Abstract
Our aim was to investigate the effects of diet supplementation with phytoestrogens on sex hormone levels, antioxidant adaptive responses and oxidative damage induced by exercise. Ten female swimmers participated for 26 days in a diet intervention with either a functional beverage rich in vitamins C and E or the same beverage but also supplemented with Lippia citriodora extract (PLX) containing 20 mg/100 ml verbascoside. After the intervention all subjects participated in a swimming session for 30 min maintaining the intensity at about 75–80% of their individual best performance time for a 50-m swim. In lymphocytes, the superoxide dismutase activity increased after exercise, with a higher increase in the PLX group. Swimming increased the erythrocyte activity of glutathione peroxidase and glutathione reductase in the PLX group. Purified glutathione reductase activity increased after an in vitro incubation with PLX. No effects were observed on the lymphocyte levels of malondialdehyde and carbonyls, but exercise increased the percentage of high-damaged lymphocytes 2.8 times in the placebo group and 1.5 times in the PLX group. PLX decreased the levels of 17-β-estradiol and testosterone and increased the levels of the sex hormone binding globulin. In conclusion, supplementation with phytoestrogens enhances the glutathione-dependent enzyme activities in erythrocytes and the superoxide dismutase activity in lymphocytes in response to exercise. PLX also shows direct antioxidant properties, by increasing glutathione reductase enzyme activity in vitro. Supplementation with phytoestrogens also decreases the plasma steroid hormone levels, pointing towards a possible agonistic effect of verbascoside in the hypothalamic regulation of estradiol synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reactive oxygen species (ROS) are a double-edged sword because they not only serve as key signaling molecules in physiological processes (Jackson et al. 2002) but also play a role in pathological processes such as those involving the upper respiratory tract diseases associated with exercise (Pedersen and Hoffman-Goetz 2000). Both exhaustive and moderate exercise may increase not only ROS production (Ji 1999) but also the antioxidant capability as occurs in lymphocytes (Ferrer et al. 2009). ROS production runs parallel to the activation of antioxidant defenses and there is a correlation between lymphocyte antioxidant enzyme activities and the markers of oxidative damage after exercise (Sureda et al. 2005). Thus, increased levels of ROS induce oxidative damage and also the expression of antioxidant enzymes in lymphocytes (Ferrer et al. 2007). A 1-h swimming session at 75–80% of maximal capacity has been shown as a good experimental model to study the blood cell and plasma oxidative stress associated with physical activity (Ferrer et al. 2009; Sureda et al. 2008a; Tauler et al. 2008a). Previous works evidence that a swimming session of these characteristics induces an activation of the antioxidant machinery in lymphocytes, although this antioxidant response to swimming is weaker in females than in males (Ferrer et al. 2009; Sureda et al. 2008a).
Diet supplementation with antioxidant nutrients could eliminate the endogenous activation of antioxidant defenses because of the direct deactivation of ROS by the exogenous antioxidants. In fact, some diet supplementation studies have shown that antioxidant nutrients attenuate the activation of antioxidant enzymes such as catalase (CAT), glutathione peroxidases (GPxs) and superoxide dismutase (SOD) (Sureda et al. 2006; Tauler et al. 2003) or inducible nitric oxide synthase (Sureda et al. 2004). Therefore, the dosage of antioxidant nutrients is crucial in order to reduce oxidative damage, and, at the same time, to maintain or enhance endogenous antioxidant defenses. Diet supplementation with an almond-based beverage enriched with vitamins E and C for 1 month has been proved to be useful in avoiding oxidative damage without intercepting the adaptive response to exercise (Sureda et al. 2008b). Higher doses of antioxidants could alter this response; thus, new studies are needed to evaluate the effects of a greater antioxidant capability of this beverage on oxidative damage and the antioxidant response to exercise. The Lippia citriodora extract (PLX) contains verbascoside and martynoside as the main compounds (Funes et al. 2009). Verbascoside and martynoside are phenylpropanoid glycosidic compounds with antioxidant activities (Seidel et al. 2000). The aforementioned compounds also exhibit antiproliferative, cytotoxic, antimetastatic and immunomodulatory properties (Li et al. 1997; Saracoglu et al. 1995; Ohno et al. 2002) but may also act as estrogen agonists/antagonists via the known estrogen receptor alpha (ERα) and beta (ERβ) subtypes (Brzezinski and Debi 1999). This nuclear receptors bind estrogens and regulate the transcription of estrogen-responsive genes by interacting directly either with DNA at particular estrogen response elements (ERE) of their promoters, or with other transcription factors, such as AP-1 or NF-κB (Papoutsi et al. 2006). The introduction of PLX extract into an almond-based beverage in order to obtain a functional food will produce a beverage not only with a higher antioxidant potential but also with estrogenic activity. In fact, the effects of phytoestrogens on the sex hormone levels need studying as several studies of estrogenic compounds present in the environment indicate the existence of effects on behavior and on the reproductive system of animals (Colborn et al. 1993).
We hypothesize that diet supplementation with antioxidant phytoestrogens could not only enhance the lymphocyte and erythrocyte antioxidant response to exercise, providing cells with increased protection against the apparition of oxidative damage, but also alter the sex hormone production in female swimmers. Therefore, our aim was to investigate the effects of diet supplementation with phytoestrogens on the sex hormone plasma levels and the lymphocyte and erythrocyte antioxidant adaptive responses and oxidative damage induced by moderate exercise in well-nourished, trained swimmers.
Materials and methods
Participants and protocol
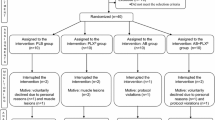
Ten girls (16.1 ± 2.2 years) volunteered for this study. They were all swimmers belonging to amateur teams. All swimmers trained 6 days/week. On Monday, Wednesday and Friday they trained 5 h each day. On Tuesday, Thursday and Saturday they trained 3 h each day. Their training was focused basically on the aerobic component. Only swimmers with regular menstrual cycles (cycle lengths between 28 and 30 days) in the first days of follicular phase of menstrual cycle (between days 4 and 8 after menstruation) were included in the study, as differences in the circulating levels of sex hormones could influence the antioxidant response to exercise and in addition the effects of the PLX supplement on the circulating levels of sex hormones were to be studied. Participants and their parents were informed of the purpose of this study and the possible risks involved before participants assented to participate and parents gave their written, informed consent to the minor taking part in the study. The study protocol was in accordance with the Declaration of Helsinki conforming to the ACSM guidelines for care of Human Subjects and was approved by the Balearic Islands Clinical Investigations bioethics committee.
This was a balanced randomized, double-blinded study in which participants were treated with either a functional beverage rich in vitamins E and C (10 and 30 mg/100 ml, respectively) or the same functional food but additionally supplemented with 200 mg/100 ml of L. citriodora extract. The beverage was manufactured by Liquats Vegetals S.L. (Viladrau, Gerona, Spain). The L. citriodora extract (PLX®), kindly provided by Monteloeder, SL, contained 10% verbascoside and up to 5–8% other phenylpropanoids, so the PLX supplement contained about 20 mg/100 ml verbascoside. Irrespective of group assignment, all participants drank 500 ml/day of the beverage for 26 days.
After the nutritional intervention all subjects participated in a customary swimming training session. The duration of the diet intervention ensured that after the supplementation period the swimmers were again in the follicular phase of the menstrual cycle, thus avoiding possible effects of the menstrual cycle on the parameters studied. The exercise session was as follows: Participants warmed up for 30 min prior to starting the exercise protocol. After warming up, the participants started with a series of intermittent 50-m swims for 30 min maintaining the intensity at about 75–80% of their individual maximal capacity, which was controlled by means of the time they took to complete each 50-m swim in relation to the best time they achieved in the preliminary tests. The swimmers were allowed 10–15 s rest between swims. Pilot tests were performed to allow the individual design of the protocol and to ensure that all the swimmers would be able to complete the exercise protocol. The mean individual best marks over 50 m was 32.3 ± 1.1 s in the placebo group and 32.3 ± 0.9 s in the PLX group. The mean speed at 80% of their own maximal mark was 1.29 ± 0.04 m/s in the placebo group and 1.29 ± 0.03 m/s in the PLX group. The swimming session meant a modest intensity workout for the participants in the study. Blood samples were obtained before (in basal conditions after overnight fasting) and 1 h after swimming since changes in antioxidant enzymes and the appearance of oxidative damage become evident 1 h after finishing the exercise bout rather than immediately after (Ferrer et al. 2007; Sureda et al. 2007).
Analytical HPLC
L. citriodora aqueous extract was analyzed using high-performance liquid chromatography (HPLC). A powered extract was dissolved 1 mg/ml in distilled water and centrifuged for 20 min at 150 g. 10 μl of the supernatant was injected into an analytical reverse-phase column LiChrospher® 100 RP-18 (5 μm, 250 × 4 mm i.d.) from Merck and subjected to HPLC analysis. The separation of the compounds was carried out in a HPLC LaChrom (Merck-Hitachi) series 7000 system, equipped with a pump, autosampler, column oven and UV–Vis diode array detector (wavelength selected at 340 nm to detect the phenylpropanoids). The chromatographic separation was performed at 25°C and a flow rate of 1 ml/min was used with a mobile phase composed of (a) ACN: phosphoric acid 0.45N (1:9) and (b) ACN: Phosphoric acid 0.45N (9:1). The multigradient solvent system was as follows: 0–25 min, from 0 to 12% B; 25–45 min, from 12 to 20% B; 45–47 min from 20 to 60% B; 47–56 min, fixed at 60% B; 56–57 min from 60 to 0% B and 13 min more for reequilibration at 0% B.
The linearity range of the responses for the standard was determined on eight concentration levels by triplicate. Calibration graphs for HPLC were recorded with a sample amount ranging from 0.25 μg/ml to 0.25 mg/ml (r 2 > 0.9999). Quantitative evaluation of verbascoside was performed by means of a six-point regression curve (r 2 > 0.996) in a concentration range between 0 and 0.1 mg/ml, using verbascoside as reference external standard and evaluated at 340 nm. Limit of detection (LOD) was 0.1 μg/ml and limit of quantification (LOQ) was 0.25 μg/ml.
Experimental procedure
Venous blood samples were obtained from the antecubital vein of swimmers in suitable vacutainers with EDTA as anticoagulant. Leukocyte counts and hematological parameters were determined in whole blood. Plasma and erythrocytes were obtained after centrifugation of the blood samples at 1,000 g, 4°C for 30 min. 17-β-estradiol, testosterone, free testosterone and sex hormone binding globulin (SHBG) levels were determined in plasma immediately after sample collection. The lymphocyte fraction was purified from whole blood following an adaptation of the method described by Boyum (1964) (Ferrer et al. 2007) using Ficoll-Paque PLUS reagent (GE Healthcare, Chalfont St Giles, UK) and samples were stored at −80°C until analysis, except for DNA damage comet assay, which was performed immediately after lymphocyte purification. CAT, GPx, glutathione reductase (GR) and SOD activities were determined in erythrocytes and lymphocytes. Malondialdehyde (MDA), protein carbonyl index and DNA damage were measured in lymphocytes. The expression of CAT, GPX, Mn-SOD, Bcl-2, UCP3 and hemeoxygenase 1 were assessed in lymphocytes.
Anthropometric data
The anthropometric variables measured in this study were height, body mass, brachial, waist, hip and thigh perimeters and body composition. Height was determined using a mobile anthropometer (Kawe 44444, France) to the nearest millimeter, with the participant’s head in the Frankfurt plane. Body mass was determined to the nearest 100 g using a digital scale (Tefal, sc 9210, France). Participants were weighed barefoot while wearing light underwear, which was accounted for by subtracting 300 g from the measured weight. Brachial, waist, hip and thigh perimeters were measured to the nearest 0.1 cm with the participant’s right arm relaxed, using a non-stretchable measuring tape (KaWe, 43972, France). Percentage body fat and mass of body fat were calculated by bioimpedance using a handheld BIA unit (Omron1BF 300 body fat monitor). All anthropometric measurements were performed by one observer to avoid inter-observer variation.
Different anthropometric indexes were calculated using these measurements: body mass index [BMI = mass (kg)/squared height (m)]; waist-hip index [waist perimeter (cm)/hip perimeter (cm)].
Dietary intake
Dietary habits were assessed using a 3-day dietary record questionnaire completed at the beginning of the study and in the week before the exercise test. A qualified dietician verified and quantified the food records. All food items consumed were transformed into nutrients using a special computerized program based on the European and Spanish food composition tables (Tauler et al. 2008a).
Clinical determinations
Leukocyte counts and erythrocyte number were determined in an automatic flow cytometer analyzer Technicon H2 (Bayer) VCS system.
Progesterone, 17-β-estradiol, testosterone and free testosterone levels were determined by standard laboratory procedures using a direct chemiluminescence immunoassay (Wood 1984) and an automatic analyzer Centauro (Siemens). SHBG levels were determined by standard laboratory procedures using an immunochemioluminescent analyzer Immulite (Siemens).
Lymphocyte and erythrocyte purification
Blood samples were processed following an adaptation of the method described by Boyum (1964). Blood was carefully introduced on Ficoll in a proportion of 1.5:1 and was then centrifuged at 900×g, at 18°C for 30 min. The lymphocyte layer was then carefully removed, washed twice with PBS and centrifuged for 10 min at 1,000×g, 4°C. The cellular precipitate of lymphocytes was lysed with distilled water.
For erythrocyte purification, blood was centrifuged at 900×g, at 4°C for 30 min. The erythrocyte phase at the bottom was washed twice with 10 ml of PBS and was finally reconstituted with distilled water in the same volume as plasma. Then the erythrocyte resuspension was hemolysed with distilled water (1:1).
Enzymatic determinations
All activities were determined in lymphocytes and erythrocytes with a Shimadzu UV-2100 spectrophotometer at 37°C. CAT activity was measured by the spectrophotometric method of Aebi based on the decomposition of H2O2 (Aebi 1984). GPx activity was measured using an adaptation of the spectrophotometric method of Flohe and Gunzler (1984) using H2O2 as substrate. GR activity was measured by a modification of the Goldberg and Spooner spectrophotometric method (Goldberg and Spooner 1985). SOD activity was measured by an adaptation of the method of McCord and Fridovich (1969). The xanthine/xanthine oxidase system was used to generate the superoxide anion. This anion produced the reduction of cytochrome c, which was monitored at 550 nm. The SOD in the sample removed the superoxide anion and produced an inhibition of the cytochrome c reduction.
Malondialdehyde determination
MDA as a marker of lipid peroxidation was analyzed in lymphocytes by a colorimetric assay kit (Calbiochem, San Diego, CA, USA). The method used is specific for MDA determination.
Protein carbonyl determination
Protein carbonyl derivatives were determined in lymphocytes by an immunological method using the OxyBlot™ Protein Oxidation Detection Kit (Chemicon International) following the manufacturer’s details. Briefly, 10 μg of protein was incubated in the presence of 2,4-dinitrophenylhydrazine (DNPH). Once derivatized, samples were transferred to a nitrocellulose membrane by the method of dot blot. The membrane was then incubated with primary antibody, specific to the DNP moiety of the proteins. This step was followed by incubation with a horseradish peroxidase-antibody (goat anti-rabbit IgG) conjugate directed against the primary antibody. The membranes were then treated with luminol, which is converted into a light-emitting form at wavelength 428 nm by the antigen/primary antibody/secondary antibody/peroxidase complex. The light was visualized and detected by short exposure to a Molecular Imager Chemidoc XRS (Bio-Rad Laboratories). Image analysis was performed using Quantity One-1D analysis software (Bio-Rad Laboratories).
Comet assay
Assessment of DNA damage was carried out in lymphocytes using the comet assay method (Ferrer et al. 2010). Briefly, slides were prepared by adding 20 μl purified lymphocytes, mixed with 80 μl 0.6% low-melting-point agarose. In order to release the DNA, cells were lysed by immersing slides in lysing solution (2.5 M NaCl, 100 mM EDTA disodium salt, 10 mM Tris, 1% Triton X-100 and 10% DMSO added freshly, pH 10) at 4°C. DNA was denatured by placing slides in an alkaline bath in the electrophoresis tank for 40 min to allow for the unwinding of the DNA and expression of alkali-labile damage. Electrophoresis was carried out with an electric current of 250 mV applied for 30 min. Tris buffer pH 7.5 was added onto the slides to neutralize excess alkali. Finally, DNA was stained by adding 80 μl ethidium bromide (20 mg/ml) to each slide.
Comet measurements were made by image analysis using a fluorescence microscope and the Comet software (TriTek CometScore™, version 1.5). Images of 100 randomly selected nuclei were analyzed for each sample.
mRNA gene expression
mRNA expressions in lymphocytes were determined by real-time PCR with 18S ribosomal as reference gene. For this purpose, mRNA was isolated from lymphocytes by extraction with Tripure (Roche). cDNA was synthesized from 1 μg total RNA using reverse transcriptase with oligo-dT primers. Quantitative PCR was performed using the LightCycler instrument (Roche Diagnostics) with DNA-master SYBR Green I. The primers used are shown in Table 1. For all PCRs there was one cycle at 95°C for 10 min, followed by 40 cycles at the conditions shown in Table 1.
The relative quantification was performed by standard calculations considering 2(−ΔΔCt). Basal mRNA levels at the beginning of the stage were arbitrarily referred to as one. The expression of the target gene was normalized with respect to ribosomal 18S.
In vitro effects of PLX on glutathione reductase activity
In order to study the possible direct effects of PLX extract on GR activity an in vitro study was performed. 6 U/ml GR in 200 mM phosphate buffer, 1 mM EDTA, pH 7.0 were incubated at 37°C for 1 h in the presence of 4, 10 and 20 mg/100 ml PLX. A parallel control incubation of enzyme in the same conditions but without PLX was carried out. After incubation, GR activity was measured by a modification of the Goldberg and Spooner spectrophotometric method (Goldberg and Spooner 1985). The study was performed by tetraplicate.
Statistical analysis
Statistical analysis was carried out using a statistical package for social sciences (SPSS 13.0 for Windows). Results are expressed as mean ± SEM and p < 0.05 was considered statistically significant. A Kolmogorov–Smirnov test was applied to assess that data followed a normal distribution. The statistical significance of the data was assessed by two-way analysis of variance (ANOVA). Concerning the anthropometric and dietary parameters, the statistical factors analyzed were (T) time, and (S) PLX supplementation. To test the effects of the swimming session and the diet supplementation on cellular and biochemical parameters, the statistical factors analyzed were (E) exercise, and (S) PLX supplementation. When significant effects were found, a one-way ANOVA was used to determine the differences between the groups involved. A one-way ANOVA was performed to test the differences between the different PLX concentrations on the GR in vitro assay.
Results
The composition of the aqueous L. citriodora extract used in this nutritional intervention was analyzed by means of HPLC-diode array. The identification of the major peaks was based on the analysis of their retention time and UV spectra by comparison with those of authentic standards or data previously reported in the literature. Figure 1 shows the HPLC profile at 340 nm of the L. citriodora extract in which verbascoside and its isomer isoverbascoside were identified as the major components, as it has been previously reported (Bilia et al. 2008; Funes et al. 2009). In addition, other minor but significant peaks showing a typical phenylpropanoid absorption spectra with maximum at 330-340 nm may correspond to methoxylated phenylpropanoids, such as eukovoside or martynoside, as it has been recently reported for L. citriodora aqueous extract (Funes et al. 2009). The rest of the minor compounds appearing in the chromatogram were identified as glycosylated flavones, probably glucuronidated derivatives of luteolin or apigenin as it has been previously shown (Carnat et al. 1995; Funes et al. 2009). The amount of verbascoside in the extract was determined as 10 ± 0.5% (w/w), using a commercially available standard.
The anthropometric characteristics of all the young women swimmers participating in the study are shown in Table 2. No effects were observed on the anthropometric characteristics as far as diet supplementation with PLX extracts for 26 days was concerned. Table 3 shows the nutritional intake by the subjects before and after the nutritional intervention. No differences were observed in nutrient intake between the placebo and PLX supplemented groups, although the nutrient intake reflected the introduction of an energy drink rich in antioxidant vitamins E and C in the nutritional habits as a supplement. During the diet intervention the energy intake increased as well as the intake of vitamins E and C, while the intake of cholesterol decreased. No differences were evidenced in the nutrient intake between the placebo and PLX supplemented groups at the end of the diet intervention. The only difference was that the PLX supplemented group ingested 2 g/day of PLX extract (200 mg/day of verbascoside and other phenylpropanoid glycosides in a minor quantity), whereas the placebo group did not.
Diet supplementation with PLX affected erythrocyte and leukocyte counts (Table 4). The group supplemented with PLX presented about 7% lower basal erythrocyte counts and about 17% lower leukocyte counts than the group supplemented without PLX. The exercise performed by the swimmers for 30 min at 80% of their maximal record maintained the same basal values of lymphocytes. Only the neutrophils were significantly affected by exercise in a similar way in the two supplemented groups, increasing about 30% after exercise.
Diet supplementation with PLX affected the activity of some antioxidant enzymes in erythrocytes (GPx and GR), but not in lymphocytes (Table 5). In the same way, the exercise significantly affected the activities of both glutathione-dependent antioxidant enzymes in erythrocytes and the SOD activity in lymphocytes. A significant interaction between exercise and supplementation was observed in the activity of GR in erythrocytes. Exercise significantly increased the erythrocyte activity of GPx (about 22%) and GR (about 25%) only in the swimmers that consumed the supplement with PLX, while the swimmers that took the placebo maintained the basal values after exercise. This different behavior in the antioxidant enzyme changes in erythrocytes produced significantly higher activities of GPx (about 20%) and reductase (about 19%) after exercise in the group supplemented with PLX than in the placebo one. In a similar way, the SOD activity of lymphocytes increased about 51% in the group supplemented with PLX and only about 32% in the placebo group after exercise, although these differences in lymphocyte SOD activity after exercise did not reach statistical significance. In order to elucidate the origin of the lymphocyte changes in SOD activity induced by exercise we determined the gene expression of this enzyme and other antioxidant genes in lymphocytes (Table 6). No effects of exercise or supplementation were observed in the gene expression of the antioxidant enzymes SOD, GPx and CAT. Other proteins with an antioxidant function such as UCP3 and Bcl-2 also maintained the same basal levels of gene expression after exercise in both the placebo and the PLX-supplemented groups. The gene expression of heme oxygenase, an indicator of oxidative stress, was not influenced by supplementation or by exercise.
The effects of supplementation and exercise on the oxidative damage of lymphocytes are shown in Table 7. No effects of exercise or supplementation were observed in the lymphocyte levels of MDA (indicator of lipid peroxidation), carbonyl index (indicator of protein modification), percentage of DNA in tail (indicator of the amount of DNA damaged in an individual lymphocyte) and in the tail moment (indicator of the degree of fragmentation of DNA in an individual lymphocyte). However, exercise significantly increased the percentage of high-damaged lymphocytes about 2.8 times in the placebo group and 1.5 times in the PLX-supplemented group. The percentage of low-damaged lymphocytes also increased after exercise but to a lesser extent than the percentage of high-damaged cells. Correspondingly, the percentage of intact cells decreased after exercise.
In view of the results obtained, an in vitro study was performed to study the possible direct effects of PLX on GR enzyme activity. Purified GR enzyme was incubated for 1 h, 37°C, in the presence of different concentrations of PLX. All PLX concentrations tested induced a significant similar increase (about 350% of control activity) in GR activity, and no differences between treatments were evidenced (Fig. 2).
In vitro effects of PLX on glutathione reductase activity purified glutathione reductase enzyme was incubated for 1 h, 37°C, in the presence of 0, 4, 10 and 20 mg/100 ml PLX and glutathione reductase activity was then determined. The results are expressed as mean ± SEM (n = 4). Statistical analysis: one-way ANOVA. *Significant differences versus control incubation (0 mg/100 ml PLX), p < 0.05
Table 8 shows the plasma levels of sex hormones. The circulating levels of progesterone and 17-β-estradiol are consistent with the subjects being in the follicular phase of the menstrual cycle in both experimental groups (Table 8). One of the main features of this experience was the effect on the sex hormone levels induced by supplementation of the diet with PLX. PLX significantly decreased the levels of 17-β-estradiol and both the total and free testosterone and significantly increased the levels of SHBG in basal conditions and after exercise. 17-β-stradiol levels in the PLX-supplemented group were about 48 and 67% lower than the placebo group in basal conditions and after exercise, respectively. In the same way, the testosterone and free testosterone levels in the PLX-supplemented group were about 25 and 48% lower than the placebo group in basal conditions and 15 and 38% lower than the placebo group after exercise. In contrast, the SHBG was about 46% higher in the PLX-supplemented group than the placebo, both in basal conditions and after exercise. Exercise significantly decreased the levels of testosterone, but did not affect the levels of 17-β-stradiol, free testosterone or SHBG. Progesterone levels were maintained at basal levels after exercise and no differences attributable to diet supplementation were observed.
Discussion
The results obtained from this study reveal that the addition of phytoestrogens to a dietary supplement already containing antioxidant vitamins can increase the antioxidant potential of the supplement, as evidenced by the higher activation of antioxidant enzymes in the erythrocytes of swimmers who ingested the PLX supplement, and attenuate the apparition of DNA oxidative damage in lymphocytes after an acute bout of intense exercise. However, the main novelty of our study is the evidence that—together with the antioxidant activation—dietary intake of phytoestrogens could alter the endogenous synthesis of estrogens and therefore alter the plasma steroid hormone levels.
The duration and intensity of the exercise test applied in the present study induced a low immune response as shown by the low increase in circulating neutrophil counts. Neutrophils only rose about 32% after 30 min of swimming at 80% of subject’s best performance time for a 50 m swim, whereas in other longer, more intense exercise tests the increase in neutrophil counts is much greater (Sureda et al. 2007; Tauler et al. 2008b). The rise in circulating neutrophils observed after the swimming session was probably associated with an immune response to exercise that included neutrophil priming to the oxidative burst, as seen after a football match or a cycling stage (Sureda et al. 2007; Tauler et al. 2008b). Diet supplementation with moderate levels of antioxidants such as vitamins E and C or ubiquinone has been shown to reduce plasma oxidative damage induced by intense exercise such as a half-marathon (Sureda et al. 2008b) or a football match (Tauler et al. 2008b), but without blocking the cellular adaptation to exercise (Sureda et al. 2008b; Tauler et al. 2008b). However, diet supplementation with high doses of vitamin C, as much as 1 g/day for 1 week, has been seen to reduce the neutrophil response to the hypoxia/reoxygenation associated with apnea diving (Sureda et al. 2004). Thus, it is evident that the antioxidant intake influences oxidative damage and cellular adaptations against the oxidative stress induced by intense exercise.
The swimmers participating in this study took high levels of antioxidants during the nutritional intervention, coming both from food and the almond-based beverage used as the placebo and the vehicle to take in the PLX extract. Supplementation with PLX slightly reduced the number of erythrocytes and leukocytes when compared with supplementation with antioxidant vitamins only. This picture is present both in basal conditions and after exercise. Supplementation with antioxidant vitamins decreases the number of circulating lymphocytes, but maintains the lymphocyte count within the normal range (Tauler et al. 2006a). Supplementation with either 1 g/day vitamin C and 500 mg/day vitamin E (Tauler et al. 2006a) or 1 g/day of vitamin C alone (Tauler et al. 2003) has also been shown to induce lymphopenia. In the present study, the PLX-supplemented group took higher levels of antioxidants than the placebo because of the presence of the PLX extract. We also find a certain degree of leukopenia in this PLX-supplemented group when compared with the placebo group. The mechanisms by which supplementation with antioxidant nutrients induces leukopenia are not clear, although a rise in the levels of corticosteroids in response to increased ascorbate levels could be involved (Richardson 1986).
Antioxidant vitamin intake (vitamins C and E) was at the same level in both the placebo and the PLX-supplemented groups, and was between 2.5 and 5 times higher than the RDA for general people (Ortega et al. 2004). We have previously observed that the oxidative damage induced by intense exercise is well balanced by the availability of antioxidant nutrients (Sureda et al. 2008b). Actually, no effects of swimming for 30 min at 80% of maximal register are observed on the markers of oxidative damage in lymphocytes of women swimmers fed with doses of vitamins C and E five times higher than their RDA. In spite of this apparent lack of oxidative damage induced by exercise in lymphocytes, the number of lymphocytes with damaged DNA was about three times higher after the swimming session than in basal conditions, although only about 4% of cells had high-damaged DNA after exercise. The additional supplementation with PLX did not affect the number of lymphocytes with damaged DNA after swimming.
In spite of the lack of oxidative damage, exercise increased the activity of SOD in lymphocytes. This enzyme is one of the first enzymes activated against exercise-induced oxidative stress (Tauler et al. 2006b). The activation was observed both in the placebo and in the PLX-supplemented group, although the increase was higher in the PLX-supplemented group. This pattern was not attributable to a change in the gene expression of Mn-SOD. Therefore, the increased activity of SOD after exercise could be attributed to the activation of preexisting SOD protein in lymphocytes. In fact, exercise has been pointed out to induce the activation of some enzymes in erythrocytes as a result of post translational regulation (Tauler et al. 2005). The lack of oxidative damage after exercise is also in accordance with the lack of effect on the antioxidant gene expression in lymphocytes, as there is a certain parallelism between the induction of oxidative damage and the induction of antioxidant enzyme expression in lymphocytes (Sureda et al. 2005).
The activities of some key antioxidant enzymes rose in erythrocytes as a result of the interaction between exercise and PLX supplementation. Intense exercise such as a duathlon race or a mountain cycling stage induces changes in the erythrocyte activity of GPx (Tauler et al. 1999, 2005). This change in antioxidant activity has to be attributed to actions on the enzymatic protein present in the erythrocyte, since erythrocytes are unable to synthesize proteins. In the view of these results, the consumption of antioxidant nutrients such as phytoestrogens seems to enhance erythrocyte endogenous antioxidant defenses through post-transcriptional mechanisms, which can operate immediately as a consequence of intense exercise. The activation of these defenses would allow a rapid avoidance of an excessive increase in ROS production. In order to evidence the possible role of PLX extract on antioxidant enzyme activity, an in vitro experiment was performed. The incubation of purified GR in the presence of PLX extracts (4-20 mg/100 ml) increased enzyme activity up to 350% of control activity. These results evidence that PLX extract has direct antioxidant properties, by increasing GR enzyme activity. The possible mechanism by which PLX modulates GR activity is still unknown, but we previously evidenced in an in vitro experiment that GR activity increased when measured in the presence of CAT (Tauler et al. 2005) which suggested that the presence of antioxidants nearby the enzyme could increase its stability against oxidation. GR is a flavoprotein which could transfer one electron to oxygen in the course of the redox reaction leading to ROS formation as occurs in other flavoproteins such as xanthine oxidase (Babior et al. 2002) and nitric oxide synthetase (Xia et al. 1998). The ROS produced by the enzyme could affect the integrity of the protein and impair its own activity; the presence of higher antioxidant levels in PLX could contribute to the scavenging of ROS and then maintain higher GR activities.
The effect of the swimming session was observed in the circulating levels of testosterone, which were decreased 1 h after exercise. These results are in contrast with the literature, as exercise is globally able to induce an increase in circulating androgens in women, after both resistance and endurance acute exercises (Enea et al. 2011). However, the testosterone response in women is equivocal as no changes have also been observed in response to a bout of heavy resistance exercise (Vingren et al. 2011). After puberty, some acute increases in testosterone from resistance exercise can be found in boys but not in girls (Vingren et al. 2011). This discrepancy in the literature might be due to differences in the types and intensities of the exercises studied, in the hormonal status of the group of women investigated and in the methods for androgen determination. In our study, post-exercise samples were taken 1 h after the swimming session was finished. As the hormonal response to exercise would have likely occurred within the first 30 min post-exercise, the sampling time might interfere with our results. Furthermore, previous studies have reported circadian variations of testosterone levels, although variable results have been reported. One study performed with girls across pubertal development (maximum age 12 years) showed that salivary testosterone levels decrease throughout the day (about 135 pg/ml at 8:30 and about 120 pg/ml at 12:00) (Matchock et al. 2007). As basal samples were drawn at 9:00 and post-exercise samples were drawn at 11:00 (30 min of warming, 30 min of exercise test and 1 h of recovery), the observed decrease in testosterone plasma levels could be attributed to circadian variations. However, other study with adult girls (mean age 19.7 years) failed to detect any effect of time of day on testosterone levels (Liening et al. 2010).
Previous studies on the effects of phytoestrogens on sex hormone levels have been mainly designed testing the effects of soy isoflavones. These previous studies suggest that soy consumption exerts small effects on the circulating levels of sex hormones in both men and premenopausal women (Kurzer 2002). However, one of the main contributions of this study is the demonstration that supplementation of the diet with PLX alters the sex hormone circulating levels in basal conditions. An agonist/antagonist action of steroid hormones has been attributed to verbascoside and martynoside, the main compounds present in the PLX extract. We here evidence that the intake of 100 mg/day verbascoside for 26 days significantly decreases the plasma levels of estradiol and testosterone and increases the level of SHBG, probably as a result of the agonist effect between verbascoside and estradiol. Thus, the reduced circulating levels of 17-β-estradiol and testosterone are accompanied with increased levels of SHBG, a transporter which keeps sex hormones in a non-bioactive form. The bioavailability of both 17-β-estradiol and testosterone is therefore widely reduced after the dietary intake of phytoestrogens such as verbascoside. The synthesis of estradiol is carried by the enzyme aromatase and regulated by negative feedback by estradiol itself, which inhibits pituitary FSH secretion by regulating GnRH and its receptor levels at the hypothalamus-pituitary level (Conn 1994; Braden and Conn 1993; Marshall et al. 1983). Several studies have demonstrated the ability of exogenous estrogens to suppress FSH and LH levels during the follicular phase of the menstrual cycle (Tsai and Yen 1971; Monroe et al. 1972; Young and Jaffe 1976; Messinis and Templeton 1990). By acting as an estrogenic agonist, verbascoside could act by inhibiting estradiol synthesis. In fact, it has been evidenced that most phytoestrogens, such as genistein and some other diphenols, interfere with aromatase expression and activity as they are competitive inhibitors and can therefore inhibit the endogenous production of 17-β-estradiol (Adlercreutz et al. 1993; Brooks and Thompson 2005). These phytoestrogens may therefore act as both estrogen agonists and antagonists via the estrogen receptor alpha (ERα) and beta (ERβ) subtypes (Kuiper et al. 1998). Estrogen receptors bind estrogens and regulate the transcription of estrogen-responsive genes by interacting directly with DNA or with other transcription factors. ERα and ERβ differ in their tissue distribution and biochemical action. Ligands that bind ERα and ERβ may exhibit varying degrees of agonism/antagonism depending on the type of the estrogen-responsive tissue and the ligand concentration. The chemical structure of a ligand is also an important determinant of its estrogen receptor affinity and to act as an estrogen agonist or antagonist in each ER subtype (Nilsson et al. 2001). In this instance, second-generation selective estrogen receptor modulators (SERMs), such as the benzothiophene raloxifene, display anti-estrogenic action in both uterus and breast, but retain agonistic activity in bone and brain (Grese and Dodge 1998). There is scant information concerning the estrogenic/antiestrogenic effects of phenylpropanoid glycosidic compounds. Our results suggest that verbascoside could agonize the effects of 17-β-estradiol in the hypothalamic regulation of estradiol synthesis, but additional studies should be performed in order to further determine the possible agonist/antagonist effects of verbascoside in other tissues and estrogen related parameters.
There are marked differences in oxidant production between males and females (Sureda et al. 2008a), and these differences have been attributed to testosterone production in males and estradiol production in females (Borras et al. 2005). It has been suggested that testosterone stimulates total body oxidative stress while estrogens decrease total body oxidative stress (Sullivan et al. 2007). As suggested by this study, phytoestrogen intake could therefore bring males the advantages of females against oxidative stress without the risk of feminizing, although additional studies with male subjects must be performed.
In summary, diet supplementation with phytoestrogens from L. citriodora such as verbascoside decreases the number of circulating erythrocytes and leukocytes and enhances glutathione-dependent enzyme activities in erythrocytes in response to short, intense exercise. PLX also shows direct antioxidant properties, by increasing GR enzyme activity in vitro. Diet supplementation with verbascoside from L. citriodora also decreases plasma steroid hormone levels, pointing towards a possible agonistic effect of verbascoside in the hypothalamic regulation of estradiol synthesis. For that reason, even though these phytoestrogens present antioxidant activity and enhance the endogenous antioxidant response to exercise, the effects of phytoestrogens, which may behave as inhibitors of endogenous estrogenic hormone synthesis, must be further studied in future experiences.
References
Adlercreutz H, Bannwart C, Wahala K, Makela T, Brunow G, Hase T, Arosemena PJ, Kellis JT Jr, Vickery LE (1993) Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol 44(2):147–153
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Babior BM, Lambeth JD, Nauseef W (2002) The neutrophil NADPH oxidase. Arch Biochem Biophys 397(2):342–344
Bilia AR, Giomi M, Innocenti M, Gallori S, Vincieri FF (2008) HPLC–DAD–ESI–MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J Pharm Biomed Anal 46(3):463–470
Borras C, Gambini J, Gomez-Cabrera MC, Sastre J, Pallardo FV, Mann GE, Vina J (2005) 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell 4(3):113–118
Boyum A (1964) Separation of white blood cells. Nature 204:793–794
Braden TD, Conn PM (1993) GnRH and its mechanism of action. In: Leung PCK, Hsueh AJW, Friesen HG (eds) Molecular basis of reproductive endocrinology. Springer, New York, pp 12–38
Brooks JD, Thompson LU (2005) Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol 94(5):461–467
Brzezinski A, Debi A (1999) Phytoestrogens: the “natural” selective estrogen receptor modulators? Eur J Obstet Gynecol Reprod Biol 85(1):47–51
Carnat A, Carnat AP, Chavignon O, Heitz A, Wylde R, Lamaison JL (1995) Luteolin 7-diglucuronide, the major flavonoid compound from Aloysia triphylla and Verbena officinalis. Planta Med 61(5):490
Colborn T, vom Saal FS, Soto AM (1993) Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 101(5):378–384
Conn PM (1994) The molecular mechanism of gonadotropin-releasing hormone action in the pituitary. In: Knobil E, Neill JD (eds) The physiology of reproduction. Raven Press, New York, pp 1815–1826
Enea C, Boisseau N, Fargeas-Gluck MA, Diaz V, Dugue B (2011) Circulating androgens in women: exercise-induced changes. Sports Med 41(1):1–15
Ferrer MD, Sureda A, Batle JM, Tauler P, Tur JA, Pons A (2007) Scuba diving enhances endogenous antioxidant defences in lymphocytes and neutrophils. Free Radic Res 41(3):274–281
Ferrer MD, Tauler P, Sureda A, Tur JA, Pons A (2009) Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J Sports Sci 27(1):49–58
Ferrer MD, Sureda A, Tauler P, Palacin C, Tur JA, Pons A (2010) Impaired lymphocyte mitochondrial antioxidant defences in variegate porphyria are accompanied by more inducible reactive oxygen species production and DNA damage. Br J Haematol 149:759–767
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Meth Enzymol 105:114–121
Funes L, Fernández-Arroyo S, Laporta O, Pons A, Roche E, Segura-Carretero A, Fernández-Gutiérrez A, Micol V (2009) Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem (in press)
Goldberg DM, Spooner RJ (1985) Glutathione Reductase. Methods in enzymatic analysis. Verlag Chemie, Basel
Grese TA, Dodge JA (1998) Selective estrogen receptor modulators (SERMs). Curr Pharm Des 4(1):71–92
Jackson MJ, Papa S, Bolanos J, Bruckdorfer R, Carlsen H, Elliott RM, Flier J, Griffiths HR, Heales S, Holst B, Lorusso M, Lund E, Oivind Moskaug J, Moser U, Di Paola M, Polidori MC, Signorile A, Stahl W, Vina-Ribes J, Astley SB (2002) Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med 23(1–3):209–285
Ji LL (1999) Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med 222(3):283–292
Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139(10):4252–4263
Kurzer MS (2002) Hormonal effects of soy in premenopausal women and men. J Nutr 132(3):570S–573S
Li J, Zheng Y, Zhou H, Su B, Zheng R (1997) Differentiation of human gastric adenocarcinoma cell line MGc80–3 induced by verbascoside. Planta Med 63(6):499–502
Liening SH, Stanton SJ, Saini EK, Schultheiss OC (2010) Salivary testosterone, cortisol, and progesterone: two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiol Behav 99(1):8–16
Marshall JC, Case GD, Valk TW, Corley KP, Sauder SE, Kelch RP (1983) Selective inhibition of follicle-stimulating hormone secretion by estradiol. Mechanism for modulation of gonadotropin responses to low dose pulses of gonadotropin-releasing hormone. J Clin Invest 71(2):248–257
Matchock RL, Dorn LD, Susman EJ (2007) Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiol Int 24(5):969–990
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Messinis IE, Templeton AA (1990) Effects of supraphysiological concentrations of progesterone on the characteristics of the oestradiol-induced gonadotrophin surge in women. J Reprod Fertil 88(2):513–519
Monroe SE, Jaffe RB, Midgley AR Jr (1972) Regulation of human gonadotropins. XII. Increase in serum gonadotropins in response to estradiol. J Clin Endocrinol Metab 34(2):342–347
Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA (2001) Mechanisms of estrogen action. Physiol Rev 81(4):1535–1565
Ohno T, Inoue M, Ogihara Y, Saracoglu I (2002) Antimetastatic activity of acteoside, a phenylethanoid glycoside. Biol Pharm Bull 25(5):666–668
Ortega RM, Requejo AM, Navia B, López Sobaler AM (2004) Nutrient and energy recommended dietary intake for the Spanish population. In: Nutrition and health, Consejería de Sanidad, Madrid, p 66
Papoutsi Z, Kassi E, Mitakou S, Aligiannis N, Tsiapara A, Chrousos GP, Moutsatsou P (2006) Acteoside and martynoside exhibit estrogenic/antiestrogenic properties. J Steroid Biochem Mol Biol 98(1):63–71
Pedersen BK, Hoffman-Goetz L (2000) Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 80(3):1055–1081
Richardson J (1986) Vitamin C and immunosuppression. Med Hypotheses 21(4):383–385
Saracoglu I, Inoue M, Calis I, Ogihara Y (1995) Studies on constituents with cytotoxic and cytostatic activity of two Turkish medicinal plants Phlomis armeniaca and Scutellaria salviifolia. Biol Pharm Bull 18(10):1396–1400
Seidel V, Verholle M, Malard Y, Tillequin F, Fruchart JC, Duriez P, Bailleul F, Teissier E (2000) Phenylpropanoids from Ballota nigra L. inhibit in vitro LDL peroxidation. Phytother Res 14(2):93–98
Sullivan JC, Sasser JM, Pollock JS (2007) Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 292(2):R764–R768
Sureda A, Batle JM, Tauler P, Aguilo A, Cases N, Tur JA, Pons A (2004) Hypoxia/reoxygenation and vitamin C intake influence NO synthesis and antioxidant defences of neutrophils. Free Radic Biol Med 37(11):1744–1755
Sureda A, Tauler P, Aguilo A, Cases N, Fuentespina E, Cordova A, Tur JA, Pons A (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 39(12):1317–1324
Sureda A, Batle JM, Tauler P, Ferrer MD, Tur JA, Pons A (2006) Vitamin C supplementation influences the antioxidant response and nitric oxide handling of erythrocytes and lymphocytes to diving apnea. Eur J Clin Nutr 60(7):838–846
Sureda A, Ferrer MD, Tauler P, Maestre I, Aguilo A, Cordova A, Tur JA, Roche E, Pons A (2007) Intense physical activity enhances neutrophil antioxidant enzyme gene expression. Immunocytochemistry evidence for catalase secretion. Free Radic Res 41(8):874–883
Sureda A, Ferrer MD, Tauler P, Tur JA, Pons A (2008a) Lymphocyte antioxidant response and H2O2 production after a swimming session: gender differences. Free Radic Res 42(4):312–319
Sureda A, Tauler P, Aguilo A, Cases N, Llompart I, Tur JA, Pons A (2008b) Influence of an antioxidant vitamin-enriched drink on pre- and post-exercise lymphocyte antioxidant system. Ann Nutr Metab 52(3):233–240
Tauler P, Gimeno I, Aguilo A, Guix MP, Pons A (1999) Regulation of erythrocyte antioxidant enzyme activities in athletes during competition and short-term recovery. Pflugers Arch 438(6):782–787
Tauler P, Aguilo A, Gimeno I, Noguera A, Agusti A, Tur JA, Pons A (2003) Differential response of lymphocytes and neutrophils to high intensity physical activity and to vitamin C diet supplementation. Free Radic Res 37(9):931–938
Tauler P, Aguilo A, Guix P, Jimenez F, Villa G, Tur JA, Cordova A, Pons A (2005) Pre-exercise antioxidant enzyme activities determine the antioxidant enzyme erythrocyte response to exercise. J Sports Sci 23(1):5–13
Tauler P, Aguilo A, Gimeno I, Fuentespina E, Tur JA, Pons A (2006a) Response of blood cell antioxidant enzyme defences to antioxidant diet supplementation and to intense exercise. Eur J Nutr 45(4):187–195
Tauler P, Sureda A, Cases N, Aguilo A, Rodriguez-Marroyo JA, Villa G, Tur JA, Pons A (2006b) Increased lymphocyte antioxidant defences in response to exhaustive exercise do not prevent oxidative damage. J Nutr Biochem 17(10):665–671
Tauler P, Ferrer MD, Romaguera D, Sureda A, Aguilo A, Tur J, Pons A (2008a) Antioxidant response and oxidative damage induced by a swimming session: Influence of gender. J Sports Sci 26(12):1303–1311
Tauler P, Ferrer MD, Sureda A, Pujol P, Drobnic F, Tur JA, Pons A (2008b) Supplementation with an antioxidant cocktail containing coenzyme Q prevents plasma oxidative damage induced by soccer. Eur J Appl Physiol 104(5):777–785
Tsai CC, Yen SS (1971) The effect of ethinyl estradiol administration during early follicular phase of the cycle on the gonadotropin levels and ovarian function. J Clin Endocrinol Metab 33(6):917–923
Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM (2011) Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med 40(12):1037–1053
Wood WG (1984) Luminescence immunoassays: problems and possibilities. J Clin Chem Clin Biochem 22(12):905–918
Xia Y, Roman LJ, Masters BS, Zweier JL (1998) Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem 273(35):22635–22639
Young JR, Jaffe RB (1976) Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. II. Effects of varying concentrations of estradiol. J Clin Endocrinol Metab 42(3):432–442
Acknowledgments
This work has been granted by the Spanish Ministry of Education, Consejo Superior de Deportes (10/UPB10/08), Generalitat Valenciana (ACOMP/2010/107) and the Ministry of Science and Innovation (DPS2008-07033-C03-03 and AGL2007-60778). A Mestre was funded by grant of the University of Balearic Islands. MD Ferrer and MM Bibiloni were funded by grant of the Spanish Ministry of Science and Education. We are thankful to trainers Rafael Huete and Fernando Gómez-Reyno for their support and collaboration in the organization of the exercise test. The authors declare that the experiments comply with the current laws of Spain. We thank Monteloeder, S.L. for providing the lemon verbena extract and liability insurance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Mestre-Alfaro, A., Ferrer, M.D., Sureda, A. et al. Phytoestrogens enhance antioxidant enzymes after swimming exercise and modulate sex hormone plasma levels in female swimmers. Eur J Appl Physiol 111, 2281–2294 (2011). https://doi.org/10.1007/s00421-011-1862-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1862-y