Abstract
Isometric muscle force after active shortening is reduced [force depression (FD)]. The mechanism is incompletely understood but work delivered during shortening has been suggested to be the main determinant of FD. However, whether muscle length affects the sensitivity of FD to work is unknown, although this information might add to the understanding of the phenomenon. The aim of this study is to investigate the length dependence of the FD/work ratio (Q). Therefore, isometric force production (ISO) of 10 incubated mouse soleus muscles was compared to isometric force after 0.6, 1.2, and 2.4 mm shortening (IAS) at different end lengths ranging from L 0 − 3 to L 0 + 1.8 mm in steps of 0.6 mm. FD was calculated as the force difference between an ISO and IAS contraction at the same activation time (6 s) and end length. We confirm the strong relation between FD and work at L 0 (R ² = 0.92) and found that FD is length dependent with a maximum of 8.8 ± 0.3% at L 0 + 1.2 mm for 0.6 mm shortening amplitude. Q was only constant for short muscle lengths (<85% L 0) but increased exponentially with increasing muscle length. The observed length dependence of Q indicates that FD is not only determined by work produced during shortening but also by a length-dependent factor, possibly actin compliance, which should be incorporated in any mechanism explaining FD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compared to the pure isometric force, isometric force production (ISO) capacity of muscle after an active shortening phase, measured at the same muscle length and same time of activation, is reduced (Josephson and Stokes 1999; Abbott and Aubert 1952). This force deficit [force depression (FD)] is not explained within the actual concept of the contraction mechanism (Huxley and Simmons 1971) and until today its origin is incompletely understood (Herzog and Leonard 2007). In the last decennium two theories received much attention (Kosterina et al. 2009). A first theory, introduced by Maréchal and Plaghki (1979) and actualised by Herzog (1998) suggests that FD results from an inhibited myosin attachment due to the entering of deformed actin in the new zone of overlap formed during the shortening phase (see Fig. 1a). In a second theory suggested by Abbott and Aubert (1952) and actualized by Morgan et al. (2000) FD is related to serial non-uniformity of sarcomeres after shortening at different regions along the muscle fiber (see Fig. 1b).
Schematic drawing of two adjacent sarcomeres explaining the origin of FD according to the actin deformation (a) and the sarcomere heterogeneity theory (b). Activated sarcomeres are shown in three conditions: isometric at long muscle length (ISO), isometric after shortening from long to short muscle length (IAS) and a reference isometric contraction at short length (REF ISO). a The free and extendible actin filament is stressed by activation (dashed pattern in ISO). FD results from a reduced number of crossbridges in the zone of overlap with deformed actin compared to the reference isometric contraction. b Active shortening creates sarcomere heterogeneity reducing interfilament overlap compared to the reference isometric contraction
Irrespective of the theory, a general feature of FD is its strong relation with work delivered during the shortening phase (Leonard and Herzog 2005). Work (W) is determined by shortening amplitude (AMP) and force during shortening (F s) and both factors have been shown to affect FD. Indeed, a strong linear relationship between AMP and FD has been observed (Abbott and Aubert 1952; Herzog and Leonard 1997; Schachar et al. 2002; Van Noten and Van Leemputte 2009) yet the slope of this relationship seems muscle length dependent (Maréchal and Plaghki 1979). The relationship between F s and FD has been studied by changing shortening velocity (Herzog et al. 2000) and activation level (Leonard and Herzog 2005), both affecting the force production during shortening. F s and consequently the resulting W, are also affected by muscle length (Josephson and Stokes 1999; Roots et al. 2007) due to the force–length relationship. The observed length dependence of FD (Edman et al. 1993; Meijer et al. 1998; Morgan et al. 2000) is therefore thought to be caused by the length dependence of W, resulting from the F s–length relation (Schachar et al. 2002). This reasonable assumption includes the hypothesis that the FD/W ratio (Q) is length independent, yet this has never been tested. Moreover, some observations indicate that FD/W is not constant for different muscle lengths. Recently, Kosterina et al. (2008) confirmed the strong relationship between FD and W for contractions ending at sub-, super- and optimal muscle lengths, however, their data suggest that the slope of the FD–work relation differs for different lengths. Furthermore, at the ascending limb of the force–length relation Meijer et al. (1998) and Bullimore et al. (2007) noticed that FD was not affected by AMP. If FD is mainly predicted by work production, a change in AMP should be accompanied by a change in F s, which is rather unlikely. These observations suggest that a muscle length dependent factor, different from work, might be involved in the mechanism of FD. To our knowledge, a systematic description of the FD/W ratio over the complete physiological force–length relationship has never been done, although this information might add to evaluate suggested mechanisms.
Therefore, we aimed to test the hypothesis that FD/work is independent of muscle length. To test his hypothesis we studied FD and work after three shortening amplitudes for contractions ending at different muscle lengths covering the functional range of the muscle. We used incubated mouse soleus muscles, a model which has been shown (Derave et al. 2005) to be valid for studying contractile properties of mixed skeletal muscle.
Methods
Protocol (Fig. 2)
Force production of incubated supramaximally electrically stimulated whole soleus muscle (n = 12) of adult mouse (male nMRI mice, weight ±36.98 g, 11 weeks old) was measured during two series of contractions: pure isometric contractions and isometric-after-isokinetic (1.5 mm/s) shortening contractions (IAS). The ISO contractions were performed between the range of 3 mm shorter and 1.8 mm longer than optimal muscle length (L 0) in nine steps of 0.6 mm. The IAS contractions were performed with 0.6, 1.2, and 2.4 mm shortening amplitudes (LOW, MID, and HIGH, respectively) ending at the same lengths as ISO. HIGH shortening amplitudes were only tested at muscle lengths ≤L 0 to avoid risk of muscle damage related to starting length (Fig. 2). The rather slow velocity of IAS was chosen to generate relatively high FD, especially at low amplitude. Additionally, in order to evaluate possible muscle damage, the protocol started and ended with ISO at L 0. If compared to the first, force of the last contraction was reduced with more than 1%, the muscle was rejected from the analysis.
Mean (n = 10) and standard error of the mean (SEM) of passive (open square, 1 s prior to stimulation) and total (open diamond, after 1 s of stimulation) forces obtained at different lengths, ranging from L 0 − 3 to L 0 + 2.4 mm in steps of 0.6 mm. Active muscle force (open triangle) was calculated as the difference between total and passive force. Forces are expressed both in absolute values and in percentage of force at L 0. Arrows indicate the start and end length of isometric contractions after shortening (IAS). FD was defined as the difference between ISO and IAS obtained at the same muscle length
After anesthesia, soleus muscles were dissected from both mice hind limbs and were suspended in thermo-controlled (25°C) organ baths (Grass tissue bath, HSE, March, Germany) filled with 10 ml Krebs–Henseleit solution (concentrations in mmol: 118 NaCl, 25 NaHCO3, 5KCl, 1 MgSO4, 1 KH2PO4, 2.5 CaCl2 and 5 glucose) and continuously gassed with 95% O2 and 5% CO2. The muscles were mounted between a force transducer (F30, HSE, March, Germany), and a position servo controlled linear actuator (MX80L, Parker Hannifin Corp, Cleveland, OH, USA), which also supported a pair of parallel platinum electrodes (G2F/Pt, HSE, March, Germany) flanking the full length of the muscle to ensure uniform activation. A Simulink (Matlab, The Mathworks, Inc., Natick, USA) program generated bipolar rectangular pulses of 0.5 ms pulse width at 80 Hz, which were amplified (UISO, HSE, March, Germany) to an intensity of 20% above the level needed to evoke maximal force. While sampling (at 2 kHz) the actuators’ position, the stimulation train and the muscle force, the Simulink program also controlled the movement of the actuator. The principles of laboratory animal care were followed and the experimental protocol was approved by the Ethics Committee for animal research of the K.U. Leuven.
After 20 min of thermo equilibration, reference length L 0 was searched by a series of five short (1 s) isometric contractions, interspersed with 2 min rest interval, in steps of 0.2 mm around the length where the passive force reached 5 mN. The length corresponding to the highest active force (force during stimulation − passive force) served as L 0.
Thereafter, all contractions (9 ISO and 9 at LOW, 8 at MID and 3 at HIGH amplitude IAS) had train durations of 6.5 s and were interspersed with 3 min rest and were performed in random order. Muscles were brought to a starting length different from L 0 20 s before stimulation started. The shortening phase of all IAS contractions ended 3.5 s after the onset of stimulation (Fig. 3).
Example of force and length trajectories of comparable ISO and IAS (after low amplitude shortening) contractions ending at L 0 and L 0 − 3 mm. Supramaximal stimulation starts after 1 s and ends after 7.5 s. FD is defined as the difference between ISO and IAS force obtained at the same muscle length and after 6 s of stimulation, corresponding to 2.5 s after the end of shortening
Data analysis
The force–length relationship (Fig. 2) was obtained from the measured maximal force during the first second of activation and the passive force 0.5 s before activation, which allowed us to add the value of L 0 + 2.4 mm within the force–length relationship. Length was scaled to length in percent of L 0 based on the data for mouse soleus muscle of Askew and Marsh (1998). FD was defined as the difference in active force between ISO and IAS contraction at the same length and stimulation time (after 6 s). Thus, steady state force was evaluated after 2.5 s of recovery after the shortening phase. Relative FD was calculated as FD/ISO at the corresponding time and length. F s was calculated as the mean active force between the start and the end of the shortening phase. Work delivered during shortening was the product of F s and AMP. The Q ratio (FD/work) was obtained for all muscle lengths and for all shortening amplitudes.
Statistics
The difference between ISO and IAS (LOW amplitude) force after 6 s of stimulation was tested with two way ANOVA [9 lengths × 2 types of contractions (repeated)]. To test the effect of muscle length on FD, work, and Q after LOW amplitude, a one way repeated measures ANOVA was used. A two way ANOVA for repeated measures was used to test the effect of muscle length on FD, work and Q for MID and HIGH amplitude (8 lengths × 2 amplitudes and 3 lengths × 3 amplitudes, respectively). All main and interaction effects were analyzed with a Bonferroni post hoc test (Statistica, StatSoft Inc., Tulsa, USA). Statistical significance level was set at p < 0.05. All values are expressed as mean ± standard error of the mean (SEM).
Results
Out of 12 muscles, two muscles were rejected because of a 7% force drop during the protocol. Active force at L 0 was 235.4 ± 7.1 mN (F 0) and dropped to 117.2 ± 7.1 mN (50% of F 0) at L 0 − 3 mm and 109.8 ± 7.6 mN (46% of F 0) at L 0 + 2.4 mm (Fig. 2). After scaling the length to the reference values of Askew and March (1998), the muscle lengths were estimated to range from 75 to 120% L 0 in steps of 5%.
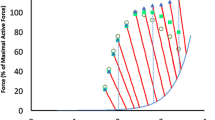
At comparable length and after 6 s stimulation, force of IAS was lower than ISO at all muscle lengths (p < 0.001) (Fig. 4). For the LOW amplitude contractions, maximal FD (16.5 ± 0.7 mN) was found at L 0 + 1.2 mm which was significantly different from FD at L 0 (11.6 ± 0.5 mN). For the MID and the HIGH amplitude contraction, maximal FD was found, respectively, at L 0 + 0.6 mm and L 0 (2.5 ± 0.8 and 35.1 ± 1.3 mN, respectively).
Mean (n = 10) and SEM of FD as a function of muscle length after LOW (open circle), MID (open triangle) and HIGH (open square) shortening amplitude. All FD measurements were significant, with significant amplitude and length effects. Asterisk indicates significant (p < 0.05) post hoc differences between adjacent lengths. Post hoc for amplitude showed no difference between LOW and MID at L 0 − 2.4 mm and L 0 + 1.2 mm
Except for equal FD production at L 0 − 2.4 and L 0 + 1.2 mm for LOW and MID amplitude, the increasing effect of shortening amplitude on FD production was found on all lengths (main effect) and this amplitude effect was length dependent (interaction effect). A maximal FD of 35.1 ± 1.3 mN (16.6 ± 0.4% of F 0) was found at L 0 after HIGH amplitude shortening. Work production was amplitude and length dependent with a maximum at L 0 − 1.2 mm (Fig. 5). The strong relationship (R ² = 0.92) between FD and work (Fig. 6) at L 0 decreased to R ² = 0.14 when data were pooled for all amplitudes and muscle lengths. The Q–length relation (Fig. 7) showed a significant length effect. Except for the two shortest lengths, where Q does not change, this relation could be well described (non-determination coefficient <0.06) by an exponential function.
Mean (n = 10) and SEM of work delivered during shortening as a function of muscle length after LOW (open circle), MID (open triangle) and HIGH (open square) shortening amplitude. Work production was length dependent (Asterisk indicates significant post hoc differences between adjacent lengths) whereas shortening amplitude was significant at all muscle lengths
Mean (n = 10) and SEM of Q (FD/work) are shown as a function of muscle length after LOW (open circle), MID (open triangle) and HIGH (open square) shortening amplitude. Asterisk indicates significant (p < 0.05) post hoc differences between adjacent lengths. For clarity the length effect is only shown for the LOW amplitude. Except for the two shortest lengths, Q was described by an exponential function (dashed line)
Discussion
A decreased force production capacity of skeletal muscle after active shortening (FD) has been observed both in muscle fibres (Edman et al. 1993; Julian and Morgan 1979) and in whole muscle in vivo (Lee and Herzog 2003; de Ruiter and de Haan 2003) and in situ (Schachar et al. 2002). The incubated mouse soleus muscle, used in this study, showed a relative FD of 16.6 ± 0.4% at L 0 after a shortening of 2.4 mm amplitude at a velocity of 1.5 mm/s. This value is within the range reported with other models, as it is higher than 10.8% reported in ‘in situ’ experiments on cats (Schachar et al. 2002) but lower than 37% (Lee and Herzog 2003), 27.1% (de Ruiter et al. 1998) and 20% (Rousanoglou et al. 2007) for human adductor pollicis muscle in vivo. At L 0 our data confirm the previously reported strong relation between FD and work delivered during shortening (Fig. 6). However, the weak relationship (R ² = 0.14) between FD and work when data were pooled for all muscle lengths (Fig. 6) and the length-dependent Q (FD/work) (Fig. 7), shows that FD is not only predicted by work production during shortening but also by a length dependent factor, representing the sensitivity of FD to work.
Effect of muscle length on force depression
Although FD after LOW amplitude shortening is small, we applied LOW amplitudes with the aim to construct a FD–length relation ranging from the ascending limb, over the plateau to the descending limb of the force–length relation. Such a relation was never reported. Yet our results (Fig. 5) are quite in agreement with combined information from different studies. FD was found at all tested muscle lengths but the amount clearly varied with muscle length. In agreement with Meijer et al. (1998), at lengths shorter than L 0 a relatively low (about 3%) but significant FD was observed (Edman et al. 1993). According to Meijer et al. (1998) and also Schachar et al. (2002), FD is maximal at L 0, whereas Kosterina et al. (2008) reported maximal FD production at suboptimal lengths (L 0 − 0.72 mm). However, and in agreement with Morgan et al. (2000), we found maximal FD (8.8 ± 0.3%) at 110% L 0, which is about 3% more than the value found at L 0 (5.9 ± 0.2%). These differences might be attributed to differences in technique to define L 0.
Muscle length affects the FD–work relation
A number of observations in this study point to an effect of muscle length on the FD–work relation. First, as generally accepted (Rassier and Herzog 2004) and here observed for multiple muscle lengths, FD was found to increase with increasing shortening amplitude. However, at long (L 0 + 1.2 mm) and short (L 0 − 2.4 mm) muscle lengths this amplitude effect were absent (Fig. 4), which confirms the results of Meijer et al. (1998). If work is the main predictor for FD (Kosterina et al. 2009), this absence of an amplitude effect should be compensated by F s to maintain an equal work output, yet a large difference is observed in work production at L 0 − 2.4 mm between LOW and MID amplitude (Fig. 5). Second, our results showed that muscle length at maximal work production (L 0 − 1.2 mm) does not correspond to the length at maximal FD production (about L 0 + 0.6 mm). Furthermore, a constant work production is observed between L 0 − 1.8 and L 0 − 0.6 mm, whereas FD was significantly different at this muscle length range for all amplitudes. These results oppose the idea by Schachar et al. (2002) that the length dependence of FD results from variations in work production. Third, although our data confirm the strong relation between FD and work delivered during shortening, especially at L 0 (R ² = 0.92), a weak (R ² = 0.14) relationship was found when data were pooled for all muscle lengths (Fig. 6), indicating that the slope of this relation differs with length. Therefore, we evaluated the length dependence of this slope, by calculating Q, which represents the length dependence of FD unexplained by work (Fig. 7). Thus, this Q-ratio expresses the sensitivity of FD to work which was found to be length-dependent. In previous studies the length dependence of FD was explained as a result of the length dependence of F s. If F s indeed explains the length dependence of FD, for contractions with constant amplitude, as in our study, F s is proportional to work and consequently the FD–work relation should be length independent. However, this was not observed in our study. Mathematically, the Q–length relation is bi-phased, with a breakpoint at about 85% L 0. For lengths shorter than 85% L 0, Q is low and rather constant and therefore the length dependence of FD in this range can be ascribed to F s, confirming previous studies. However, for lengths longer than 85% L 0, Q exponentially increases with length. This explains the finding that maximal FD was found not at L 0, but at a length (110% of L 0) where the product of two length dependent determinants of FD (F s and Q) is maximal. From these observations we conclude that, aside from work delivered during shortening, a length dependent factor should be included in any mechanism aimed to explain FD.
Impact of length dependence on proposed mechanisms of FD
The obtained Q can hardly be considered as a residual, because of its clear exponential profile with low variability, except for the extreme long length, and its consistency for the three amplitudes. Therefore, a speculation about the possible physical meaning of Q, when integrated in the actually proposed mechanisms of FD, is appealing.
According to the actin deformation theory, FD is proportional to the product of AMP and the amount of actin deformation. If actin is modelled as a highly compliant elastic element (Liu and Pollack 2002), then actin deformation is the product of F s and its compliance. Thus, actin compliance is proportional to FD/(AMP × F s) which is the Q-ratio. Therefore, within this theory, the Q-ratio has the physical meaning of being proportional to actin compliance. In this regard, the obtained length dependence of Q might be attributed to an exponentially length-dependent compliance of the free actin segment (the I-band), as indeed observed on rabbit skeletal muscle (Liu and Pollack 2002; Higuchi et al. 1995) and ascribed to its differential binding to titin (Nagy et al. 2004). The observation of a low and rather constant Q at short muscle lengths might be explained by the inter actin overlap in this length range, compromising the relation between compliance and sarcomere length. Within this reasoning, our findings support the actin deformation theory, if a length dependent compliance is taken into account.
According to the sarcomere heterogeneity theory (Morgan et al. 2000), no FD production should be observed at the ascending limb due to theoretical sarcomere length stability (Allinger et al. 1996), which is not in line with our observation and the findings of Meijer et al. (1998). On the other hand, the observed increasing FD with increasing length at the descending limb of the force–length relation confirms the length dependent sarcomere heterogeneity observed by Edman et al. (1993). Therefore, within this theory, the obtained Q might reflect the length dependent sarcomere non-uniformity, however, limited to the descending limb of the force–length relation.
Aside from an explanation within these two theories, other mechanisms might be involved in the length dependence of FD. Indeed, muscle membrane excitability might be affected by prior isometric contractions at various lengths (Ruff 1996). Although in our study muscles were supra-maximally stimulated we did not control muscle activation level. However, the consistency of the length dependent factor (Fig. 7) obtained from different shortening amplitudes and shortening duration does not indicate that membrane excitability was altered by shortening. In this regard further investigation, including M-wave recordings, is warranted.
Conclusions
In conclusion, this study on incubated mouse soleus muscle confirms the strong relation between force depression after shortening and work delivered during the shortening phase. As a new finding, the slope of this relation is partly muscle length dependent: for lengths shorter than 85% L 0 the slope is constant, but for longer lengths the slope exponentially increases with length. These findings might be explained within the theory that force depression results from actin deformation, if a length-dependent actin compliance is taken into account.
Reference
Abbott BC, Aubert XM (1952) The force exerted by active striated muscle during and after change of length. J Physiol 117:77–86
Allinger TL, Epstein M, Herzog W (1996) Stability of muscle fibers on the descending limb of the force–length relation. A theoretical consideration. J Biomech 29:627–633
Askew GN, Marsh RL (1998) Optimal shortening velocity (V/V max) of skeletal muscle during cyclical contractions: length–force effects and velocity-dependent activation and deactivation. J Exp Biol 201:1527–1540
Bullimore SR, Leonard TR, Rassier DE, Herzog W (2007) History-dependence of isometric muscle force: effect of prior stretch or shortening amplitude. J Biomech 40:1518–1524
de Ruiter CJ, de Haan A (2003) Shortening-induced depression of voluntary force in unfatigued and fatigued human adductor pollicis muscle. J Appl Physiol 94:69–74
de Ruiter CJ, de Haan A, Jones DA, Sargeant AJ (1998) Shortening-induced force depression in human adductor pollicis muscle. J Physiol 507(Pt 2):583–591
Derave W, Eijnde BO, Ramaekers M, Hespel P (2005) Soleus muscles of SAMP8 mice provide an accelerated model of skeletal muscle senescence. Exp Gerontol 40:562–572
Edman KA, Caputo C, Lou F (1993) Depression of tetanic force induced by loaded shortening of frog muscle fibres. J Physiol 466:535–552
Herzog W (1998) History dependence of force production in skeletal muscle: a proposal for mechanisms. J Electromyogr Kinesiol 8:111–117
Herzog W, Leonard TR (1997) Depression of cat soleus-forces following isokinetic shortening. J Biomech 30:865–872
Herzog W, Leonard TR (2007) Residual force depression is not abolished following a quick shortening step. J Biomech 40:2806–2810
Herzog W, Leonard TR, Wu JZ (2000) The relationship between force depression following shortening and mechanical work in skeletal muscle. J Biomech 33:659–668
Higuchi H, Yanagida T, Goldman YE (1995) Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J 69:1000–1010
Huxley AF, Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233:533–538
Josephson RK, Stokes DR (1999) Work-dependent deactivation of a crustacean muscle. J Exp Biol 202:2551–2565
Julian FJ, Morgan DL (1979) The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. J Physiol 293:379–392
Kosterina N, Westerblad H, Lannergren J, Eriksson A (2008) Muscular force production after concentric contraction. J Biomech 41:2422–2429
Kosterina N, Westerblad H, Eriksson A (2009) Mechanical work as predictor of force enhancement and force depression. J Biomech 42:1628–1634
Lee HD, Herzog W (2003) Force depression following muscle shortening of voluntarily activated and electrically stimulated human adductor pollicis. J Physiol 551:993–1003
Leonard TR, Herzog W (2005) Does the speed of shortening affect steady-state force depression in cat soleus muscle? J Biomech 38:2190–2197
Liu X, Pollack GH (2002) Mechanics of F-actin characterized with microfabricated cantilevers. Biophys J 83:2705–2715
Maréchal G, Plaghki L (1979) The deficit of the isometric tetanic tension redeveloped after a release of frog muscle at a constant velocity. J Gen Physiol 73:453–467
Meijer K, Grootenboer HJ, Koopman HF, van der Linden BJ, Huijing PA (1998) A Hill type model of rat medial gastrocnemius muscle that accounts for shortening history effects. J Biomech 31:555–563
Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U (2000) Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J Physiol 522(Pt 2):503–513
Nagy A, Cacciafesta P, Grama L, Kengyel A, Malnasi-Csizmadia A, Kellermayer MS (2004) Differential actin binding along the PEVK domain of skeletal muscle titin. J Cell Sci 117:5781–5789
Rassier DE, Herzog W (2004) Considerations on the history dependence of muscle contraction. J Appl Physiol 96:419–427
Roots H, Offer GW, Ranatunga KW (2007) Comparison of the tension responses to ramp shortening and lengthening in intact mammalian muscle fibres: crossbridge and non-crossbridge contributions. J Muscle Res Cell Motil 28:123–139
Rousanoglou EN, Oskouei AE, Herzog W (2007) Force depression following muscle shortening in sub-maximal voluntary contractions of human adductor pollicis. J Biomech 40:1–8
Ruff RL (1996) Effects of length changes on Na+ current amplitude and excitability near and far from the end-plate. Muscle Nerve 19:1084–1092
Schachar R, Herzog W, Leonard TR (2002) Force enhancement above the initial isometric force on the descending limb of the force–length relationship. J Biomech 35:1299–1306
Van Noten P, Van Leemputte M (2009) Shortening amplitude affects the incomplete force recovery after active shortening in mouse soleus muscle. J Biomech 42:2636–2641
Acknowledgments
The study was supported by a grant from the Fonds voor Wetenschappelijk Onderzoek (Grant No. 1.5.151.04). J. Lebacq (Université Catholique de Louvain) is greatly acknowledged for his valuable suggestions.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Alain Martin.
Rights and permissions
About this article
Cite this article
Van Noten, P., Van Leemputte, M. The effect of muscle length on force depression after active shortening in soleus muscle of mice. Eur J Appl Physiol 111, 1361–1367 (2011). https://doi.org/10.1007/s00421-010-1760-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1760-8