89:675–689
Reeves GV, Kraemer RR, Hollander DB, Clavier J, Thomas C, Francois M, Castracane VD (2006) Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise without occlusion. J Appl Physiol 101:1616–1622
Skelton DA, Greig CA, Davies JM, Young A (1994) Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 23:371–377
Steensberg A, Fischer CP, Keller C, Moller K, Pederson BK (2003) IL-6 enhances plasma IL-1ra, IL-10 and cortisol in humans. Am J Physiol Endocrinol Metab 285:433–437
Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund PB (2000) Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529:237–242
Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, Hirose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y, Nakajima T (2005) Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 95:65–73
Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N (2000a) Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol 88:61–65
Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N (2000b) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88:2097–2106
Takarada Y, Sato Y, Ishii N (2002) Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 86:308–314
Umbel JD, Hoffman RL, Dearth DJ, Chleboun GS, Manini TM, Clark BC (2009) Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Eur J Appl Physiol 107:687–695
Wernbom M, Paulsen G, Nilsen TS, Hisdal J, Raastad T (2012) Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur J Appl Physiol 112:2051–2063
Zhang J, Silva T, Yarovinsky T, Manes TD, Tavakoli S, Nie L, Tellides G, Pober JS, Bender JR, Sadeghi MM (2010) VEGF blockade inhibits lymphocyte recruitment and ameliorates immune-mediated vascular remodelling. Circ Res 107:408–417
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Patterson, S.D., Leggate, M., Nimmo, M.A. et al. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur J Appl Physiol 113, 713–719 (2013). https://doi.org/10.1007/s00421-012-2479-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2479-5