Abstract
This study sought to determine the effect of the pre-transition work rate (WR) and WR transition magnitude on the adjustment of pulmonary oxygen uptake (VO2p kinetics) in older men. Seven men (69 ± 5 years; mean ± SD) each performed 4–6 cycling transitions from 20 W to either a WR corresponding to 90% estimated lactate threshold (full step, FS) or 2 equal-step transitions (lower step, LS; upper step, US) to the same end-exercise WR as in FS. Gas exchange was analysed breath-by-breath and muscle deoxygenation (∆[HHb]) was measured with NIRS. The time constant (τ) for VO2p was greater in US (53 ± 17 s) and FS (44 ± 11 s) compared to LS (37 ± 9 s); τVO2p for US also trended (p = 0.05) towards being greater than FS. The VO2p gain in US (9.97 ± 0.41 mL/min/W) was greater than LS (9.06 ± 1.17; p = 0.06) and FS (9.13 ± 0.54; p < 0.05). The O2 deficit was greater in US (0.25 ± 0.08 L) than LS (0.19 ± 0.06 L); yet the ‘accumulated O2 deficit’ (0.44 ± 0.13 L; O2 deficit from LS + US) was similar to that of FS (0.42 ± 0.13 L; p = 0.38). The effective Δ[HHb] response time (τ′∆[HHb]) for US (36 ± 12 s) was greater than LS (27 ± 6 s; p = 0.07) and FS (26 ± 4 s; p < 0.05), suggesting that the slowed adjustment of muscle O2 extraction was associated with the slowed VO2 kinetics of the US. Despite already slowed VO2p kinetics, older men exhibit further slowing when small WR transitions are initiated from an elevated pre-transition WR, yet this results in no cumulative impact on O2 deficit. This slowing in US compared to LS does not appear to be related to local O2 availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An approximately linear relationship exists between work rate (WR) and oxygen consumption during steady-state, constant-load exercise performed within the moderate-intensity domain; yet it is during non-steady state transitions between WRs, when this linear relationship is temporarily challenged (Hughson et al. 2001; Poole et al. 2007; Whipp and Wasserman 1972), where insights can be gained into the control mechanisms governing the delivery and consumption of oxygen (VO2). Thus, the study of pulmonary VO2 (VO2p) kinetics has become an area of interest for exercise physiologists. Following an abrupt increase in WR within the moderate-intensity domain (i.e., below the lactate threshold), VO2p exhibits an exponential increase, after a brief period (i.e., the “cardiodynamic” phase), eventually resulting in the attainment and maintenance of a steady-state VO2p (Whipp and Ward 1990; Whipp and Wasserman 1972). Outside of these tightly controlled laboratory situations, however, humans rarely perform prolonged bouts of constant WR exercise, but instead experience frequent fluctuations in exercise intensity and metabolic rate even during activities of daily living. As such, both the WR prior to a transition and the magnitude of the change in WR are practical concerns that have been considered within the literature. Several studies (Brittain et al. 2001; Hughson and Morrissey 1982; MacPhee et al. 2005) have demonstrated a slowed VO2p adjustment and increased O2 cost per unit increase in WR (VO2 gain; ΔVO2p/ΔWR) in young adults when transitions were performed in the upper (i.e., from an elevated initial WR and metabolic rate) compared with the lower region of the moderate-intensity exercise domain.
Hughson and Morrissey (1982) proposed a model whereby the slowed VO2p adjustment observed in transitions performed from a higher initial WR was limited by bulk O2 delivery. Specifically, a rapid withdrawal of parasympathetic neural activity would explain similarly rapid adjustments in the heart rate (HR) response during rest-to-work transitions, whereas the relatively slower sympathetic activation during work-to-work transitions may underlie the observation of slower HR adjustments. A role for O2 transport in limiting the adjustment of muscle O2 utilization in the upper region of the moderate-intensity domain was further supported by the findings of MacPhee et al. (2005), who reported slowed femoral (conduit) artery blood flow and slowed HR kinetics in transitions performed from an elevated compared to a lower baseline metabolic and work rate. Whether this suggested relationship between slowed bulk delivery of O2 and slowed adjustment of VO2p has implications for the matching of O2 distribution within the microvasculature, however, remains unclear. In addition to slowed VO2p kinetics in the upper region of the moderate-intensity domain, MacPhee et al. also observed a greater mean response time for near-infrared spectroscopy (NIRS) derived muscle deoxygenation (Δ[HHb]), reflecting the balance between O2 delivery and O2 utilization within the microvasculature, possibly suggesting that local muscle O2 delivery was improved relative to metabolic demand and that a greater reliance on O2 extraction was not required to meet the O2 requirements of the muscle. This latter observation of a slowed Δ[HHb] adjustment from an elevated baseline may, in fact, support the proposal of Brittain et al. (2001), that a hierarchical recruitment pattern exists which favours recruitment of the most efficient (i.e., lowest VO2 gain) fibres with inherently fast kinetics during transitions from low pre-transition WRs, and therefore only less efficient, slower adjusting fibres are available to address the added demands of a subsequent transition to a higher WR within the moderate-intensity domain. It is conceivable that the faster and slower VO2p profiles observed in the lower and upper regions of the moderate-intensity domain, respectively, are governed by independent physiological mechanisms.
Advanced age has been linked to a slowed VO2p response during moderate-intensity exercise transitions (Babcock et al. 1994; Bell et al. 1999; DeLorey et al. 2004; Murias et al. 2010; Scheuermann et al. 2002). At present it is unknown whether the already slowed VO2p kinetics in older adults are further slowed when transitions are performed from an elevated initial WR as is the case in younger adults. Such a response could potentially result in a greater accumulated O2 deficit and disruption to cellular metabolic stability (Zoladz et al. 2006), which may compromise exercise tolerance. From a practical perspective in older adults, it may be that transitioning from near rest, to a lower moderate-intensity (functionally serving as a low-intensity “warm-up”) and then to a higher moderate-intensity of exercise may not be beneficial, particularly if the work in the higher ranges of the moderate-intensity domain is performed by fibres that had not previously been recruited; on the other hand, it may also be that this type of incremental “warm-up” exercise may favour improved local muscle blood flow and O2 delivery. Therefore, the purpose of the present study was to investigate the effect of the pre-transition WR and metabolic rate (since these were not dissociated from one another in the present study), and WR transition magnitude (i.e., a “full step” to 90% of lactate threshold versus two “half steps” to the same end-exercise WR) on the parameters of VO2p and Δ[HHb] kinetics in older men. It was hypothesized that: (1) the adjustment of VO2p following a small increase in WR within the moderate-intensity domain from an elevated baseline WR would be slower and have a larger VO2 gain than either large or small magnitude changes in WR performed from a low baseline WR; (2) small WR transitions performed from a low baseline WR would result in faster VO2p kinetics and a smaller VO2 gain than large WR transitions performed from an identical low baseline WR; (3) the adjustment of Δ[HHb] would be slower in response to transitions performed from an elevated baseline compared to a lower baseline metabolic and work rate.
Methods
Participants
Seven older men (69 ± 5 years; mean ± SD; Table 1) volunteered and gave written consent to participate in the study. All procedures were approved by The University of Western Ontario Research Ethics Board for Health Sciences Research Involving Human Subjects. All participants were recreationally active and non-smokers. Additionally, no participants were taking medications that would affect the cardiorespiratory or haemodynamic responses to exercise.
Protocol
On day one, participants reported to the laboratory to perform a ramp incremental test (20–25 W/min) to the limit of tolerance on a cycle ergometer (model: H-300-R Lode; Lode B.V., Groningen, Holland) for determination of peak VO2 (VO2peak) and the estimated lactate threshold (θ L). θ L was determined by visual inspection as the VO2 at which CO2 output (VCO2) began to increase out of proportion in relation to VO2 with a systematic rise in minute ventilation-to-VO2 ratio and end-tidal PO2, whereas minute ventilation-to-VCO2 ratio and end-tidal PCO2 were stable (Beaver et al. 1986).
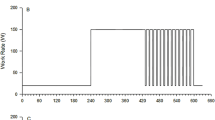
Subsequent to the incremental test, participants completed 4–6 square-wave transitions within the moderate-intensity domain in each of two upright leg cycling exercise protocols (Fig. 1). One of these protocols required participants to perform transitions consisting of 6 min of baseline cycling at 20 W, followed by 2 equal 6 min step transitions (lower step, LS; upper step, US) to a final WR corresponding to 90% θ L (n = 6 repetitions of this protocol) while the second protocol required participants to perform 6-min transitions from 20 W to a WR corresponding to 90% θ L (full step, FS; n = 4 repetitions of this protocol). Each visit to the laboratory was separated by at least 24 h.
Measurements
Gas exchange measurements were similar to those previously described (Babcock et al. 1994). Briefly, inspired and expired flow rates were measured using a low dead space (90 mL) bidirectional turbine (Alpha Technologies VMM 110) which was calibrated before each test using a syringe of known volume. Inspired and expired gases were continuously sampled (50 Hz) at the mouth and analysed for concentrations of O2, CO2, and N2 by mass spectrometry (Innovision, AMIS 2000, Lindvedvej, Denmark) after calibration with precision-analysed gas mixtures. Changes in gas concentrations were aligned with gas volumes by measuring the time delay for a square-wave bolus of gas passing the turbine to the resulting changes in fractional gas concentrations as measured by the mass spectrometer. Data were transferred to a computer, which aligned concentrations with volume information to build a profile of each breath. Breath-by-breath alveolar gas exchange was calculated by using algorithms of Beaver et al. (1981).
HR was monitored continuously by electrocardiogram (three-lead arrangement) using PowerLab (ML132/ML880; ADInstruments, Colorado Springs, CO). Data were recorded using LabChart v4.2 (ADInstruments, Colorado Springs, CO) on a separate computer.
Local muscle deoxygenation profiles of the quadriceps vastus lateralis muscle were made with NIRS (Hamamatsu NIRO 300, Hamamatsu Photonics, Hamamatsu, Japan). Optodes were placed on the belly of the muscle midway between the lateral epicondyle and greater trochanter of the femur. The optodes were housed in an optically dense plastic holder and secured on the skin surface with tape and then covered with an optically dense, black vinyl sheet, thus minimizing the intrusion of extraneous light. The thigh was wrapped with an elastic bandage to minimize movement of the optodes.
The physical principles of tissue spectroscopy and the manner in which these are applied have been explained by DeLorey et al. (2003). Briefly, one fiber optic bundle carried the NIR-light produced by the laser diodes to the tissue of interest while a second fiber optic bundle (interoptode spacing = 5 cm) returned the transmitted light from the tissue to a photon detector (photomultiplier tube) in the spectrometer. Four laser diodes (λ = 775, 810, 850, and 910 nm) were pulsed in a rapid succession and the light was detected by the photomultiplier tube for online estimation and display of the concentration changes from the resting baseline for oxyhaemoglobin (Δ[HbO2]), Δ[HHb], and total haemoglobin (Δ[Hbtot]). Changes in light intensities were recorded continuously at 2 Hz and transferred to a computer for later analysis. The NIRS-derived signal was zero set with the subject sitting in a resting steady-state on the cycle ergometer prior to the onset of baseline exercise and changes in the concentration are reported as a delta (∆) in arbitrary units (a.u.).
Data analysis
VO2p and HR data were filtered by removing aberrant data points that lay outside 4 SD of the local mean. Data for each repetition of a similar protocol were then linearly interpolated to 1 s intervals, time-aligned such that time zero represented the first transition and ensemble-averaged to yield a single averaged response for each subject for a given exercise protocol. These averaged responses were further time-averaged into 5-s bins. The on-transient responses for VO2p and HR were modelled using the following equation:
where Y(t) represents the VO2p or HR at any given time (t); YBSLN is the steady-state baseline value of Y before an increase in WR; A is the amplitude of the increase in Y above YBSLN; τ represents the time required to attain 63% of the steady-state amplitude; and TD represents the mathematically generated time delay through which the exponential model is predicted to intersect the baseline. After excluding the initial 20 s of data from the model, while still allowing TD to vary freely (in order to optimize accuracy of parameter estimates), VO2p data were modelled to the end of the 6-min exercise transition; HR data were modelled from the first datum after a transition to the end of the 6-min exercise transition. The model parameters were estimated by least-squares nonlinear regression (Origin, OriginLab Corp., Northampton, MA, USA) in which the best fit was defined by minimization of the residual sum of squares and minimal variation of residuals around the Y-axis (Y = 0). The 95% confidence interval (CI95) for the estimated time constant was determined after preliminary fit of the data with YBSLN, A, and TD constrained to the best-fit values and the τ allowed to vary. In addition, a value for the mean response time (Linnarsson 1974) or effective response time (Whipp and Ward 1990) of VO2p (τ′VO2p) was estimated using the function described in Eq. 1, but with data from the initial 20 s following exercise onset included in the model and TD constrained to 0 s. This approach characterizes the entire response (i.e., Phases I, II and III) and allows for an accurate estimate of the O2 deficit (Rossiter et al. 1999), computed as the product of τ′VO2p and the amplitude of the VO2p response from this alternate model (A′).
The Δ[HHb] profile has been described to consist of a time delay at the onset of exercise, followed by an increase in the signal with an “exponential-like” time course. The time delay for the ∆[HHb] response (TD Δ[HHb]) was determined using second-by-second data (DeLorey et al. 2003) and corresponded to the time, after the onset of exercise, at which the ∆[HHb] signal began a systematic increase from its nadir value. Determination of the TD ∆[HHb] was made on individual trials and averaged to yield three values (i.e., LS, US, FS) for each individual. The ∆[HHb] data were modelled using Eq. 1; the fitting window for the “exponential” response spanned from the end of the TD ∆[HHb] to 90 s into each transition. As described previously (duManoir et al. 2010), different fitting strategies ranging from 90 to 180 s into a transition resulted in minimal differences in estimates of τ[HHb]. The early exponential increase in ∆[HHb] was well-characterized in the 90 s following exercise onset in participants from the present study, whereas longer fitting windows risked poorer fitting of the early transient. Baseline ∆[HHb] (∆[HHb]BSLN) values were computed as the mean value in the 60 s prior to a transition, and ∆[HHb]BSLN for the US were calculated independently from the steady state predicted by the exponential fit from the LS. Whereas the τΔ[HHb] described the time course for the increase in ∆[HHb], the overall change of the effective ∆[HHb] (τ′∆[HHb] = TD ∆[HHb] + τ∆[HHb]) described the overall time course of the ∆[HHb] from the onset of each step transition.
Statistics
Data are presented as means ± SD. Repeated measures analyses of variance (ANOVA) were used to determine statistical significance for the dependent variables. A Tukey post hoc analysis was used when significant differences were found for the main effects of each dependent variable. All statistical analyses were performed using SPSS Version 16.0, (SPSS Inc., Chicago, IL). Statistical significance was declared when p < 0.05.
Results
Figures 2 and 3 display the VO2p and Δ[HHb] responses, respectively, to each of the two exercise protocols from a representative subject. Group mean parameter estimates for VO2p, HR and Δ[HHb] kinetics are presented in Table 2. τVO2p was greater (p < 0.05) in the US (53 ± 17 s) and FS (44 ± 11 s) compared to the LS (37 ± 9 s); τVO2p for the US also trended towards being greater (p = 0.05) than the FS. The trend of smallest, intermediate and greatest τVO2p values in the LS, FS and US, respectively, was observed in six of seven participants (Fig. 4). The VO2p gain in the US (9.97 ± 0.41 mL/min/W) was greater (p < 0.05) than that observed in the FS (9.13 ± 0.54 mL/min/W; p < 0.05) and trended towards being greater (p = 0.06) than in the LS (9.06 ± 1.17 mL/min/W). By design, the US was initiated from an elevated VO2p baseline (VO2pBSLN; 1.07 ± 0.09 L/min) compared to the FS (0.85 ± 0.09 L/min) and LS (0.83 ± 0.06 L/min). The steady-state VO2p (VO2pSS) attained in the US and FS was identical (1.32 ± 0.17 and 1.32 ± 0.18 L/min, respectively), while the VO2pSS (1.07 ± 0.09 L/min; Table 2) required by the LS was lower (p < 0.05). The O2 deficit (τ′VO2p × A′) was greater (p < 0.05) in the US (0.25 ± 0.08 L) compared to the LS (0.19 ± 0.06 L; Fig. 5); this was observed in all seven participants. The ‘accumulated O2 deficit’ (0.44 ± 0.13 L), calculated as the sum of the O2 deficit from the LS and US, was not different from the O2 deficit calculated for the FS (0.42 ± 0.13 L).
VO2p (empty circle) response (5-s average) in a representative subject with mono-exponential models superimposed. Residuals to the fitted functions are shown as fluctuating “randomly” around zero error. a Displays a response to the two-step protocol depicted in Fig. 1a; b displays a response to the FS protocol depicted in Fig. 1b). Dashed line represents the beginning of new WR
The HR response was similar to that of VO2p in that differences in HRBSLN, HRAMP and HRSS were identified depending on the condition (Table 2); yet, τHR remained unchanged across the three different exercise intensity transitions (p = 0.11; Table 2).
The adjustment of muscle deoxygenation was slowest (p < 0.05) in the US; specifically, τΔ[HHb] was greater (p < 0.05) in the US (22 ± 10 s) compared to the FS (13 ± 4 s) or the LS (11 ± 5 s), and τ′Δ[HHb] was greater (p < 0.05) in the US (36 ± 12 s) than the FS (26 ± 4 s) and trended towards being greater (p = 0.07) than the LS (27 ± 6 s). Finally, the index of the steady-state, end-exercise Δ[HHb] amplitude (Δ[HHb]EE-AMP) to VO2p amplitude (VO2pAMP) did not differ amongst the three conditions (LS, 14.3 ± 11.8; US, 12.5 ± 8.9; FS, 13.1 ± 9.3).
Discussion
This study investigated the effects of the pre-transition WR and metabolic rate, and WR transition magnitude on the adjustment of VO2p, Δ[HHb] and HR during the exercise on-transient in a group of older men. The main findings were as follows: (1) moderate-intensity step transitions initiated from an elevated baseline WR and metabolic rate (i.e., US) resulted in a greater τVO2p and greater VO2 gain than step transitions initiated from a baseline WR of 20 W (i.e., LS and FS); (2) the slowed VO2p kinetics of the US were accompanied by a slowed adjustment of Δ[HHb] in comparison to the LS and FS; (3) the ‘accumulated O2 deficit’ for two equal step transitions did not differ from the O2 deficit incurred for a single-step transition to the same end-exercise WR despite being elevated in the US compared to LS. Collectively, these findings suggest that the physiological response of older adults to these perturbations may comprise: (1) an improved local blood flow or O2 availability during the US; (2) a systematic, hierarchal recruitment pattern that favours recruitment of highest efficiency, fastest kinetic fibres to perform the work demanded by the LS, with lower-efficiency, slower kinetic fibres to address the energy demands of the US, and a mixture of these fibres (with intermediate efficiency and kinetic properties) to perform the work required by the FS; and (3) no net effect on the proportion of energy that is derived through non-aerobic pathways.
The participants tested in the present study were comparable to similar groups of older men tested in our laboratory; the τVO2p values in the full step (44 ± 11 s), which are greater than those generally reported for healthy younger adults (~20–30 s) (DeLorey et al. 2004; Gurd et al. 2006, 2008; Murias et al. 2010), are similar to pre-training values recently reported (43 ± 11 s) by Murias et al. (2010) in a study of older men of the same age and fitness (age, 68 ± 7 years, VO2peak, 2.3 ± 0.5 L/min). Therefore, in agreement with the stated hypothesis, despite the already slowed VO2p kinetics in these older men (compared to the ~25 s τVO2p values observed in younger adults), the adjustment of VO2p in response to the US was slowed even further and with a larger VO2p gain than in either the FS or LS in this group of older men; yet, as stated, the cumulative effect of this slow, inefficient adjustment is negated by the more rapid and efficient response to the LS, such that the accumulated O2 deficit did not differ from the O2 deficit accrued in the FS. These results also agree with those reported in younger individuals who performed similar exercise protocols (Brittain et al. 2001; Hughson and Morrissey 1982; MacPhee et al. 2005).
Historically, the major hypotheses presented to explain the primary limitation to VO2 kinetics are (1) that the rate of adjustment of VO2 at exercise onset is limited by the availability of O2 at active muscle sites (Tschakovsky and Hughson 1999), and (2) that VO2 kinetics is limited by a ‘sluggish’ activation of metabolic pathways and availability of metabolic substrates (other than O2) to the mitochondria [previously referred to as a “metabolic inertia” (Grassi 2001; Poole et al. 2007)]. The ‘double-step’ exercise protocol employed in the present study has been used to investigate the mechanisms that govern the rate of adjustment of muscle O2 utilization (as reflected by the phase II pulmonary O2 uptake) under conditions of different metabolic demand. Participants in the present study attained identical VO2pSS in both the US and FS; however, the τVO2p in the US was ~10 s greater than in the FS, suggesting that even within an individual there may not be a single “rate-limiting factor” that governs this response under differing conditions.
The focus of studies using the ‘double-step’ protocol has been the slowed VO2p adjustment in the US compared to the LS. While this slower response was observed in the US compared to the LS in the present study, it also should be emphasized that when exercise was initiated from the same starting WR (and metabolic rate), τVO2p was appreciably faster in the LS than in the FS. Hughson and Morrissey (1982, 1983) suggested that the reduced τVO2p in the LS was likely the result of a faster adjustment of bulk O2 delivery (i.e., faster HR kinetics); this idea was supported by MacPhee et al. (2005) who observed both faster HR and femoral (conduit) artery blood flow kinetics in the LS compared to US during knee-extension exercise. Contrary to these observations in young adults, in the present study no differences were observed in τHR amongst the three conditions in older men. When taken together, the altered τVO2p values across the three conditions, combined with the unaffected τHR estimates reinforces the idea that central, bulk, delivery of O2 does not appear to limit the Phase II VO2p response to moderate-intensity exercise. This statement assumes that τHR approximates the time course of adjustment for cardiac output which was not measured.
Brittain et al. (2001) discussed the possibility that higher-efficiency muscle fibres (i.e., smallest VO2 gain) with inherently faster kinetic properties (i.e., smallest τVO2p) may be preferentially recruited over lower-efficiency, slower kinetic fibres. As such, supposing that the most efficient fibres with the fastest kinetics were recruited to perform the work required by the LS, only those fibres with a greater VO2 gain and τVO2p would be available to address the additional metabolic demands imposed by the US. In this model, the work demanded by the FS would be accomplished by a mixture of fibres with these properties, thereby resulting in an intermediate VO2p kinetics profile relative to the LS and US. Indeed, data from the present study could be used to support this suggestion, as the τVO2p was fastest in the LS, intermediate in the FS and slowest in the US, while the VO2 gain also tended to conform to the proposed model.
The notion that fibre recruitment patterns might underlie the slowed VO2 kinetics (and increased VO2 gain) observed in the US has recently been examined by DiMenna et al. (2010). These authors reported similar phase II τVO2p values when transitioning from a raised metabolic rate (approximating 95% of θ L) but not a raised pre-transition WR (i.e., following an incomplete recovery from a prior bout of heavy-intensity exercise) as compared to a control bout of heavy-intensity exercise; this finding was in opposition to the condition in which the transition to heavy-intensity exercise was initiated from both a raised metabolic rate and WR which yielded a markedly slower response. Since the theoretical model described above would allow for recruitment of muscle fibres from the same pool in both the control condition and the raised metabolic rate condition, but not the raised WR condition, owing to the fact that exercise was initiated from the same pre-transition WR in the first two, these results seem to lend support to the notion of a hierarchal recruitment pattern. The impact of these conclusions on the interpretation of the present data is unclear, however, due to the fact that the exercise bouts spanned two different intensity domains. An important consideration, for instance, is the notion that during the initial heavy-intensity exercise bout, the onset of muscle fatigue may occur within the first minute (Sargeant and Dolan 1987), thereby potentially altering recruitment patterns in the subsequent bout.
Another possible explanation for the relatively slowed adjustment of VO2p (and presumably muscle VO2) of the US is a potentially less favourable energetic status at the transition onset in comparison to the LS or FS. The elevated pre-transition WR characteristic of the US is associated with a reduced cellular energetic state in active fibres (i.e., reduced PO2 and [PCr], increased [ADP], and less negative ΔGATP) which has the potential to slow the VO2p on-transient response to any subsequent increase in energy demand (Barstow et al. 1994; Kemp 2008). Further, that a trend for a greater VO2 gain was observed in the US compared to LS—two steps of equal WR magnitude, likely requiring the same change in ATP demand—suggests the possibility of a lower P/O ratio of oxidative phosphorylation (ATP produced per atom of O2 reduced). A reduced P/O ratio would indicate reduced efficiency within the mitochondria during the on-transient of the US; this along with the reduced cellular energetic state in active fibres could potentially lead to both higher τVO2p and VO2 gain values which are consistent with the findings in the present study. However, it also is possible that the ATP requirement for the same change in WR is not the same, but instead was greater in the US compared to LS due to the accumulation of metabolites and fall in free energy release with ATP hydrolysis (Zoladz et al. 2006). In this case, the P/O would be similar in the LS and US, and the greater O2 requirement (perhaps relating to a slower adjustment) for a given change in WR would be a consequence of the greater ATP requirement.
The overall adjustment of muscle deoxygenation (as assessed by τ′Δ[HHb]) was slower in the US compared to either the LS (p = 0.07) or FS (p < 0.05). The TD Δ[HHb] remained unchanged amongst the three transition types, likely indicating that an early increase in local blood flow (and O2 availability) resulting from contributions of the muscle pump and some rapid vasodilation (Tschakovsky and Hughson 1999; Tschakovsky et al. 2004) shared a similar time course in all conditions. As such, the differences observed in τ′Δ[HHb] can be explained by underlying differences in the τΔ[HHb] response across the three conditions. Other studies from our laboratory have consistently shown τ′Δ[HHb] values of ~20–25 s (as was observed in the LS and FS) in older adults during upright cycling (DeLorey et al. 2004; Murias et al. 2010), so the lengthened response observed in the US is intriguing. Whereas differences were observed with respect to the time course of the Δ[HHb] adjustment, no differences were seen in the steady-state reliance on O2 extraction for a given VO2p as indicated by the similar Δ[HHb]EE-AMP/VO2pAMP amongst the three conditions; this differs from the greater Δ[HHb]AMP/VO2pAMP in the US compared to LS that was reported by MacPhee et al. (2005) in young adults performing knee-extension exercise. A similar index of Δ[HHb]AMP/VO2pAMP amongst the three conditions implies that the steady-state reliance on O2 extraction for a given metabolic demand is stable across conditions; so, for example, transitions requiring a greater VO2 gain are accomplished in part by a greater O2 extraction. However, since the time course of adjustment of Δ[HHb] relative to VO2 may differ during the on-transient, this relationship can be described as unstable during that period of adjustment. To this end, the present data, reinforce the notion that it is during the non-steady-state on-transient where considering differences in the adjustments of VO2p and Δ[HHb] may be useful in providing meaningful insights into the physiological mechanisms underlying the regulation of oxidative phosphorylation.
The slower adjustment of Δ[HHb] observed in the US may suggest an improved O2 availability prior to and throughout the transition relative to metabolic demand. The Δ[HHb] profile is thought to mirror the drop in the microvascular PO2 (PO2mv) at exercise onset, and as such, a slow fall in PO2mv (or Δ[HHb]) might be expected with an attenuated reliance on O2 extraction, a consequence of convective O2 delivery being in excess relative to the metabolic demand. Such a scenario is conceivable if the local matching of O2 delivery to O2 demand (i.e., microvascular O2 distribution) during the steady state in the LS was poor, thereby creating a condition where adequate O2 supply to active fibres was accomplished by over-perfusing the muscle as a whole. In such a scenario, availability of O2 would necessarily be adequate to inactive fibres as well as active fibres; thus, upon initiation of the US, those fibres that were not recruited to perform the work required of the LS would have to supply all of the metabolic substrates other than O2 (i.e., ADP, Pi, NADH, H+). This interpretation of the Δ[HHb] data has implications for the greater τVO2p observed in the US. Since a slowed Δ[HHb] adjustment (perhaps reflecting a slowed adjustment of PO2mv and therefore a preserved O2 driving pressure) would be expected when convective O2 delivery at exercise onset was in excess of O2 demand, the fact that τVO2p in the US was greater than that observed in the LS implies that O2 availability may not play a rate-limiting role in the regulation of VO2 kinetics under conditions of elevated pre-transition metabolic and work rates. Using this rationale, and particularly when also considering the greater VO2 gain values observed in the US, the present data support the model proposed by Brittain et al. (2001) which suggests that higher-efficiency muscle fibres (i.e., smallest VO2 gain) with inherently faster kinetic properties (i.e., smallest τVO2p) may be preferentially recruited over lower-efficiency, slower kinetic fibres.
The trend for speeded and slowed VO2p kinetics in the LS and US (relative to the FS), respectively, persisted when the VO2p data were modelled with TD VO2p constrained to 0 s. The product of greater τ′VO2p and A′ values in the US compared to the LS resulted in a greater O2 deficit in the US. Since the O2 deficit closely reflects the contribution of non-oxidative energy sources, an inflated O2 deficit would necessarily imply a greater accumulation of metabolic by-product and greater disturbance of intracellular homeostasis. As first reported by Brittain et al. (2001) in younger adults, the ‘accumulated O2 deficit,’ calculated as the sum of the O2 deficit conferred in the LS and US, did not differ from that observed in the FS in the present study. This finding may suggest that ‘progressive’ warm-up activities offer little cardiovascular benefit to older adults when they are performing exercise within the moderate-intensity domain.
It is important to recognize potential limitations associated with the methodology used in the present study. When modelling the on-transient VO2p response, the confidence in parameter estimates is largely dependent upon the underlying signal-to-noise ratio. Small magnitude WR increments, which are to be expected in older adults, but which were exacerbated in the present study’s “double-step” protocol, are likely to give rise to physiological responses with small amplitudes, and as a result, the potentially low signal-to-noise ratio must be considered. In an effort to address potential concerns, several repeats were performed for each of the two exercise protocols (four for FS and six for LS + US). We have recently determined that at least three repetitions are required for young adults exercising in the moderate-intensity domain to effectively improve day-to-day reproducibility of both τVO2p and τ′Δ[HHb] (Spencer et al. 2010). Given the smaller amplitude of response in older adults, a fourth repetition was added for FS data, and six repetitions were performed for protocols involving the smaller steps (i.e., LS and US). The consistency of findings within subjects resulted in statistically significant findings when comparing the different step transitions. Secondly, concerns may arise regarding the appropriate selection of WRs. Although blood lactate measures were not made in the present study, careful inspection of individual data confirmed the absence of a VO2p slow component (which would be expected had exercise been performed within the heavy-intensity domain). For each individual, a 20-s average taken from t = 4τ was used to predict end-exercise VO2p values by dividing by 0.98 (i.e., given that 4τ represents the time required to attain ~98% of steady-state amplitude). These ‘predicted end-exercise’ values were then compared to the observed 20 s end-exercise values. This resulted in individual differences that ranged from −0.057 mL/min (indicating a “decrease” from predicted end-exercise values) to 0.026 mL/min (mean = −0.017 ± 0.026 mL/min). As such we are confident that all transitions were within the moderate-intensity domain.
In conclusion, this study showed that despite presenting with slowed VO2p kinetics (in the FS compared to values typically reported for healthy, young adults), the VO2p kinetics in older men was slowed even further during exercise transitions in the US, and that compared to the FS, the VO2 kinetics was faster in the LS. Additionally, the VO2 gain tended to be greater in the US compared to the LS (p = 0.06) and FS (p < 0.05). Consequently, the O2 deficit was greater in the US compared to LS, but the overall ‘accumulated O2 deficit’ (from LS and US) was similar to the resultant O2 deficit conferred by the FS. Interestingly, the combination of slowed Δ[HHb] and VO2p adjustments that were observed in the US may suggest that local O2 availability does not limit VO2p kinetics in this unique condition. Collectively, these data support the proposed model of preferential recruitment of the most efficient fibres with inherently fast kinetic properties during the LS, and thus only less efficient, slower adjusting fibres are available to meet the demands of the US.
References
Babcock MA, Paterson DH, Cunningham DA, Dickinson JR (1994) Exercise on-transient gas exchange kinetics are slowed as a function of age. Med Sci Sports Exerc 26:440–446
Barstow TJ, Buchthal SD, Zanconato S, Cooper DM (1994) Changes in potential controllers of human skeletal muscle respiration during incremental calf exercise. J Appl Physiol 77:2169–2176
Beaver WL, Lamarra N, Wasserman K (1981) Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol 51:1662–1675
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60:2020–2027
Bell C, Paterson DH, Kowalchuk JM, Cunningham DA (1999) Oxygen uptake kinetics of older humans are slowed with age but are unaffected by hyperoxia. Exp Physiol 84:747–759
Brittain CJ, Rossiter HB, Kowalchuk JM, Whipp BJ (2001) Effect of prior metabolic rate on the kinetics of oxygen uptake during moderate-intensity exercise. Eur J Appl Physiol 86:125–134
DeLorey DS, Kowalchuk JM, Paterson DH (2003) Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95:113–120
DeLorey DS, Kowalchuk JM, Paterson DH (2004) Effect of age on O2 uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J Appl Physiol 97:165–172
DiMenna FJ, Bailey SJ, Vanhatalo A, Chidnok W, Jones AM (2010) Elevated baseline VO2 per se does not slow O2 uptake kinetics during work-to-work exercise transitions. J Appl Physiol 109:1148–1154
duManoir GR, DeLorey DS, Kowalchuk JM, Paterson DH (2010) Kinetics of VO2 limb blood flow and regional muscle deoxygenation in young adults during moderate intensity, knee-extension exercise. Eur J Appl Physiol 108:607–617
Grassi B (2001) Regulation of oxygen consumption at exercise onset: is it really controversial? Exerc Sport Sci Rev 29:134–138
Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM (2006) Prior heavy exercise elevates pyruvate dehydrogenase activity and speeds O2 uptake kinetics during subsequent moderate-intensity exercise in healthy young adults. J Physiol 577:985–996
Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM (2008) O2 uptake kinetics, pyruvate dehydrogenase activity, and muscle deoxygenation in young and older adults during the transition to moderate-intensity exercise. Am J Physiol Regul Integr Comp Physiol 294:R577–R584
Hughson RL, Morrissey M (1982) Delayed kinetics of respiratory gas exchange in the transition from prior exercise. J Appl Physiol 52:921–929
Hughson RL, Morrissey MA (1983) Delayed kinetics of VO2 in the transition from prior exercise. Evidence for O2 transport limitation of VO2 kinetics: a review. Int J Sports Med 4:31–39
Hughson RL, Tschakovsky ME, Houston ME (2001) Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev 29:129–133
Kemp G (2008) Physiological implications of linear kinetics of mitochondrial respiration in vitro. Am J Physiol Cell Physiol 295:C844–C846 (author reply C847–C848)
Linnarsson D (1974) Dynamics of pulmonary gas exchange and heart rate changes at start and end of exercise. Acta Physiol Scand Suppl 415:1–68
MacPhee SL, Shoemaker JK, Paterson DH, Kowalchuk JM (2005) Kinetics of O2 uptake, leg blood flow, and muscle deoxygenation are slowed in the upper compared with lower region of the moderate-intensity exercise domain. J Appl Physiol 99:1822–1834
Murias JM, Kowalchuk JM, Paterson DH (2010) Speeding of VO2 kinetics with endurance training in old and young men is associated with improved matching of local O2 delivery to muscle O2 utilization. J Appl Physiol 108:913–922
Poole DC, Ferreira LF, Behnke BJ, Barstow TJ, Jones AM (2007) The final frontier: oxygen flux into muscle at exercise onset. Exerc Sport Sci Rev 35:166–173
Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ (1999) Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol 518:921–932
Sargeant AJ, Dolan P (1987) Effect of prior exercise on maximal short-term power output in humans. J Appl Physiol 63:1475–1480
Scheuermann BW, Bell C, Paterson DH, Barstow TJ, Kowalchuk JM (2002) Oxygen uptake kinetics for moderate exercise are speeded in older humans by prior heavy exercise. J Appl Physiol 92:609–616
Spencer MD, Murias JM, Lamb HP, Kowalchuk JM, Paterson DH (2010) Are the parameters of VO2, heart rate and muscle deoxygenation kinetics affected by serial moderate-intensity exercise transitions in a single day? Eur J Appl Physiol
Tschakovsky ME, Hughson RL (1999) Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol 86:1101–1113
Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM (2004) Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96:639–644
Whipp BJ, Ward SA (1990) Physiological determinants of pulmonary gas exchange kinetics during exercise. Med Sci Sports Exerc 22:62–71
Whipp BJ, Wasserman K (1972) Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol 33:351–356
Zoladz JA, Korzeniewski B, Grassi B (2006) Training-induced acceleration of oxygen uptake kinetics in skeletal muscle: the underlying mechanisms. J Physiol Pharmacol 57(Suppl 10):67–84
Acknowledgments
We would like to express our gratitude to the participants in this study and to acknowledge the assistance provided by Brad Hansen. This study was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) research and equipment grants. Additional support was provided by Standard Life Assurance Company of Canada. J.M. Murias was supported by a doctoral research scholarship from the Canadian Institutes of Health Research (CIHR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan A. Ward.
Rights and permissions
About this article
Cite this article
Spencer, M.D., Murias, J.M., Kowalchuk, J.M. et al. Pulmonary O2 uptake and muscle deoxygenation kinetics are slowed in the upper compared with lower region of the moderate-intensity exercise domain in older men. Eur J Appl Physiol 111, 2139–2148 (2011). https://doi.org/10.1007/s00421-011-1851-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1851-1