Abstract
The aim of the study was to examine the association of muscular strength with markers of insulin resistance in European adolescents. The study comprised a total of 1,053 adolescents (499 males; 12.5–17.5 years) from ten European cities participating in the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) Cross-Sectional Study. Muscular strength was measured by the handgrip strength and standing long jump tests. Cardiorespiratory fitness was measured by the 20-m shuttle run test. Fasting insulin and glucose were measured and the homeostasis model assessment (HOMA) and quantitative insulin sensitivity check index (QUICKI) indices were calculated. Weight, height, waist circumference and skinfold thickness were measured, and body mass index (BMI) was calculated. In males, the handgrip strength and standing long jump tests were negatively associated with fasting insulin and HOMA (all P < 0.05) after controlling for pubertal status, country and BMI or waist circumference. When skinfold thickness was included in the model, the association became non-significant. In females, the standing long jump test was negatively associated with fasting insulin and HOMA (all P < 0.001) after controlling for pubertal status, country and surrogate markers of total or central body fat (BMI, waist circumference or skinfold thickness). Findings were retained in males, but not in females after controlling for cardiorespiratory fitness. The findings of the present study suggest that preventive strategies should focus not only on decreasing fatness and increasing cardiorespiratory fitness but also on enhancing muscular strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence of the precursors of type 2 diabetes (insulin resistance and glucose intolerance) has been observed in children and adolescents (Karam and McFarlane 2008; Tresaco et al. 2003) making them an increasing public health concern in most of regions of the world (van Dieren et al. 2010; Wild et al. 2004).
Obesity is strongly associated with insulin resistance in children and adolescents (Srinivasan et al. 2002). Likewise, cardiorespiratory fitness has shown to be inversely associated with insulin resistance in children (9–10 years) with high levels of total and central body fat (Ruiz et al. 2007), as well as in obese (Allen et al. 2007; Bell et al. 2007) and non-obese young people (Carrel et al. 2009). These studies highlight the protective role of cardiorespiratory fitness on the association between body fat and insulin levels (Carrel et al. 2009; Carrel and Allen 2009; Steele et al. 2008; Shaibi et al. 2008; Ruiz et al. 2007; Allen et al. 2007; Bell et al. 2007). Other studies have shown, however, that the association between cardiorespiratory fitness and insulin is independent of adiposity (Srinivasan et al. 2002; Allen et al. 2007; Carrel and Allen 2009), suggesting that both cardiorespiratory fitness and adiposity are related to insulin resistance and that their effects could be independent.
Muscular strength is also considered an emergent fitness component in relation to health parameters in young population groups (Ruiz et al. 2009) and adults (Ruiz et al. 2008). Several studies examined the association of muscular strength with metabolic syndrome and insulin resistance in adults (Jurca et al. 2004, 2005; Wijndaele et al. 2007; Chen et al. 2008; Unni et al. 2009) but less is known in young people (Steene-Johannessen et al. 2009; Benson et al. 2006). Benson et al. (2006) examined the association between muscular strength and insulin resistance in a relative small sample (n = 126) of children and adolescents from New Zeland (Benson et al. 2006). They observed that children in the highest and middle tertiles of absolute upper body muscular strength were less likely to have high insulin resistance than those in the lowest muscular strength tertile (Benson et al. 2006). More recently, Steene-Johannessen et al. (2009) reported that muscular fitness (a computed score) was negatively associated with a cardiovascular disease risk score including insulin resistance, in a large sample (n = 1,592) of Norwegian children and adolescents (Steene-Johannessen et al. 2009). Other studies also analysed the association of several muscular strength indexes with metabolic risk scores (Garcia-Artero et al. 2007; Mota et al. 2010), body composition and physical activity (Moliner-Urdiales et al. 2010).
It is of public health interest to better understand whether the association between muscular strength and insulin resistance in young populations is affected by gender, as well as to know more in detail the pattern of this association in a large sample of current generations of European adolescents.
The purpose of this study was to examine the association of muscular strength with markers of insulin resistance after controlling for several potential confounders including total and central body fat in European adolescents from nine different countries.
Methods
The Healthy Lifestyle in Europe by Nutrition in Adolescence Cross-sectional study (HELENA-CSS) is a multi-centre study conducted in ten European cities from nine countries designed to obtain reliable and comparable data on nutrition and health-related parameters of a sample of European adolescents aged 12.5–17.5 years (Beghin et al. 2008). Data collection took place between October 2006 and October 2007. The total sample of the HELENA-CSS included 3,546 adolescents with a subset of 1,089 of them providing blood sample. A total of 1,053 adolescents with complete data on serum insulin concentrations, glucose, body mass index (BMI) and waist circumference were included in the present study. The group of adolescents included in the present study was similar when compared to the whole HELENA-CSS group in terms of age, sex, weight, height and muscular strength tests (all P > 0.2), except for cardiorespiratory fitness which was significantly higher in the group of females excluded than in those females included (3.9 vs. 3.4 stages, respectively; P < 0.001). The study was performed following the ethical guidelines of the Declaration of Helsinki 1964 (revision of Edinburgh 2000), the Good Clinical Practice, and the legislation about clinical research in humans in each of the participating countries. The protocol was approved by the Human Research Review Committees of the involved centres (Beghin et al. 2008).
Physical examination
The protocol for the anthropometric measurements has been described in details elsewhere (Nagy et al. 2008). Weight was measured with an electronic scale (Type SECA 861) to the nearest 0.05 kg in underwear and without shoes. Height was measured with a telescopic height measuring instrument (Type SECA 225) to the nearest 0.1 cm barefoot in the Frankfort plane. BMI was calculated as body weight (kg) divided by height (m) squared (kg/m2). BMI-z scores were subsequently calculated using the LMS Growth method (Cole et al. 1998). Skinfold thickness was measured with a Holtain Caliper to the nearest 0.2 mm in triplicate in the left side at biceps, triceps, subscapular, suprailiac, thigh, and medial calf (Crymych, UK) (Lohman et al. 1988). Body fat percentage was estimated using the equation reported elsewhere (Slaughter et al. 1988). The same trained investigator made all skinfold thickness measurements in every centre. The intraobserver technical errors of measurement were smaller than 1 mm and reliability greater than 95%. Waist circumference was measured in triplicate at the midpoint between the lowest rib and the iliac crest with an anthropometric tape SECA 200 (Lohman et al. 1988), and was used as a surrogate marker of central body fat. The same trained investigator made all waist circumference measurements in every centre, and the reliability was greater than 95%. Inter-observer reliability for skinfold and circumferences was higher than 90% (Nagy et al. 2008). Pubertal stage was assessed by a physician according to Tanner and Whitehouse (1976).
Muscular strength
Physical fitness characteristics of the study sample, as well as the procedures used for assessing it in the HELENA-CSS, have been published elsewhere (Ortega et al. 2011). The tests are valid, reliable and feasible to be used in population-based studies (Artero et al. 2010; Ortega et al. 2008; Ruiz et al. 2009; Castro-Pinero et al. 2010). We assessed upper and lower body muscular strength with the following tests:
Handgrip strength test (upper body muscular strength)
A hand dynamometer with adjustable grip was used (TKK 5101 Grip D; Takey, Tokio Japan). The adolescent squeezed gradually and continuously for at least 2 s, performing the test with the right and left hand alternatively, using the optimal grip-span. The optimal grip-span was calculated according to hand size using an equation that we developed specifically for adolescents (Ruiz et al. 2006). The maximum score in kilograms for each hand was recorded. The sum of the maximum scores achieved by left and right hands was used in the analysis.
Standing long jump test (lower body explosive muscular strength)
The participant stands behind the starting line and was instructed to push off vigorously and jump as far as possible. The distance was measured from the take-off line to the point where the back of the heel nearest to the take-off line lands on the mat. The result was recorded in centimeters (cm).
Both tests were performed twice and the best score was retained.
Cardiorespiratory fitness
It was measured by the progressive 20-m shuttle run test (Leger et al. 1984). This test required subjects to run back and forth between two lines set 20 m apart following a running pace determined by audio signals and with an initial speed of 8.5 km h−1 increasing by 0.5 km h−1 every minute (1 min equals 1 stage). The test was finished when the adolescent failed to reach the end lines concurrent with the audio signals on two consecutive occasions and the final score was computed as the number of stages completed (precision of 0.5 stages).
Blood samples
A detailed description of the blood analysis has been reported elsewhere (Gonzalez-Gross et al. 2008). Serum concentrations of glucose and insulin were measured after an overnight fast. The homeostasis model assessment (HOMA) was calculated (Matthews et al. 1985) as fasting insulin (μIU/mL) × fasting glucose (mmol/l)/22.5. To convert glucose in mg/dL to mmol/L, the value of glucose in mg/dL multiplied by a factor of 0.05551 and to convert insulin values in μlU/mL to pmol/l multiply by 6.94, before insertion into the HOMA equation. A quantitative insulin sensitivity check index (QUICKI) was calculated as QUICKI = 1/[log insulin (μIU/mL) + log glucose (mg/dL)] (Katz et al. 2000).
Statistical analysis
The data are presented as mean ± standard deviation, unless stated otherwise. To achieve normality, insulin, waist circumference and total body fat were transformed to the natural logarithm, and HOMA was raised to the power of 1/3. To examine the association between muscular strength, markers of insulin resistance and surrogate markers of body fat, we conducted partial correlation analyses controlling for pubertal status.
Multiple linear regression models were used to study the association of muscular strength with markers of insulin resistance after controlling for pubertal status, country and surrogate markers of total and central body fat. Country was entered into the models as a dummy variable. Interaction products of gender and surrogate markers of body fat in the association of muscular strength with markers of insulin resistance were explored. There was an interaction effect for gender but not for surrogate markers of body fat, therefore all the analyses were performed separately for females and males but not for body fat levels. Three different models were conducted: model I included pubertal status, country and BMI as covariates, model II included pubertal status, country and waist circumference as covariates, model III included pubertal status, country and skinfold thickness as covariates.
The association between muscular strength (quartiles) and markers of insulin resistance was assessed by one-way analysis of covariance with handgrip/body weight and standing long jump as fixed factors, and HOMA as dependent variables. Pubertal status, country, and surrogates markers of body fat were entered as covariates.
Additional analyses further controlling for cardiorespiratory fitness (stages) were performed. BMI-z scores were included into the models instead of BMI in secondary analyses. The analyses were conducted using the Statistical Package for Social Science (SPSS, v. 15.0 for Windows; SPSS Inc., Chicago, IL, USA) and the level of significance was set to 0.05.
Results
Valid data on the handgrip strength, standing long jump tests and skinfold thickness were available in 93% (n = 975), 91% (n = 963) and 93% (n = 981) of adolescents, respectively. Table 1 shows the descriptive characteristics of the study sample. Males had significantly higher waist circumference, glucose and muscular strength than females. Females had significantly higher levels of insulin and skinfold thickness than males. HOMA and QUICKI mean levels were similar for both males and females. Partial correlations among the study variables are displayed in Table 2.
The results of the linear regression models showing the association of muscular strength with markers of insulin resistance are presented in Tables 3 and 4 for males and females, respectively. In males, the handgrip strength test was negatively associated with fasting insulin and HOMA after controlling for pubertal status, country and BMI (Model I) or waist circumference (Model II) (all P < 0.05). The standing long jump test was negatively associated with fasting insulin and HOMA after controlling for waist circumference (Model II). There were no significant associations between muscular strength and markers of insulin resistance after controlling for skinfold thickness (Table 3, Model III). In females, the standing long jump test was negatively associated with fasting insulin and HOMA and positively associated with QUICKI (all P < 0.001) after controlling for pubertal status, country and surrogate markers of body fat (Models I, II and III) (Table 4).
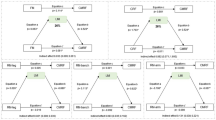
HOMA was analyzed by quartiles of handgrip/body weight (Fig. 1a) and standing long jump tests (Fig. 1b). The values (range) for the handgrip strength and standing long jump tests were 28.70–127.10 kg and 75–270 cm, respectively, in males, and 18.40–89.20 kg and 46–217 cm, respectively, in females. The cut offs for the handgrip/body weight (quartiles) were 1.01, 1.20 and 1.35 in males, and 0.82, 0.93 and 1.05 in females.
Differences in HOMA across muscular strength quartiles (a handgrip strength test; b standing long jump test) in males and females. Model I included pubertal status, country and body mass index as covariates. Model II included pubertal status, country and waist circumference as covariates. Model III included pubertal status, country and skinfold thickness as covariates
HOMA showed a negative association with muscular strength in both genders. Particularly in females, HOMA was significantly higher in quartile 1 compared to quartiles 3 or 4 for handgrip/body weight (Fig. 1a) and standing long jump (Fig. 1b) after controlling for several confounders including all the surrogate markers of body fat (all P < 0.05). In males, a similar trend was observed across quartiles of both muscular strength tests but statistically significant differences were observed after controlling for waist circumference (in both tests) and for BMI (in the standing long jump test) (all P < 0.05).
To account for the effect of weight-bearing tests, additional analyses using standing long jump multiplied by weight were undertaken; this, however, did not affect the results (data not shown). When the analyses were additionally controlled for cardiorespiratory fitness the associations of handgrip with insulin and HOMA remained significant in males (models I and II; all P < 0.05), while in females, the associations became non-significant. The results remain the same after the inclusion of BMI-z scores into the models instead of BMI (data not shown).
Discussion
The main findings of this study indicate that, in females, muscular strength, especially lower body muscular strength, is negatively associated with markers of insulin resistance after controlling for several confounders including total and central body fat (BMI and skinfold thickness, and waist circumference, respectively), yet, these associations become non-significant when cardiorespiratory fitness is taken into account. Muscular strength in males, especially upper body muscular strength, is also negatively associated with markers of insulin resistance after controlling for several confounders including BMI or waist circumference, yet the association is attenuated once the analyses are controlled for skinfold thickness.
Our findings agree with other studies (Benson et al. 2006; Steene-Johannessen et al. 2009). Benson et al. (2006) analyzed the association between muscular strength and insulin resistance in a relative small sample (n = 126) of children and adolescents (12.1 years; range 10–15 years) from New Zealand (Benson et al. 2006). Upper body muscular strength was assessed with 1 repetition maximum (RM) supine bench press (progressive repetition maximal lift), and lower body strength was assessed with a 1RM incline seated leg press. They observed a negative association between muscular strength (a combination of the upper and lower body muscular strength) and insulin resistance (HOMA) (Benson et al. 2006). However, they did not conduct gender-specific analysis, probably, due to the relative small sample size. In our study, the analyses were performed separately by gender (n = 499 males and n = 554 females) and using two relatively simple, valid, reliable, and safe field-based muscular strength tests (Ruiz et al. 2011a). Our findings showed a negative association of muscular strength (upper body muscular strength in males and lower body muscular strength in females) with markers of insulin resistance in both genders. These results suggest a gender-specific pattern for the association between muscular strength and insulin resistance due to the fact that different tests were associated for each sex. However, the difference in insulin levels between sexes as well as the fact that insulin resistance was associated with upper body strength in males and with upper and lower body strength in females is intriguing and warrants further investigation (Benson et al. 2006). It is possible that the well known higher physical activity levels in males versus females (Ruiz et al. 2011b), the differences in hormone levels and maturation between sexes (Rogol 2010), and the higher amount of body fat in females compare to males (Jiménez-Pavón et al. 2011; Rogol 2010) could affect, to some extent, the association of muscular strength with insulin resistance. In fact, it is possible that the underlying physiologic mechanism could be the joint effect of the mentioned factors and a higher fat-free mass in males compared to females (Moliner-Urdiales et al. 2010).
To note is that in males, the association between upper body muscular strength and insulin resistance disappeared after controlling for skinfold thickness, which suggest that handgrip strength could partially represents a surrogate of lean body mass. However, it is of interest that those males being in the first quartile of upper body muscular strength had a biological higher insulin resistance level compared to those in the last quartile after controlling for skinfold thickness. Steene-Johannessen et al. (2009) observed an inverse association between muscular strength (a score computed from the handgrip, standing long jump, sit-up and Biering-SØrensen tests) and a cardiovascular disease risk score including insulin resistance (HOMA), in a large sample (n = 1,592) of Norwegian children and adolescents. This inverse association was independent of cardiorespiratory fitness in both males and females. By contrast, our study showed that after controlling for cardiorespiratory fitness the associations between muscular strength and insulin resistance remained significant only in males. The discrepancies between studies might be attributable to the choice of the dependent variable (cardiovascular disease risk score vs. a single component such as insulin resistance). Artero et al. (2011) observed in the HELENA-CSS that muscular (a score of several tests) and cardiorespiratory fitness are inversely and independently associated with clustered metabolic risk, and specially those adolescents in the lower quartile of muscular fitness had a higher clustered metabolic risk than their counterparts (Artero et al. 2011). Our findings show a similar pattern for insulin resistance and add the gender-specific dimension, as well as controls for more potential confounders such as surrogate markers of body fat.
In adults, it has been suggested that resistance exercises might protect against insulin resistance by an increase in muscle quantity and an increase in skeletal muscle insulin action, indicating qualitative muscle adaptations (Koopman et al. 2006). Several studies examined the effect of resistance exercise and/or nutrition (Shaibi et al. 2006; Davis et al. 2009) on insulin sensitivity in overweight adolescents, and showed concomitant increases of muscular strength and insulin sensitivity after a 16-week intervention training in the pilot study (Shaibi et al. 2006) but not in the overall study (Davis et al. 2009). In adults, it has been suggested that higher muscular strength and muscle mass might be relevant factors against insulin resistance (Holten et al. 2004; Koopman et al. 2006; Rattarasarn et al. 2010), while in adolescents, the concrete mechanisms are not yet established.
Skeletal muscle is the major site of glucose disposal in the euglycemic state, and muscular strength is related to muscle size (Rattarasarn et al. 2010). We hypothesized that those adolescents with a higher levels of muscular strength are also those playing activities that increase muscular strength and consequently insulin sensitivity (Shaibi et al. 2006). The possibility to increase muscular strength without a concomitant increase in muscle mass in adolescents has also been stated (Shaibi et al. 2006) which could partially explain the different pattern observed in our study regarding the relationship of muscular strength with insulin resistance between genders. However, our study was not designed to analyse this issue.
The present study has several limitations. The cross-sectional nature of this study limits the ability to determine any causality in the results. Randomized controlled trials and prospective studies focused on clarifying the specific role of changes in muscular strength manifestations are needed. Moreover, it would also be useful to better understand the possible different patterns of this relationship between ethnicities. The strengths of our study include the availability of standardize measures of insulin resistance, muscle strength, cardiorespiratory fitness and fat mass in a well sex-balanced and heterogeneous sample of European healthy adolescents from nine countries.
Conclusion
Our cross-sectional observations suggest that muscular strength is negatively associated with markers of insulin resistance after controlling for several confounders including total and central body fat (BMI and waist circumference in both genders and skinfold thickness only in females). Specifically, upper body muscular strength in males and lower body muscular strength in females show the strongest association with markers of insulin resistance. In males, however, the association between muscular strength and markers of insulin resistance disappears when controlling for skinfold thickness. In addition, these associations in males are independent of cardiorespiratory fitness, while in females the associations of lower body strength and cardiorespiratory fitness with markers of insulin resistance seem to be collinear.
Preventive strategies should focus not only on decreasing fat mass and increasing cardiorespiratory fitness but also on enhancing muscular strength. In fact, the recent position statement from the National Strength and Conditioning Association and the American Academy of Pediatrics recommends a resistance training frequency of 2–3 times per week on non-consecutive days for children and adolescents (Faigenbaum et al. 2009; McCambridge and Stricker 2008).
Ethical standard The study complies with the current laws of the countries involved.
References
Allen DB, Nemeth BA, Clark RR, Peterson SE, Eickhoff J, Carrel AL (2007) Fitness is a stronger predictor of fasting insulin levels than fatness in overweight male middle-school children. J Pediatr 150(4):383–387
Artero EG, Espana-Romero V, Castro-Pinero J, Ortega FB, Suni J, Castillo-Garzon MJ, Ruiz JR (2010) Reliability of field-based fitness tests in youth. Int J Sports Med 32(3):159–169
Artero EG, Ruiz JR, Ortega FB, Espana-Romero V, Vicente-Rodriguez G, Molnar D, Gottrand F, Gonzalez-Gross M, Breidenassel C, Moreno LA, Gutierrez A (2011) Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes. doi:10.1111/j.1399-5448.2011.00769.x
Beghin L, Castera M, Manios Y, Gilbert CC, Kersting M, De Henauw S, Kafatos A, Gottrand F, Molnar D, Sjostrom M, Leclercq C, Widhalm K, Mesana MI, Moreno LA, Libersa C (2008) Quality assurance of ethical issues and regulatory aspects relating to good clinical practices in the HELENA Cross-Sectional Study. Int J Obes (Lond) 32(Suppl 5):S12–S18
Bell LM, Watts K, Siafarikas A, Thompson A, Ratnam N, Bulsara M, Finn J, O’Driscoll G, Green DJ, Jones TW, Davis EA (2007) Exercise alone reduces insulin resistance in obese children independently of changes in body composition. J Clin Endocrinol Metab 92(11):4230–4235
Benson AC, Torode ME, Singh MA (2006) Muscular strength and cardiorespiratory fitness is associated with higher insulin sensitivity in children and adolescents. Int J Pediatr Obes 1(4):222–231
Carrel AL, Allen DB (2009) The influence of fitness on insulin resistance in obese children. Rev Endocr Metab Disord 10(3):189–196
Carrel AL, McVean JJ, Clark RR, Peterson SE, Eickhoff JC, Allen DB (2009) School-based exercise improves fitness, body composition, insulin sensitivity, and markers of inflammation in non-obese children. J Pediatr Endocrinol Metab 22(5):409–415
Castro-Pinero J, Artero EG, Espana-Romero V, Ortega FB, Sjostrom M, Suni J, Ruiz JR (2010) Criterion-related validity of field-based fitness tests in youth: a systematic review. Br J Sports Med 44(13):934–943
Chen CN, Chuang LM, Wu YT (2008) Clinical measures of physical fitness predict insulin resistance in people at risk for diabetes. Phys Ther 88(11):1355–1364
Cole TJ, Freeman JV, Preece MA (1998) British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 17(4):407–429
Davis JN, Kelly LA, Lane CJ, Ventura EE, Byrd-Williams CE, Alexandar KA, Azen SP, Chou CP, Spruijt-Metz D, Weigensberg MJ, Berhane K, Goran MI (2009) Randomized control trial to improve adiposity and insulin resistance in overweight Latino adolescents. Obesity (Silver Spring) 17(8):1542–1548
Faigenbaum AD, Kraemer WJ, Blimkie CJ, Jeffreys I, Micheli LJ, Nitka M, Rowland TW (2009) Youth resistance training: updated position statement paper from the national strength and conditioning association. J Strength Cond Res 23(5 Suppl):S60–S79
Garcia-Artero E, Ortega FB, Ruiz JR, Mesa JL, Delgado M, Gonzalez-Gross M, Garcia-Fuentes M, Vicente-Rodriguez G, Gutierrez A, Castillo MJ (2007) Lipid and metabolic profiles in adolescents are affected more by physical fitness than physical activity (AVENA study). Rev Esp Cardiol 60(6):581–588
Gonzalez-Gross M, Breidenassel C, Gomez-Martinez S, Ferrari M, Beghin L, Spinneker A, Diaz LE, Maiani G, Demailly A, Al-Tahan J, Albers U, Warnberg J, Stoffel-Wagner B, Jimenez-Pavon D, Libersa C, Pietrzik K, Marcos A, Stehle P (2008) Sampling and processing of fresh blood samples within a European multicenter nutritional study: evaluation of biomarker stability during transport and storage. Int J Obes (Lond) 32(Suppl 5):S66–S75
Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F (2004) Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 53(2):294–305
Jiménez-Pavón D, Castillo MJ, Moreno LA, Kafatos A, Manios Y, Kondaki K, BG L, Zaccaria M, de Henauw S, Widhalm K, Moln RD, Sj Str MM, Gonzalez-Gross M, Ruiz JR (2011) Fitness and fatness are independently associated with markers of insulin resistance in European adolescents; the HELENA study. Int J Pediatr Obes 6(3–4):253–260
Jurca R, Lamonte MJ, Church TS, Earnest CP, Fitzgerald SJ, Barlow CE, Jordan AN, Kampert JB, Blair SN (2004) Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc 36(8):1301–1307
Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN (2005) Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc 37(11):1849–1855
Karam JG, McFarlane SI (2008) Prevention of type 2 DM: implications for adolescents and young adults. Pediatr Endocrinol Rev 5(Suppl 4):980–988
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85(7):2402–2410
Koopman R, Manders RJ, Jonkers RA, Hul GB, Kuipers H, van Loon LJ (2006) Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Eur J Appl Physiol 96(5):525–534
Leger L, Lambert J, Goulet A, Rowan C, Dinelle Y (1984) Aerobic capacity of 6 to 17-year-old Quebecois—20 meter shuttle run test with 1 minute stages. Can J Appl Sport Sci 9(2):64–69
Lohman TG, Roche AF, Martorell R (1988) Anthropometric standardization reference manual. Human Kinetics Books, Champaign
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
McCambridge TM, Stricker PR (2008) Strength training by children and adolescents. Pediatrics 121(4):835–840
Moliner-Urdiales D, Ortega FB, Vicente-Rodriguez G, Rey-Lopez JP, Gracia-Marco L, Widhalm K, Sjostrom M, Moreno LA, Castillo MJ, Ruiz JR (2010) Association of physical activity with muscular strength and fat-free mass in adolescents: the HELENA study. Eur J Appl Physiol 109(6):1119–1127
Mota J, Vale S, Martins C, Gaya A, Moreira C, Santos R, Ribeiro JC (2010) Influence of muscle fitness test performance on metabolic risk factors among adolescent girls. Diabetol Metab Syndr 2:42
Nagy E, Vicente-Rodriguez G, Manios Y, Beghin L, Iliescu C, Censi L, Dietrich S, Ortega FB, De Vriendt T, Plada M, Moreno LA, Molnar D (2008) Harmonization process and reliability assessment of anthropometric measurements in a multicenter study in adolescents. Int J Obes (Lond) 32(Suppl 5):S58–S65
Ortega FB, Artero EG, Ruiz JR, Vicente-Rodriguez G, Bergman P, Hagstromer M, Ottevaere C, Nagy E, Konsta O, Rey-Lopez JP, Polito A, Dietrich S, Plada M, Beghin L, Manios Y, Sjostrom M, Castillo MJ (2008) Reliability of health-related physical fitness tests in European adolescents. The HELENA Study. Int J Obes (Lond) 32(Suppl 5):S49–S57
Ortega FB, Artero EG, Ruiz JR, Espana-Romero V, Jimenez-Pavon D, Vicente-Rodriguez G, Moreno LA, Manios Y, Beghin L, Ottevaere C, Ciarapica D, Sarri K, Dietrich S, Blair SN, Kersting M, Molnar D, Gonzalez-Gross M, Gutierrez A, Sjostrom M, Castillo MJ (2011) Physical fitness levels among European adolescents: the HELENA study. Br J Sports Med 45(1):20–29
Rattarasarn C, Leelawattana R, Soonthornpun S (2010) Contribution of skeletal muscle mass on sex differences in 2-hour plasma glucose levels after oral glucose load in Thai subjects with normal glucose tolerance. Metabolism 59(2):172–176
Rogol AD (2010) Sex steroids, growth hormone, leptin and the pubertal growth spurt. Endocr Dev 17:77–85
Ruiz JR, Rizzo NS, Hurtig-Wennlof A, Ortega FB, Warnberg J, Sjostrom M (2006) Relations of total physical activity and intensity to fitness and fatness in children: the European Youth Heart Study. Am J Clin Nutr 84(2):299–303
Ruiz JR, Rizzo NS, Ortega FB, Loit HM, Veidebaum T, Sjostrom M (2007) Markers of insulin resistance are associated with fatness and fitness in school-aged children: the European Youth Heart Study. Diabetologia 50(7):1401–1408
Ruiz JR, Sui X, Lobelo F, Morrow JR Jr, Jackson AW, Sjostrom M, Blair SN (2008) Association between muscular strength and mortality in men: prospective cohort study. BMJ 337:a439
Ruiz JR, Castro-Pinero J, Artero EG, Orgeta FB, Sjostrom M, Suni J, Castillo MJ (2009) Predictive validity of health-related fitness in youth: a systematic review. Br J Sports Med 43(12):909–923
Ruiz JR, Castro-Pinero J, Espana-Romero V, Artero EG, Ortega FB, Cuenca MM, Jimenez-Pavon D, Chillon P, Girela-Rejon MJ, Mora J, Gutierrez A, Suni J, Sjostrom M, Castillo MJ (2011a) Field-based fitness assessment in young people: the ALPHA health-related fitness test battery for children and adolescents. Br J Sports Med 45(6):518–524
Ruiz JR, Ortega FB, Martinez-Gomez D, Labayen I, Moreno LA, De Bourdeaudhuij I, Manios Y, Gonzalez-Gross M, Mauro B, Molnar D, Widhalm K, Marcos A, Beghin L, Castillo MJ, Sjostrom M (2011b) Objectively measured physical activity and sedentary time in European adolescents: the HELENA study. Am J Epidemiol 174(2):173–184
Shaibi GQ, Cruz ML, Ball GD, Weigensberg MJ, Salem GJ, Crespo NC, Goran MI (2006) Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc 38(7):1208–1215
Shaibi GQ, Faulkner MS, Weigensberg MJ, Fritschi C, Goran MI (2008) Cardiorespiratory fitness and physical activity in youth with type 2 diabetes. Pediatr Diabetes 9(5):460–463
Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA (1988) Skinfold equations for estimation of body fatness in children and youth. Hum Biol 60(5):709–723
Srinivasan SR, Myers L, Berenson GS (2002) Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes 51(1):204–209
Steele RM, Brage S, Corder K, Wareham NJ, Ekelund U (2008) Physical activity, cardiorespiratory fitness, and the metabolic syndrome in youth. J Appl Physiol 105(1):342–351
Steene-Johannessen J, Anderssen SA, Kolle E, Andersen LB (2009) Low muscle fitness is associated with metabolic risk in youth. Med Sci Sports Exerc 41(7):1361–1367
Tanner JM, Whitehouse RH (1976) Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 51(3):170–179
Tresaco B, Bueno G, Moreno LA, Garagorri JM, Bueno M (2003) Insulin resistance and impaired glucose tolerance in obese children and adolescents. J Physiol Biochem 59(3):217–223
Unni US, Ramakrishnan G, Raj T, Kishore RP, Thomas T, Vaz M, Kurpad AV (2009) Muscle mass and functional correlates of insulin sensitivity in lean young Indian men. Eur J Clin Nutr 63(10):1206–1212
van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B (2010) The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil 17(Suppl 1):S3–S8
Wijndaele K, Duvigneaud N, Matton L, Duquet W, Thomis M, Beunen G, Lefevre J, Philippaerts RM (2007) Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc 39(2):233–240
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000, projections for 2030. Diabetes Care 27(5):1047–1053
Acknowledgments
We gratefully acknowledge all participating children and adolescents, and their parents and teachers for their collaboration. We also acknowledge all the members involved in the field work for their efforts and great enthusiasm. The HELENA study takes place with the financial support of the European Community Sixth RTD Framework Programme (Contract FOOD-CT-2005-007034). This analysis was also supported by grants from the Spanish Ministry of Education (JCI-2010-07055, AP-2005-4358, RYC-2010-05957, EX-2008-0641, AGL2007-29784-E/ALI), Universidad Politécnica de Madrid (CH/018/2008), the Swedish Council for Working Life and Social Research, and the ALPHA study, an European Union-funded study, in the framework of the Public Health Programme (Ref: 2006120). This study was also supported by a grant from the Spanish Ministry of Health: Maternal, Child Health and Development Network (number RD08/0072) (LMA) and Science-FEDER funds (Acciones Complementarias DEP2007-29933-E). Many thanks to Petra Pickert and Anke Carstensen for their contribution to laboratory work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan A. Ward.
on behalf of the HELENA Study group. The Members of the HELENA study group are given in Appendix.
Appendix: HELENA Study Group
Appendix: HELENA Study Group
Co-ordinator: Luis A. Moreno.
Core Group members: Luis A. Moreno, Fréderic Gottrand, Stefaan De Henauw, Marcela González-Gross, Chantal Gilbert.
Steering Committee: Anthony Kafatos (President), Luis A. Moreno, Christian Libersa, Stefaan De Henauw, Jackie Sánchez, Fréderic Gottrand, Mathilde Kersting, Michael Sjöstrom, Dénes Molnár, Marcela González-Gross, Jean Dallongeville, Chantal Gilbert, Gunnar Hall, Lea Maes, Luca Scalfi.
Project Manager: Pilar Meléndez.
-
1.
Universidad de Zaragoza (Spain)
Luis A. Moreno, Jesús Fleta, José A. Casajús, Gerardo Rodríguez, Concepción Tomás, María I. Mesana, Germán Vicente-Rodríguez, Adoración Villarroya, Carlos M. Gil, Ignacio Ara, Juan Revenga, Carmen Lachen, Juan Fernández Alvira, Gloria Bueno, Aurora Lázaro, Olga Bueno, Juan F. León, Jesús Mª Garagorri, Manuel Bueno, Juan Pablo Rey López, Iris Iglesia, Paula Velasco, Silvia Bel, Theodora Mouratidou.
-
2.
Consejo Superior de Investigaciones Científicas (Spain)
Ascensión Marcos, Julia Wärnberg, Esther Nova, Sonia Gómez, Esperanza Ligia Díaz, Javier Romeo, Ana Veses, Mari Angeles Puertollano, Belén Zapatera, Tamara Pozo.
-
3.
Université de Lille 2 (France)
Laurent Beghin, Christian Libersa, Frédéric Gottrand, Catalina Iliescu, Juliana Von Berlepsch.
-
4.
Research Institute of Child Nutrition Dortmund, Rheinische Friedrich-Wilhelms-Universität Bonn (Germany)
Mathilde Kersting, Wolfgang Sichert-Hellert, Ellen Koeppen.
-
5.
Pécsi Tudományegyetem (University of Pécs) (Hungary)
Dénes Molnar, Eva Erhardt, Katalin Csernus, Katalin Török, Szilvia Bokor, Mrs. Angster, Enikö Nagy, Orsolya Kovács, Judit Répasi.
-
6.
University of Crete School of Medicine (Greece)
Anthony Kafatos, Caroline Codrington, María Plada, Angeliki Papadaki, Katerina Sarri, Anna Viskadourou, Christos Hatzis, Michael Kiriakakis, George Tsibinos, Constantine Vardavas Manolis Sbokos, Eva Protoyeraki, Maria Fasoulaki.
-
7.
Institut für Ernährungs- und Lebensmittelwissenschaften–Ernährungphysiologie. Rheinische Friedrich Wilhelms Universität (Germany)
Peter Stehle, Klaus Pietrzik, Marcela González-Gross, Christina Breidenassel, Andre Spinneker, Jasmin Al-Tahan, Miriam Segoviano, Anke Berchtold, Christine Bierschbach, Erika Blatzheim, Adelheid Schuch, Petra Pickert.
-
8.
University of Granada (Spain)
Manuel J. Castillo Garzón, Ángel Gutiérrez Sáinz, Francisco B. Ortega Porcel, Jonatan Ruiz Ruiz, Enrique García Artero, Vanesa España Romero, David Jiménez-Pavón, Cristóbal Sánchez Muñoz, Victor Soto, Palma Chillón, Jose M. Heredia, Virginia Aparicio, Pedro Baena, Claudia M. Cardia, Ana Carbonell.
-
9.
Istituto Nazionalen di Ricerca per gli Alimenti e la Nutrizione (Italy)
Davide Arcella, Giovina Catasta, Laura Censi, Donatella Ciarapica, Marika Ferrari, Cinzia Le Donne, Catherine Leclerq, Luciana Magrì, Giuseppe Maiani, Rafaela Piccinelli, Angela Polito, Raffaela Spada, Elisabetta Toti.
-
10.
University of Napoli “Federico II” Dept of Food Science (Italy)
Luca Scalfi, Paola Vitaglione, Concetta Montagnese.
-
11.
Ghent University (Belgium)
Ilse De Bourdeaudhuij, Stefaan De Henauw, Tineke De Vriendt, Lea Maes, Christophe Matthys, Carine Vereecken, Mieke de Maeyer, Charlene Ottevaere, Inge Huybrechts.
-
12.
Medical University of Vienna (Austria)
Kurt Widhalm, Katharina Phillipp, Sabine Dietrich, Birgit Kubelka
Marion Boriss-Riedl.
-
13.
Harokopio University (Greece)
Yannis Manios, Eva Grammatikaki, Zoi Bouloubasi, Tina Louisa Cook, Sofia Eleutheriou, Orsalia Consta, George Moschonis, Ioanna Katsaroli, George Kraniou, Stalo Papoutsou, Despoina Keke, Ioanna Petraki, Elena Bellou, Sofia Tanagra, Kostalenia Kallianoti, Dionysia Argyropoulou, Katerina Kondaki, Stamatoula Tsikrika, Christos Karaiskos.
-
14.
Institut Pasteur de Lille (France)
Jean Dallongeville, Aline Meirhaeghe.
-
15.
Karolinska Institutet (Sweden)
Michael Sjöstrom, Patrick Bergman, María Hagströmer, Lena Hallström, Mårten Hallberg, Eric Poortvliet, Julia Wärnberg, Nico Rizzo, Linda Beckman, Anita Hurtig Wennlöf, Emma Patterson, Lydia Kwak, Lars Cernerud, Per Tillgren, Stefaan Sörensen.
-
16.
Asociación de Investigación de la Industria Agroalimentaria (Spain)
Jackie Sánchez-Molero, Elena Picó, Maite Navarro, Blanca Viadel, José Enrique Carreres, Gema Merino, Rosa Sanjuán, María Lorente, María José Sánchez, Sara Castelló.
-
17.
Campden BRI (United Kingdom)
Chantal Gilbert, Sarah Thomas, Elaine Allchurch, Peter Burguess.
-
18.
SIK - Institutet foer Livsmedel och Bioteknik (Sweden)
Gunnar Hall, Annika Astrom, Anna Sverkén, Agneta Broberg.
-
19.
Meurice Recherche & Development asbl (Belgium)
Annick Masson, Claire Lehoux, Pascal Brabant, Philippe Pate, Laurence Fontaine.
-
20.
Campden & Chorleywood Food Development Institute (Hungary)
Andras Sebok, Tunde Kuti, Adrienn Hegyi.
-
21.
Productos Aditivos SA (Spain)
Cristina Maldonado, Ana Llorente.
-
22.
Cárnicas Serrano SL (Spain)
Emilio García.
-
23.
Cederroth International AB (Sweden)
Holger von Fircks, Marianne Lilja Hallberg, Maria Messerer
-
24.
Lantmännen Food R&D (Sweden)
Mats Larsson, Helena Fredriksson, Viola Adamsson, Ingmar Börjesson.
-
25.
European Food Information Council (Belgium)
Laura Fernández, Laura Smillie, Josephine Wills.
-
26.
Universidad Politécnica de Madrid (Spain)
Marcela González-Gross, Jara Valtueña, Ulrike Albers, Raquel Pedrero, Agustín Meléndez, Pedro J. Benito, David Cañada, David Jiménez-Pavón, Alejandro Urzanqui, Juan Carlos Ortiz, Francisco Fuentes, Juan José Gómez Lorente, Rosa María Torres, Paloma Navarro
Rights and permissions
About this article
Cite this article
Jiménez-Pavón, D., Ortega, F.B., Valtueña, J. et al. Muscular strength and markers of insulin resistance in European adolescents: the HELENA Study. Eur J Appl Physiol 112, 2455–2465 (2012). https://doi.org/10.1007/s00421-011-2216-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2216-5