Abstract
Near-infrared spectroscopy (NIRS) is a continuous and noninvasive technology that measures regional tissue oxygen saturation (rSO2). A new 4-wavelength generation of NIRS monitors is now available. We aimed to compare peripheral somatic rSO2 values given by the 4-wavelength EQUANOX™ 7600 device (Nonin Medical Inc., Plymouth, Mn) and O3™ device (Masimo Corporation, Irvine, CA). Twenty adult patients scheduled for conventional elective cardiac surgery with cardiopulmonary bypass over a 4-month period were included after local Ethics Committee approval. For each patient, 2 NIRS sensors (EQUANOX and O3) were placed over the medial part of the forearm. Thirteen couples of measurements were performed at predefined intraoperative time points. We compared 260 couples of absolute intraoperative rSO2 values. No significant difference was found between both monitors: EQUANOX median rSO2 60% (95% CI 57–62) versus O3 median rSO2 62% (95% CI 61–64), P = 0.103. Bias was 4.0% and limits of agreement were ±26.3%. Significant correlations were evidenced between EQUANOX and O3 rSO2 absolute values: rho = 0.758 (95% CI 0.701–0.806), P < 0.0001, and rSO2 percent maximum difference versus baseline: rho = 0.582 (95% CI 0.188–0.815), P = 0.007. While absolute values of rSO2 given by both devices were equivalent and well correlated, the clinical agreement is probably not acceptable, meaning that EQUANOX and O3 are not interchangeable in routine practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Near-infrared spectroscopy (NIRS) is a continuous and non-invasive technology that measures regional tissue oxygen saturation (rSO2) [1]. Initially marketed for cerebral oximetry, peripheral measurements have also been validated [2, 3]. Overall, rSO2 can be considered as a meta-parameter influenced by oxygenation, ventilation, hemoglobin, regional perfusion and metabolism [4]. Whatever the site of measurement, a normal value of rSO2 would suggest adequacy between oxygen supply and consumption at the regional level [5]. NIRS is increasingly used in perioperative clinical practice to optimize hemodynamics in high-risk patients. Thus, absolute rSO2 values below 50% or a 20% decrease from baseline have been considered as valuable triggers to initiate therapeutic interventions [6]. There is however no well-established reference values for cerebral and peripheral rSO2, and the commercially available devices differ in numerous technical aspects, explaining why they are not clinically interchangeable [7]. These inter-device technologic differences suggest a potential for variation in the ability to acquire and spatially resolve rSO2 signals [7, 8]. Two and 3-wavelength NIRS devices were first available and generally used as trend monitors. A new 4-wavelength generation of NIRS devices proposing a reliable real-time assessment of absolute values of rSO2 has recently emerged. These new monitors could be of paramount importance to help practitioners to apply therapeutic strategies including predefined rSO2 threshold values at the bedside. The O3™ NIRS monitor (Masimo Corporation, Irvine, CA) is the most recent. The system uses NIRS, interrogating tissue by transmitting light of four different wavelengths through the tissue and processing the received light waveforms, to provide continuous measurement of rSO2. No independent comparative data have been yet published in human.
Therefore, the objectives of the present study were to compare simultaneous peripheral rSO2 absolute values given by two different 4-wavelength NIRS devices, namely the EQUANOX™ 7600 (Nonin Medical, Inc, Plymouth, MN) and the O3™ monitor during conventional cardiac surgery with cardiopulmonary bypass. We also compared dynamic changes in rSO2 between predefined intraoperative time points. We tested the hypothesis that both absolute values and changes in rSO2 could be comparable and both monitors would be interchangeable.
2 Materials and methods
Twenty adult patients undergoing conventional elective cardiac surgery with cardiopulmonary bypass (coronary artery bypass grafting, aortic valve replacement, or combined cardiac surgery) were prospectively included at the Louis Pradel Teaching University Hospital (Lyon, France) over a 4-month period according to the availability of the investigators. Institutional approval was obtained from the local Ethics Committee (Comité de Protection des Personnes Sud-Est III, Groupement Hospitalier Est, Lyon, France; Ref#QH 10/2015, approved on September 29, 2015). As the design of the study was purely observational, waive written informed consent was authorized. Verbal information was however given to all patients. The study was registered on ClinicalTrials.gov under the number NCT02847273. Patients were not included if they were younger than 18 years, have a body mass index >30 kg/m2, underwent urgent (<24-h) or redo surgery and surgery without cardiopulmonary bypass. Pregnant women, nonwhite people, and patients with chronic anemia were also not included in the study.
2.1 Perioperative management
General anesthesia, myocardial protection, cardiopulmonary bypass, and postoperative management followed institutional standards. Briefly, all patients were premedicated with oral hydroxyzine (1 mg/kg) or lorazepam (1 mg) on the evening before surgery and on the morning of surgery. Preoperative betablockers and statins were given systematically until the morning of surgery in chronically treated patients. Oral antiplatelet agents were managed as follows: aspirin was continued and clopidogrel was discontinued 5 days before surgery. Standardized total intravenous anesthesia (i.e., target-control propofol and remifentanil or sufentanil infusion, and cisatracurium) and monitoring techniques (i.e., 5-lead electrocardiogram with computerized analysis of repolarization, invasive arterial blood pressure by means of a radial artery catheter on the opposite hand of the NIRS optode, and central venous pressure by means of a jugular venous central catheter) were used in all patients. Remote ischemic preconditioning was systematically performed before the start of cardiopulmonary bypass, and consisted in four consecutive brief periods of 5 min each of ischemia and reperfusion by means of an inflatable cuff positioned at the upper extremity of the ipsilateral upper limb [9].
Antifibrinolytic therapy with tranexamic acid (15 mg/kg twice) routinely was administered. Anticoagulation was obtained during cardiopulmonary bypass with an initial bolus of heparin (300 UI/kg) to maintain activated coagulation time more than 450 s. Reversion was systematically performed with protamine. Cardiopulmonary bypass was performed under normothermia and myocardial protection was achieved by intermittent cold crystalloid cardioplegia. Boluses of ephedrine and/or phenylephrine were given intraoperatively to maintain mean arterial pressure between 50 and 80 mmHg. The heart was defibrillated after aortic unclamping, if sinus rhythm did not resume spontaneously. After the termination of cardiopulmonary bypass, norepinephrine was used to maintain the mean arterial pressure >65 mmHg, and the trigger for transfusion of packed erythrocytes was set to a hematocrit of 21% in all patients and complied with routine practice at the study institution. In the postoperative period, all patients were admitted to the cardiac surgical intensive care unit (ICU). Postoperative care was delivered by cardiac anesthesiologists in the ICU. Extubation was performed after completion of the institutional weaning protocol. Standard postoperative care included blood glucose control <10 mM and a low-molecular-weight heparin, beginning 6 h after surgery in the absence of significant mediastinal bleeding. Betablockers, renin-angiotensin system inhibitors, and statins were given as soon as possible postoperatively in chronically treated patients.
2.2 Study protocol
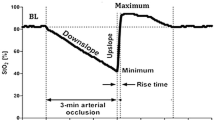
After rubbing and cleaning the skin with an alcohol swab, two sensors (EQUANOX Advance sensor adult model 8004CA; Nonin Medical, Inc, Plymouth, MN, and O3 sensor MasimoSet; Masimo, Irvine, CA) were carefully placed over the medial part of the skeletal muscle of the left or right forearm, 5 cm below the elbow, allowing measurements of peripheral rSO2. The optodes were attached to the skin with opaque adhesive stickers so the angle and position of the optodes were kept constant. The sensors were connected both to the 4-wavelength NIRS EQUANOX and O3 monitors. All rSO2 values were recorded continuously and read every second. Data were recorded online and stored for further analysis. Thirteen couples of simultaneous measurements were performed at predefined intraoperative time points for each patient. The first-time point was collected before the induction of general anesthesia and served as baseline rSO2 values for both monitors. Then, the following 8 time points were assessed during the four-consecutive remote ischemic preconditioning dynamic maneuvers immediately before ischemia and at the nadir of rSO2, immediately before reperfusion. The remaining time points were assessed 10 min after the beginning of cardiopulmonary bypass, 10 min after the termination of cardiopulmonary bypass and at the time of chest and skin closures. The different time points of the study for each patient are depicted in Fig. 1.
2.3 Endpoints
The primary endpoint of the study was the agreement between the 4-wavelength NIRS EQUANOX and O3 monitors in assessing absolute values of peripheral rSO2. Secondary endpoints were the relationships between absolute values and changes in rSO2 among intraoperative time points.
2.4 Statistical analysis
The number of patients was fixed empirically at 20. Data are presented as mean ± standard deviation (SD) or median (25th–75th) for non-normally distributed variables (Kolmogorov–Smirnov test) or number (%), as appropriate. Continuous variables were analyzed with the Mann–Whitney U test to compare absolute rSO2 values and percent maximum difference versus baseline values for both NIRS devices. Areas under the curves (AUC) of rSO2 were calculated via serial measurements integrating the number of intraoperative time points below baseline values over the time. A modified Bland–Altman analysis for repeated measurements was used to assess bias and limits of agreement (bias ± 1.96 SD) between rSO2 given by both NIRS devices [10]. Correlations between absolute values of peripheral rSO2 and between percent maximum difference versus baseline values given by the 4-wavelength NIRS EQUANOX and O3 monitors were determined by the Spearman correlation coefficient rho and its 95% confidence interval (CI).
All tests were two-tailed, and a P value <0.05 was considered as statistically significant. Statistical analyses were performed using MedCalc Statistical Software version 14.10.2 (MedCalc Software bvba, Ostend, Belgium).
3 Results
Twenty adult patients were prospectively included in the study from October 2015 to January 2016. Patients demographic and pre- and intraoperative clinical characteristics are reported in Table 1. We compared 260 couples of absolute intraoperative peripheral rSO2 values. No significant difference was found between both monitors: EQUANOX median rSO2 60% (95% CI 57–62) [range 4–93] versus O3 median rSO2 62% (95% CI 61–64) [range 19–95], P = 0.103 (Fig. 2). No difference was also found between EQUANOX and O3 median rSO2 values at baseline: 70% (95% CI 61–78) versus 68% (95% CI 63–74), P = 0.766 (N = 20 couples of measurements). As the primary endpoint, the agreement between the two devices is represented in Fig. 3. Bias was 4.0% and limits of agreements were ±26.3%. A significant positive relationship was evidenced between EQUANOX and O3 absolute values of rSO2: rho = 0.758 (95% CI 0.701–0.806), P < 0.001 (Fig. 4).
Marked intraoperative changes in peripheral rSO2 values were observed during the study. Especially, a deep decrease followed by a rapid increase overshooting baseline values were found during remote ischemic preconditioning, whatever the NIRS technology. The rSO2 percent maximum difference versus baseline was statistically different between EQUANOX and O3 NIRS devices: 66% (95% CI 49–82) versus 44% (95% CI 36–54), respectively (P = 0.003, N = 20 couples of measurements). The percentage of intraoperative time below rSO2 baseline value was similar for EQUANOX and O3: 88% (95% CI 66–92) versus 87% (95% CI 66–91), P = 0.841 (N = 20 couples of measurements). AUC of rSO2 were also similar between both monitors: EQUANOX median AUC 149 (95% CI 56–235) versus O3 median AUC 112 (95% CI 35–159), P = 0.204 (N = 20 couples of measurements). A moderate positive relationship was observed between intraoperative rSO2 percent maximum difference versus baseline given by both NIRS devices: rho = 0.582 (95% CI 0.188–0.815), P = 0.007 (Fig. 5).
4 Discussion
The main results of the present study are that intraoperative values of peripheral rSO2 given by the two 4-wavelength NIRS devices EQUANOX and O3 are similar and well correlated. However, the limits of agreement are large, suggesting that both devices are not interchangeable in routine clinical practice. Moreover, intraoperative percent maximum changes in rSO2 values were significantly different and moderately correlated, reinforcing the fact that a single monitor should always be used in a single patient.
NIRS devices differ in numerous important aspects, and all of these interdevice technologic differences suggest a potential for variation in the ability to acquire and spatially resolve rSO2 signals [7, 11]. Both EQUANOX and O3 monitors belong to a new generation of 4-wavelength NIRS devices. They also differ in several points, as the distance between light emitters and detectors, the depth of light penetration inside the tissue, and the built-in proprietary algorithm used to assess oxygen saturation. To date, a single study reported a clinical evaluation of absolute and trend accuracy of the O3 monitor in assessing cerebral rSO2 with interesting results [12]. In healthy volunteers undergoing controlled hypoxia, the authors found an absolute root-mean-squared error of 4% and a relative root-mean-squared error of 2.1% when compared to a reference value combining arterial and central venous oxygen saturations [12]. Numerous studies compared commercially available NIRS devices between them [7, 11, 13,14,15], but no independent comparative data regarding the O3 monitor have been yet published in human, fully justifying the current work. We found that O3 and 4-wavelength EQUANOX were not interchangeable when measuring peripheral rSO2 in the cardiac surgical setting, as recently reported with older generations of NIRS devices [16]. These results could be of paramount importance when considering algorithms aiming to reverse a regional desaturation (peripheral or cerebral) below pre-defined absolute threshold values in outcomes multicenter trials.
The utility of a vascular occlusion test (VOT) in addition to the measurement of rSO2 has been suggested to assess the microcirculatory response to an ischemic stress in critically ill patients [17]. Different parameters can be derived from the NIRS oxygen saturation monitoring, as the rate of desaturation during ischemia, the rate of resaturation, and the peak value of rSO2 during the initial phase of reperfusion [18]. These dynamic indices markedly differed between male and female [19] and also when they are calculated with different NIRS devices [7, 16]. Furthermore, the absence of automatized calculation—at the exception of the InSpectra™ device (Hutchinson, MN, USA)—strongly limits their interest at the bedside for routine clinical practice. In the present observational study, we routinely used remote ischemic preconditioning before cardiopulmonary bypass, an intervention that has been shown to reduce the extent of perioperative myocardial injury in patients undergoing cardiac surgery [20]. It resulted in major changes in intraoperative rSO2 absolute values, responsible for the very large range of rSO2 observed with both devices. Then, we compared the percent maximum difference with baseline values and we found significant differences, mainly related to differences in minimum values during ischemia, and only a moderate correlation between O3 and EQUANOX, even if changes in rSO2 values moved in the same way. Again, these results suggest that rSO2 variations cannot be extrapolated from one device to another.
Some comments are necessary concerning the limitations of the present study. First, the number of patients we investigated was low and fixed empirically, so that no definitive conclusion can be drawn from our work. Further studies comparing O3 with both reference methods and other 4-wavelength NIRS monitors in various subsets of surgical patients are mandatory before recommending a wider use of O3 for routine practice. Second, we did not compare peripheral rSO2 absolute values with a reference method, namely the gradient between arterial and central venous oxygen saturation [12, 21]. While encouraging results have been previously reported in healthy volunteers with cerebral rSO2 [12], it remains to be established in cardiac surgical patients with peripheral rSO2. Third, we placed two different NIRS sensors on the same forearm and we cannot formally exclude interferences between both NIRS signals, even if we respected a minimal distance between them and if opaque adhesive stickers were systematically used. Fourth, we did not use multimodal O3 NIRS monitoring aiming to compare regulated and non-regulated regional perfusions by simultaneous measurements in a single patient of cerebral and somatic rSO2 values. To date, no definitive conclusion can however be drawn regarding potential routine benefits in cardiac surgical patients of such a multimodal strategy. Finally, no published data showed the superiority of 4-wavelength NIRS rSO2 -based algorithms in helping to manage high-risk surgical patients or clinical decision making at the bedside.
5 Conclusions
In conclusion, the new 4-wavelength NIRS device O3 used to assess peripheral tissue oxygenation is not interchangeable with the 4-wavelength NIRS device EQUANOX in cardiac surgery patients. These results should be taken into account for both clinical research and routine practice.
References
Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3–i13.
Bartels SA, Bezemer R, de Vries FJW, Milstein DMJ, Lima A, Cherpanath TGV, Anton Van den Meiracker H, Jasper Van Bommel J, Heger M, Karemaker JM, Ince C. Multi-site and multi-depth near-infrared spectroscopy in a model of simulated (central) hypovolemia: lower body negative pressure. Intensive Care Med. 2011;37(4):671–7.
Cole AL, Herman RA, Heimlich JB, Ahsan S, Freedman BA, Shuler MS. Ability of near infrared spectroscopy to measure oxygenation in isolated upper extremity muscle compartments. J Hand Surg. 2012;37(2):297–302.
Siegenthaler N, Giraud R, Piriou V, Bendjelid K. Near-infrared spectroscopy monitoring during cardiac surgery: a promising concept? Ann Fr Anesth Réanim. 2011;30(7–8):531–2.
Scheeren TWL. Journal of Clinical Monitoring and Computing 2015 end of year summary: tissue oxygenation and microcirculation. J Clin Monit Comput. 2016;30:141–6.
Scheeren TWL, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput. 2012;26(4):279–87.
Fellahi J-L, Butin G, Fischer M-O, Zamparini G, Gérard J-L, Hanouz J-L. Dynamic evaluation of near-infrared peripheral oximetry in healthy volunteers: a comparison between INVOS and EQUANOX. J Crit Care. 2013;28(5):881.e1-881.e6.
Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology. 2012;116(4):834–40.
Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhäuser M, Peters J, Jakob H, Heusch G. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet Lond Engl. 2013;382(9892):597–604.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–82.
Bickler PE, Feiner JR, Rollins MD. Factors affecting the performance of 5 cerebral oximeters during hypoxia in healthy volunteers. Anesth Analg. 2013;117(4):813–23.
Redford D, Paidy S, Kashif F. Absolute and trend accuracy of a new regional oximeter in healthy volunteers during controlled hypoxia. Anesth Analg. 2014;119(6):1315–9.
Pisano A, Galdieri N, Iovino TP, Angelone M, Corcione A. Direct comparison between cerebral oximetry by INVOSTM and EQUANOXTM during cardiac surgery: a pilot study. Heart Lung Vessels. 2014;6(3):197–203.
Cournoyer A, Denault A, Cossette S, Fortier A, Daoust R, Iseppon M, Chauny JM, Notebaert E. Reproducibility, interchangeability of measures, time to measure stabilization, and reference values of two tissue oximeters in healthy volunteers. J Biomed Opt. 2016;21(9):97003.
Douds MT, Straub EJ, Kent AC, Bistrick CH, Sistino JJ. A systematic review of cerebral oxygenation-monitoring devices in cardiac surgery. Perfusion. 2014;29(6):545–52.
Steenhaut K, Lapage K, Bové T, De Hert S, Moerman A. Evaluation of different near-infrared spectroscopy technologies for assessment of tissue oxygen saturation during a vascular occlusion test. J Clin Monit Comput. 2016. doi:10.1007/s10877-016-9962-1.
Gómez H, Torres A, Polanco P, Kim HK, Zenker S, Puyana JC, Pinsky MR. Use of non-invasive NIRS during a vascular occlusion test to assess dynamic tissue O(2) saturation response. Intensive Care Med. 2008;34(9):1600–7.
Mayeur C, Campard S, Richard C, Teboul J-L. Comparison of four different vascular occlusion tests for assessing reactive hyperemia using near-infrared spectroscopy. Crit Care Med. 2011;39(4):695–701.
Fellahi J-L, Butin G, Zamparini G, Fischer M-O, Gérard J-L, Hanouz J-L. Lower limb peripheral NIRS parameters during a vascular occlusion test: an experimental study in healthy volunteers. Ann Fr Anesth Réanim. 2014;33(1):e9–e14.
Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet Lond Engl. 2007;370(9587):575–9.
Kim MB, Ward DS, Cartwright CR, Kolano J, Chlebowski S, Henson LC. Estimation of jugular venous O2 saturation from cerebral oximetry or arterial O2 saturation during isocapnic hypoxia. J Clin Monit Comput. 2000;16(3):191–9.
Funding
The study was supported entirely by departmental sources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Research involving human participants
As the design of the study was purely observational, waive written informed consent was authorized (local Ethics Committee). Verbal information was given to all patients.
Rights and permissions
About this article
Cite this article
Ferraris, A., Jacquet-Lagrèze, M. & Fellahi, JL. Four-wavelength near-infrared peripheral oximetry in cardiac surgery patients: a comparison between EQUANOX and O3. J Clin Monit Comput 32, 253–259 (2018). https://doi.org/10.1007/s10877-017-0025-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-017-0025-z