Abstract
The Athlete Blood Passport is the most recent tool adopted by anti-doping authorities to detect athletes using performance-enhancing drugs such as recombinant human erythropoietin (rhEPO). This strategy relies on detecting abnormal variations in haematological variables caused by doping, against a background of biological and analytical variability. Ten subjects were given twice weekly intravenous injections of rhEPO for up to 12 weeks. Full blood counts were measured using a Sysmex XE-2100 automated haematology analyser, and total haemoglobin mass via a carbon monoxide rebreathing test. The sensitivity of the passport to flag abnormal deviations in blood values was evaluated using dedicated Athlete Blood Passport software. Our treatment regimen elicited a 10% increase in total haemoglobin mass equivalent to approximately two bags of reinfused blood. The passport software did not flag any subjects as being suspicious of doping whilst they were receiving rhEPO. We conclude that it is possible for athletes to use rhEPO without eliciting abnormal changes in the blood variables currently monitored by the Athlete Blood Passport.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recent introduction of the Athlete Biological Passport (ABP) by the World Anti-Doping Agency (WADA) is intended to equip anti-doping organisations with a robust framework in which to pursue anti-doping rule violations and to support intelligent, targeted testing of athletes (WADA 2009). The hypothesis underpinning this strategy is that biological variables, otherwise maintained in homeostasis, will be disturbed when an athlete dopes. Longitudinal evaluation of an athlete’s results is intended to identify abnormal changes caused by doping against a background of normal biological variability.
Some insight into the normal biological variability encountered in professional endurance athletes have been described. For cyclists (their federation, the International Cycling Union or UCI, was the first to formally implement a passport approach) haemoglobin concentration ([Hb]) decreased marginally (1.3 g/dL) during the competition season, whilst exercise-induced hypervolemia reduced [Hb] values by 11.5% during the Tour de France itself (Morkeberg et al. 2009d). Although Banfi et al. (2010) confirmed that seasonal variations in [Hb] were also apparent in cross country skiers, they endorsed the validity of using reticulocyte levels for anti-doping purposes.
Retrospective analyses of databanks have revealed some of the cat-and-mouse nuances of the interplay between blood monitoring and athlete’s responses. In the years immediately after the introduction of an enlarged blood testing programme by the International Ski Federation (FIS), the unexpected high mean [Hb] values encountered in 1997–1999 (i.e., 16.2 g/dL) declined closer to normal values (14.8 g/dL). However, in subsequent years, the [Hb] began to creep upwards again whilst reticulocyte levels fell, which the authors cautioned was likely a sign that skiers had adopted new strategies to manipulate their blood values (Morkeberg et al. 2009b). It is disconcerting to note that cycling, which also introduced blood monitoring during this period, reported a similar ‘evolution’ in blood values (Zorzoli 2005).
Against this backdrop, anti-doping researchers have also evolved their (detection) strategies, most notably with the progression of longitudinal monitoring of blood values (Sharpe et al. 2006). However, these refinements have been criticised in some quarters. Borno et al. (2010) interrogated blood samples collected from 24 healthy subjects who received injections of recombinant human erythropoietin (rhEPO) every second day or once weekly. They were able to indicate rhEPO use in 58% of subjects, but questioned whether that sensitivity would be apparent when athletes were tested in a field setting as opposed to their intensive laboratory protocol. The authors also concluded that the OFF-hr model was ineffective and suggested that this parameter be dropped from anti-doping activities.
A major limitation of Borno’s study was that it evaluated z-scores, and not the mathematics actually utilised by the ABP software. Although the ABP software and z score approach can be tweaked to yield similar outcomes (Morkeberg et al. 2009c), this requires that the same between- and within-subject variances be adopted with each of the calculations. This was not done in Borno’s study, subsequently, although a theoretical insight, Borno’s findings cannot be extrapolated to evaluate the true efficacy of WADA’s ABP protocol.
Athletes have migrated to injecting frequent ‘microdoses’ of rhEPO in order to minimise the persistence of rhEPO in their system and thereby lessen the risk of being caught via conventional anti-doping controls (Ashenden et al. 2006). In the present study, we injected 10 healthy subjects with a microdose regimen and monitored blood values using the ABP software. Our aim was to assess whether the software would flag the subject’s blood results as abnormal during the midst of a rhEPO microdose programme.
Methods
Subjects
We recruited ten healthy male subjects (mean ± SD age 31.3±6.9 year, height 180.8±7.2 cm, weight 79.8±8.4 kg) who participated in regular endurance training (at least 3 sessions per week, >2 hours per session). No subject was a member of a state or national sporting squad nor scholarship holder at the Australian Institute of Sport. Subjects were requested to maintain their normal training throughout the study period and were provided and signed informed consent forms, following procedures approved by the Australian Institute of Sport Ethics Committee.
Study design
Subjects provided medical history and underwent screening for illness, injury and high blood pressure. Injections of rhEPO (Neorecormon, Roche Diagnostics, Australia) were given intravenously twice per week (Monday and Thursday). Subjects took oral iron tablets (Ferro-Grad C, Abbott Australasia Pty Ltd, Australia), which provided 105 mg of elemental iron per day. Venous blood samples for passport evaluation were also obtained on these days but always prior to rhEPO injections.
The primary aim of the extended rhEPO microdose treatment was to increase haemoglobin mass whilst deliberately avoiding large fluctuations in reticulocyte production. Since an overshoot would have been irretrievable (i.e., in the context of a flagged result) but an undershoot could be gradually compensated, we incrementally increased dosages over a period of weeks, using the data derived from haemoglobin mass estimations plus reticulocyte values from blood tests as feedback mechanisms. During the initial ‘titration’ phase (Phase I), four subjects were given 10 IU/kg for 4 weeks whilst the remaining six received twice this dosage but over half the time (2 weeks). Thus, all subjects received the same total quantity of rhEPO (adjusted for body weight) during the titration phase.
With the feedback information ‘in hand’ from the titration phase, the dosage of rhEPO was subsequently standardised for all 10 subjects and gradually elevated so that subjects received 20 IU/kg for 4 weeks (Phases II and III), then injections of 30 IU/kg for the final 4-week period (Phases IV and V; three subjects were given 40 IU/kg for their final three injections). Total haemoglobin mass was measured immediately after the titration phase, then every fortnight (i.e., at the end of each Phase) until completion of the study.
Haemoglobin mass estimation
Total haemoglobin mass (Hbmass) was used as an index to confirm that our rhEPO treatment regimen caused a measurable increase in circulating red cell numbers. Hbmass was estimated using a carbon monoxide rebreathing technique (Schmidt and Prommer 2005). Briefly, a dose of 99.9% pure CO was administered and rebreathed for 2 min. Capillary fingertip blood samples were taken before the start of the test and at 7 min post-administration of CO. These samples were assayed for %HbCO (average of ten replicates) using an OSM 3 hemoximeter (Radiometer, Copenhagen). Hbmass was calculated from the mean change in %HbCO before and after rebreathing CO. The same technician performed all estimations of Hbmass. The typical error (TE) for Hbmass quantification, based on duplicate measures performed on 14 subjects was 2.8% (90% confidence interval of 2.1–4.1%).
Blood analysis
Venous blood samples drawn into K3EDTA vacutainers were refrigerated until analysis (samples always assayed within 24 h of collection). Full blood counts were conducted on two side-by-side Sysmex XE-2100 instruments located in a commercial pathology laboratory, which participated in an external quality assurance scheme (Royal College of Pathologists of Australasia QAP programme). Both instruments operated within the Allowable Limits of Performance of the programme.
In accordance with WADA Athlete Blood Passport Guidelines (WADA 2009), samples were homogenised for at least 15 min prior to analysis using a roller mixer then measured in duplicate. In order for the results to be deemed acceptable, the absolute difference between duplicates had to be equal or <0.1 g/dL for [Hb] or 0.15 absolute difference for reticulocyte percentage (0.25 difference if the reticulocyte percentage was >1.00%). Satisfactory differences meant that only the first data point was included or else the entire analyses were discarded then repeated.
Statistical analysis
To assess the sensitivity of the Passport approach to detect our rhEPO treatment regimen, results for blood samples were analysed using the Athlete Biological Passport software as recommended by the WADA guidelines (WADA 2009). The software is subject to licensing agreements, although access can be requested by written application to the World Anti-Doping Agency (WADA). Briefly, using the nomenclature utilised by the guidelines, results for seven variables (HGB, HCT, MCV, MCH, MCHC, % RET and RET#) are interrogated by the ‘adaptive’ statistical approach built into the programme. This generates tolerance thresholds for [Hb], reticulocyte percentage and OFF-hr score which, although initially based on population-derived thresholds, ‘adapt’ as soon as data from the subject is introduced. Individual values that fall outside of these tolerance limits are considered as being abnormal.
In detail, the adaptive model operates as follows. Before any observation, the mean of the predictive distribution of expected values for an athlete is the population mean (POPmean) and its variance is the sum of the between-subject (BS) and within-subject (WS) variance:
Thereafter, the predictive distribution of expected values is computed iteratively as a function of the athlete’s test history and the information above. For a normally distributed marker and a universal WS variance, where RES_N is the result for the observation number ‘n’, the iteration is the following:
For illustrative purposes, an example of calculations when an athlete’s first two [Hb] values are 134 and 142 is:
Since the presenting RES_1 = 134, it falls within the thresholds.
With RES_1 = 134, the calculations for the predictive distribution of expected values for the second observation are:
Since the presenting value RES_2 is equal to 142, the value falls within the thresholds.
Specificity levels in the ABP software were set at 99.9%, which is the threshold beyond which a value is deemed to be ‘abnormal’. As well, the ABP software permits not only individual values but also the sequence of values to be evaluated (i.e., a blood profile can show unusual fluctuations over time without any single value exceeding the thresholds). The outcome is reported as a percentile of the expected distributions, and we used a 99.9% percentile as a threshold to declare the sequence abnormal.
If sampling frequency is too high, the resulting individual limits will ‘follow’ the increase/decrease in test results, which will cause a substantial loss in sensitivity (Sottas PE, personal communication). Therefore, we elected to include only data collected once per month from the subjects during the treatment phase. This subjective decision was a reasonable mimicry of the frequency with which cyclists are currently tested in the International Cycling Unions (UCI) Blood Passport programme (i.e., 8–12 tests per year).
Although quite separate and not to be confused with the ABP calculations, changes in blood variables across time were tested for statistical significance using Tukey’s HSD test (JMP 8.0, SAS Institute, P < 0.05).
Results
Compliance and efficacy
All subjects received the allotted dosage of rhEPO at each timepoint (100% compliance). However, one subject withdrew prior to the final test session due to personal reasons unrelated to the study. The baseline Hbmass (976 ± 140 g, n = 10) increased steadily throughout the treatment but the 113 g change did not reach statistical significance by the final measurement (1,089 ± 173 g, n = 9). A 113 g difference is equivalent to the haemoglobin contained in two or three 450 ml bags of liquid- or frozen-stored blood (Morkeberg et al. 2009a).
Sensitivity of the Athlete Passport to detect rhEPO treatment
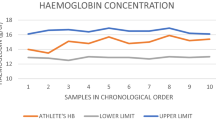
To achieve a sensible estimation of the frequency of testing likely in a passport scenario, and to avoid ‘oversampling’, we evaluated results every 4 weeks. A blood value from one subject exceeded the 99.9% threshold (OFF-hr score) at baseline prior to receiving rhEPO. Throughout the remainder of the study no individual values were flagged as abnormal by the ABP software. Two other subjects had sequences of blood profiles that approached but did not exceed the 99.9% percentile (99.88% for [Hb] and 99.87% for OFF-hr score).
Haematological response to rhEPO injections
Changes in the two key variables required to exceed 99.9% thresholds in order to be defined as abnormal according to the ABP Operating Guidelines ([Hb] and OFF-hr score) are depicted in Table 1. Although tending to be higher the [Hb] at the conclusion of the administration phase (164.3 ± 5.4 g/L, n = 9) the difference from baseline was not statistically significant (155.3 ± 11.6 g/L, n = 10) but approached statistical significance compared with Phase II (154.4 ± 6.5 g/L, p = 0.06).
The OFF score at the end of treatment (116.1 ± 7.8 units, n = 9) was significantly higher than at Phase I (101.3 ± 9.1 units, p = 0.045), approached statistical significance compared to Phase II (102.2 ± 9.9 units, p = 0.07) but was not statistically different from baseline (108.3 ± 16.8 units).
Reticulocyte percentages tended to increase at Phase I (0.88 ± 0.26%, n = 10, p = 0.10), which coincided with the beginning of treatment with rhEPO, but were unchanged at the end of treatment (0.66 ± 0.19%, n = 9) compared to baseline (0.63 ± 0.21%, n = 10).
Discussion
There exists compelling circumstantial evidence that the introduction of ceiling values for haemoglobin and haematocrit precipitated a change in how professional cyclists and cross-country skiers manipulated their blood (Morkeberg et al. 2009b; Zorzoli 2005). It is a reasonable premise that introduction of the Athlete Biological Passport, which flags unusual increases or decreases in blood variables (as opposed to just high values), would be accompanied by evasive tactics tailored to this new circumstance. The outcomes from this study suggest that at least one evasive tactic (to use small, frequent injections of rhEPO) does not perturb blood variables in a manner that would be flagged as abnormal by the ABP software.
Three parameters can be adjusted with regard to how rhEPO treatment is administered—the route (intravenous or subcutaneous), the frequency of injections and the dosage used. We chose the intravenous route of administration since the decreased half-life compared to subcutaenous administration (Halstenson et al. 1991) would be considered attractive to an athlete wishing to minimise the risk of failing a doping control (i.e., remnant traces of rhEPO would almost certainly lead to imposition of an anti-doping rule violation). Twice weekly injections were considered to be a reasonable balance between the competing considerations of subject compliance (less frequent injections are better) and pharmacokinetics (more frequent injections are better since it enables lower dosages to be used). Having selected the first two parameters, the final parameter (dosage) was increased incrementally over time.
Our dosages commenced at 10 IU/kg rhEPO twice weekly, corresponding to approximately 1,500 IU per week in absolute terms for a typical body mass of 75 kg. In subsequent phases, this dosage increased to 3,000 IU per week (20 IU/kg), 4,500 IU per week (30 IU/kg) and peaked at 6,000 IU per week (40 IU/kg) for several subjects. This dosage/frequency combination spanned a range shown previously to enhance maximal oxygen uptake: 20–40 IU/kg (Berglund and Ekblom 1991); 20–50 IU/kg (Russell et al. 2002); 60 IU/kg (Thomsen et al. 2007), however our focus was not on physiological but rather haematological endpoints. As such, our incremental approach deliberately contrasted with previous studies that began with a more aggressive treatment strategy; we surmised that in an environment where rhEPO is unreservedly acknowledged to be performance enhancing and thus highly coveted, an athlete selecting a doping regimen would place greater emphasis on finding the highest dosage which evaded detection, rather than the lowest dosage which yielded a performance enhancement. In terms of a risk analysis from the perspective of the athlete, a mistaken calculation with the former may yield an abnormal blood result, but a mistake with the latter may yield a positive analytical test and almost certain sanction. Moreover, given the elastic nature of ‘elite performance’ and the difficulty quantifying changes either in the laboratory or on the sporting field (e.g., compared with easily measured blood variables), we believe our approach to use incremental dosages and haematology as the endpoint more faithfully reproduces the most likely modus operandi of athletes seeking to evade detection.
Throughout our administration protocol, the reticulocyte response was muted. Our findings agree with reticulocyte data published by Russell et al. (2002) who injected (subcutaneously) 20 IU/kg thrice weekly (i.e., ~4,500 IU per week) for 5 weeks. During what they termed a ‘maintenance phase’ the reticulocyte percentages in treated subjects were indistinguishable from baseline values (and placebo subjects). Similarly, when Borno et al. (2010) injected eight subjects with 5,000 IU (one subcutaneous injection per week) during a 7-week ‘maintenance’ phase, the reticulocyte levels were strikingly similar to baseline. Two other cohorts in Borno’s study also showed similar reticulocyte values to baseline during a 2-week maintenance phase of 5,000 IU once weekly, however, it is unclear what remnant influence the prior aggressive dosing (four injections per week) may have had on these values. One obvious difference between our protocol and Russell and Borno’s protocols is that their ‘maintenance’ phases were each preceded by several weeks of more aggressive dosing (three of four injections per week). These data suggests that, whether or not a ‘microdosing’ regimen is preceded by a more aggressive dosing strategy, reticulocyte levels remain similar to baseline values when dosages of ~5,000 IU are administered intravenously or subcutaneously, either once or twice per week.
Reticulocytes are a cornerstone of the Athlete Biological Passport. The presence of reticulocytes in circulation increases following injection of rhEPO (Audran et al. 1999; Parisotto et al. 2001) or phlebotomy (Damsgaard et al. 2006; Morkeberg et al. 2009a), and decrease after rhEPO treatment ceases (Gore et al. 2003) or blood is reinfused (Morkeberg et al. 2009c). Thus anti-doping authorities carefully scrutinise reticulocyte levels for signs of doping. However, since the current ABP Operating Guidelines do not permit changes in reticulocyte levels by themselves to serve as a basis for a profile to be deemed abnormal, the strategic role of reticulocytes is instead manifest via the OFF-hr score. The OFF-hr score is an algorithm that combines [Hb] and reticulocyte percentage into a single value (Gore et al. 2003). Deviations in the OFF-hr score can be deemed as abnormal and are potential grounds for imposition of an anti-doping rule violation.
None of the individual OFF-hr values collected from our subjects during treatment with rhEPO were flagged as abnormal by the ABP software. Similarly, the sequence of blood results collected once monthly did not exceed the requisite percentile to be deemed abnormal. In this context, ‘abnormal’ must be strictly interpreted as a <1 in 1,000 likelihood of encountering such a value (or sequence) in a population of elite athletes who did not dope (Sharpe et al. 2006). In other words, even though an OFF-hr score may deviate noticeably from previous values, the ABP software does not flag the value as being abnormal unless it exceeds a generous allowance for both biological and analytical variations (Sottas et al. 2008).
It is therefore striking that one OFF-hr score value collected before rhEPO treatment began was designated as ‘abnormal’ by the ABP software. The OFF-hr score of 138 was derived by combining [Hb] of 16.9 g/dL with 0.26% reticulocytes. In the context of normal population reference ranges for the XE-2100 model used in our study (from n = 93 healthy males as reported to the U.S. Food and Drug Administration by Sysmex Corporation), the [Hb] from our subject was within the 95% reference interval (13.03–17.35 g/dL) but the reticulocyte value was not (0.48–2.11%). Whereas 5% of values would normally fall outside that range, we found two of our ten subjects (20%) had reticulocyte levels below 0.48% at baseline.
A close scrutiny of quality control records (both internal and external QCs) confirmed the instruments were operating within both the manufacturer’s and external QC programme’s guidelines, and therefore there was no objective basis to exclude these data. However, the average reticulocyte value for our ten endurance-trained subjects at baseline (0.65 ± 0.2%) was marginally lower than the 0.76 ± 0.2% reported in a cohort of 20 endurance-trained subjects tested using the same XE-2100 instrument model (Schumacher et al. 2010). Similarly, a cohort of 41 non-endurance-trained subjects we immediately tested on our instrument were marginally lower than the comparative non-endurance cohort (n = 16) in Schumacher’s study (0.79 ± 0.3 vs. 0.94 ± 0.4%, respectively). Taken together, these comparisons of fresh blood data suggested existence of an instrument bias (Ashenden et al. 2004).
To assess what affect this apparent bias may have had on our assessment of ABP software sensitivity, we made a paper adjustment to increase our entire reticulocyte database by the 0.11% difference indicated in endurance-trained athletes. For didactic purposes, we assumed a uniform instrument bias across the 0.26–1.23% range in our data; uniformity is considered to be a reasonable assumption given that the typical range of Retic-Chex Linearity material used to establish linearity of the Sysmex instrument typically spans a range 0.4–24%. This adjustment made no difference to sensitivity as no adjusted values nor sequences were flagged as abnormal post-adjustment. Interestingly, the adjusted OFF-hr score of 132 was still deemed to be abnormal, however, only 1 of the 10 subjects had reticulocyte values below the 95% reference boundary of 0.48%.
It was beyond the scope of this study to partition out definitively whether naturally low reticulocyte values amongst our cohort, or instrument bias, caused the flagged baseline OFF-hr score. Our data do not exclude that there was a combination of these two possibilities. With regard to the former, in the hypothetical scenario where an athlete with inherently low values (i.e., among the 5% of subjects expected to fall outside the reference range) were to present a flagged result in the ABP programme, follow-up tests would quickly inform the expert panel that this was most likely attributable to biological not doping causes. In the case of the latter (instrument bias), it is highly improbable that a 0.11% deviation between tests (i.e., if one result was derived from a biased instrument and one was not) would ever be used to underpin an anti-doping rule violation. Nevertheless, our current findings emphasise previous suggestions (Ashenden et al. 2004) that further research is warranted to quantify inter-instrument bias.
Limitations
One limitation of the present study was the absence of urinalyses to assess the presence of rhEPO in urine samples. Ideally, any blood sample collected for the ABP programme would be accompanied by a corresponding urine sample and isoelectric focussing test (Lasne 2001). However, during out of competition testing in 2009, the UCIs Biological Passport programme collected 6,165 blood samples but only 2,165 accompanying urine samples were tested for rhEPO (Zorzoli and Rossi 2010), dictating that in many circumstances blood values must be interpreted in isolation. It was this latter reality we sought to reproduce in the current study. We are mindful that the IEF sensitivity depends on factors including the dosage used and the interval between injection and sample collection. However, some insight can be gleaned from a previous publication where a cohort of eight healthy subjects were given subcutaneous injections of 5,000 IU (Lundby et al. 2008), which elicited an overall increase in haemoglobin mass of 92 g (compared to our increase of 113 g). All urine samples collected 2 days post-injection were positive when injections were given every second day, whilst one quarter of samples collected during the maintenance phase (i.e., one injection per week) were declared positive (it is noteworthy that a second laboratory analysing duplicate samples yielded no positive results during either phase). An imperfect bookend for Lundby’s study may be the research reported previously where an (undisclosed) microdose of rhEPO was not detectable via IEF analyses 12–18 h post-injection (Ashenden et al. 2006), however, it is noteworthy that study utilised ‘first generation’ positivity criteria, which have since been modified to yield better sensitivity. In summary, we would speculate that the likely sensitivity of urinalyses to detect our protocol of once weekly injections of 1,500–6,000 IU per week, assuming samples were collected 2 days post-injection, would probably range between 0 and 25% (i.e., a continuum correlated with the dosage used). Incidentally, our blood passport findings do not affirm Lundby’s prediction that longitudinal monitoring of blood variables would surpass urinalyses’ lack of sensitivity (Lundby et al. 2008).
Another limitation of the current protocol was the absence of a washout period. Extending the protocol by a further 3–4 weeks was deemed to place an unwarranted burden on participants, and primacy was instead given to extending the treatment phase for as long as possible. This trade-off almost certainly diminished the sensitivity of the ABP component monitoring OFF-hr score, since it has been well-documented that a nadir in OFF-hr score is reached 10–14 days after rhEPO injections cease (Gore et al. 2003). We assume this signature would have held also in our subjects, whose OFF-hr scores would have increased markedly and most likely been flagged by the software as being abnormal during the weeks after injections ceased. Similarly, we speculate that the sequence analysis used by the ABP software, which explicitly evaluates variations in the data, would have yielded greater sensitivity if washout data had been included as these data would have varied substantially from both baseline and treatment phases.
Finally, an inevitable limitation when structured laboratory studies seek to mimic how athletes dope ‘in the field’ is the inability to faithfully replicate that setting. For example, our protocol incorporated regular twice weekly injections over a three month period. It could be disputed whether an athlete would adhere to that regimen, and less consistent dosing could result in larger fluctuations in blood variables.
One implication of this study is that careful attention should be paid to when and how unannounced urine controls are collected from high risk groups. Given that at least one microdosing strategy can be utilised without being flagged by the ABP software, conventional urinalyses will remain a crucial and perhaps the only avenue open to authorities to remedy microdosing. However, this observation should only temper, not redact, implementation of the Athlete Biological Passport: sport federations or national anti-doping agencies without a coherent blood testing strategy may well house a population of athletes whose blood values resemble cycling and cross-country skiing circa 1997 (i.e., unsophisticated doping regimens with commensurately extreme blood values). In that environment we speculate the ABP software would wield an unprecedented deterrent/detection effect (at least until the athletes revised their doping strategies).
The passport’s utility as an intelligence-gathering tool should not be understated. Perhaps additional biomarkers, with increased sensitivity but reduced specificity, might be introduced to magnify the passport’s utility as a targeting strategy (low specificity in this setting is immaterial when the data are used only for intelligence gathering).
Conclusions
There is a substantial financial burden, and significant logistical obstacles, associated with collecting, transporting and analysing fresh blood samples in compliance with the WADA guidelines. Meanwhile, anecdotal evidence and admissions from several athletes suggest that athletes have already learnt to avoid detection by using small, frequent injections of rhEPO.
Our findings demonstrate that it is possible to inject rhEPO without triggering Passport thresholds. On one hand, this underlines the need to retain existing analytical procedures (‘direct detection’) in support of the Athlete Blood Passport, and on the other hand, emphasises the need for recruitment of novel strategies to hopefully close current loopholes.
References
Ashenden M, Varlet-Marie E, Lasne F, Audran M (2006) The effects of microdose recombinant human erythropoietin regimens in athletes. Haematologica 91:1143–1144
Ashenden MJ, Sharpe K, Damsgaard R, Jarvis L (2004) Standardization of reticulocyte values in an antidoping context. Am J Clin Pathol 121:816–825
Audran M, Gareau R, Matecki S, Durand F, Chenard C, Sicart M-T, Marion B, Bressolle F (1999) Effects of erythropoietin administration in training athletes and possible indirect detection in doping control. Med Sci Sports Exerc 31:639–645
Banfi G, Tavana R, Freschi M, Lundby C (2010) Reticulocyte profile in top-level alpine skiers during four consecutive competitive seasons. Eur J Appl Physiol 109:561–568
Berglund B, Ekblom B (1991) Effect of recombinant human erythropoietin treatment on blood pressure and some haematological parameters in healthy men. J Intern Med 229:125–130
Borno A, Aachmann-Andersen NJ, Munch-Andersen T, Hulston CJ, Lundby C (2010) Screening for recombinant human erythropoietin using [Hb], reticulocytes, the OFF(hr score), OFF(z score) and Hb(z score): status of the Blood Passport. Eur J Appl Physiol 109:537–543
Damsgaard R, Munch T, Moerkeberg J, Mortensen SP, Gonzalez-Alonso J (2006) Effects of blood withdrawal and reinfusion on biomarkers of erythropoiesis in humans: implications for anti-doping strategies. Haematologica 91:1006–1008
Gore CJ, Parisotto R, Ashenden MJ, Stray-Gundersen J, Sharpe K, Hopkins W, Emslie KR, Howe C, Trout GJ, Kazlauskas R, Hahn AG (2003) Second-generation blood tests to detect erythropoietin abuse by athletes. Haematologica 88:333–344
Halstenson CE, Macres M, Katz SA, Schnieders JR, Watanabe M, Sobota JT, Abraham PA (1991) Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther 50:702–712
Lasne F (2001) Double-blotting: a solution to the problem of non-specific binding of secondary antibodies in immunoblotting procedures. J Immunol Methods 253:125–131
Lundby C, Achman-Andersen NJ, Thomsen JJ, Norgaard AM, Robach P (2008) Testing for recombinant human erythropoietin in urine: problems associated with current anti-doping testing. J Appl Physiol 105:417–419
Morkeberg J, Belhage B, Ashenden M, Borno A, Sharpe K, Dziegiel MH, Damsgaard R (2009a) Screening for autologous blood transfusions. Int J Sports Med 30:285–292
Morkeberg J, Saltin B, Belhage B, Damsgaard R (2009b) Blood profiles in elite cross-country skiers: a 6-year follow-up. Scand J Med Sci Sports 19:198–205
Morkeberg J, Sharpe K, Belhage B, Damsgaard R, Schmidt W, Prommer N, Gore CJ, Ashenden MJ (2009c) Detecting autologous blood transfusions: a comparison of three passport approaches and four blood markers. Scand J Med Sci Sports. doi:10.1111/j.1600-0838.2009.01033.x
Morkeberg JS, Belhage B, Damsgaard R (2009d) Changes in blood values in elite cyclist. Int J Sports Med 30:130–138
Parisotto R, Wu M, Ashenden MJ, Emslie KR, Gore CJ, Howe C, Kazlauskas R, Sharpe K, Trout GJ, Xie M (2001) Detection of recombinant human erythropoietin abuse in athletes utilizing markers of altered erythropoiesis. Haematologica 86:128–137
Russell G, Gore CJ, Ashenden MJ, Parisotto R, Hahn AG (2002) Effects of prolonged low doses of recombinant human erythropoietin during submaximal and maximal exercise. Eur J Appl Physiol 86:442–449
Schmidt W, Prommer N (2005) The optimised CO-rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol 95:486–495
Schumacher YO, Wenning M, Robinson N, Sottas PE, Ruecker G, Pottgiesser T (2010) Diurnal and exercise-related variability of haemoglobin and reticulocytes in athletes. Int J Sports Med 31:225–230
Sharpe K, Ashenden MJ, Schumacher YO (2006) A third generation approach to detect erythropoietin abuse in athletes. Haematologica 91:356–363
Sottas P-E, Robinson N, Saugy M (2008) A forensic approach to the interpretation of blood doping markers. Law, Probability Risk 7:191–210
Thomsen JJ, Rentsch RL, Robach P, Calbet JA, Boushel R, Rasmussen P, Juel C, Lundby C (2007) Prolonged administration of recombinant human erythropoietin increases submaximal performance more than maximal aerobic capacity. Eur J Appl Physiol 101:481–486
WADA (2009) Athlete biological passport operating guidelines
Zorzoli M (2005) Blood monitoring in anti-doping setting. In: Schanzer WHG, Gotzmann A (eds) Recent advances in doping analysis. Sport und Buch Strauss, Koln, pp 255–264
Zorzoli M, Rossi F (2010) Implementation of the biological passport: the experience of the International Cycling Union. Drug Test Anal. doi:10.1002/dta.173
Acknowledgments
We sincerely thank the subjects for their participation and adherence to this extended research protocol. We are grateful for the professional expertise demonstrated by Dr Melissa Arkinstall and Ms Kiara Johnson at Exercise Research Australia. The assistance of Andrew McKechnie, Juanita Mac, Shaun Kinna and Leigh Branagan was integral to the outcome of this project. This research was supported by the Australian Government through the Anti-Doping Research Programme of the Department of Health and Ageing. This study complied with the current laws of the country in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Guido Ferretti.
Rights and permissions
About this article
Cite this article
Ashenden, M., Gough, C.E., Garnham, A. et al. Current markers of the Athlete Blood Passport do not flag microdose EPO doping. Eur J Appl Physiol 111, 2307–2314 (2011). https://doi.org/10.1007/s00421-011-1867-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1867-6