Abstract
Maximal active force of skeletal muscle contraction occurs at a sarcomere length where overlap of thick and thin filaments is optimal. However, the interaction of muscle length and active force is complicated. Active force, is the force generated by energy-requiring processes. To calculate active force, passive force provided by in-parallel structures must be subtracted from total force. Sarcomere length will change during a contraction with constant muscle–tendon length, due to tendon stretch. Passive force therefore changes during the contraction. Taking this into account, it has been demonstrated that there is less length dependence of fatigue than previously thought. The remaining difference may be associated with length dependence of activation, a property that is evident with submaximal activation. The sarcomere length at which peak contraction occurs is longer than the length that gives optimal overlap of the filaments and this shift of optimal length appears to be due to increased Ca2+ sensitivity. The increased Ca2+ sensitivity occurs because at longer lengths, the myofilaments are closer together allowing greater force than expected. However, the potential for length-dependent activation has been challenged. Submaximal contractions obtained by recruitment of fewer motor units but with maximal stimulation across different muscle lengths still demonstrate length-dependent activation. In contrast, contractions with similar absolute electromyographic signal magnitude at different lengths do not demonstrate length-dependent activation. Recent work has improved our understanding of how sarcomere length impacts the force of contraction but also reveals inadequacies in our knowledge that need to be addressed by additional research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle has been studied in the intact human (in vivo), partially isolated in living animals (in situ) and removed from the animal (in vitro). In vivo, the effective moment that can be exerted in any joint configuration is dependent on moment arm and the force that the muscle exerts in that position. The sarcomere length of the muscle fibers is a major factor influencing the force of contraction, and this length depends on initial sarcomere length as well as the tendon stiffness which dictates the amount of fiber shortening that will occur, even when joint angle does not change. Although advances have been made, our knowledge about muscle properties that dictate the length dependence of force production is still incomplete.
Early observations of the skeletal muscle force–length relationship
The force–length relationship of skeletal muscle was first described in the nineteenth century by Blix (1891, 1892, 1894), elaborated by Ramsey and Street (1940), and Hill (1953) and refined by Gordon et al. (1966). This fundamental property of muscle seems simple enough: maximally activated muscle generates the greatest active isometric force when sarcomere length allows optimal overlap of the myofilaments. Active force decreases when contraction is performed at shorter or longer lengths than this optimum. The force–length relationship describes only the isometric property of muscle related to the sarcomere length at which the contraction is accomplished when the contraction is performed with maximal activation.
Isometric is a term that refers to a constant length. A truly isometric muscle contraction would not allow shortening of the muscle fibers. However, the term is regularly used to refer to constant length of the muscle–tendon unit. In this case, muscle fiber length changes as the tendon is stretched. The magnitude of muscle fiber shortening is dependent on the force of contraction and the stiffness of the tendon. The sarcomere length change depends on the amount of shortening and the number of sarcomeres in series. In this paper, the term, isometric, will be used when fiber length change is prevented. The term “fixed-end contraction” will be used to refer to a contraction where the muscle–tendon unit length is kept constant.
A summary of the last review
The last major review of the force–length relationship was by Rassier et al. (1999) and some of the key points of that review will be reiterated here before considering the advances in our understanding of this complex property of skeletal muscle. In most cases, only active force is presented in the force–length relationship (see Fig. 1a). Passive force, the force measured at different lengths when there is no activation of the muscle, is sometimes presented and occasionally total force, the actual peak force reached when the muscle is activated, is also presented (see Fig. 1b). Active force is calculated in an effort to represent the involvement of active processes in generating muscle force. According to the cross-bridge theory of muscle contraction (Huxley 1957), active force is generated by the myosin heads while energy is provided by adenosine triphosphate hydrolysis. Although we like to separate active and passive force, it needs to be remembered that, in vivo, it is total force that is the effective force operating at the ends of the muscle.
A theoretical force–sarcomere length relationship for maximal contractions of human muscle is shown (a). This relationship is based on thin filament lengths of 1.3 µm and thick filament length of 1.6 µm and a bare zone in the middle of the thick filament of 0.2 µm. In b, passive (blue solid line), total (triangles) and active force measured by subtracting passive force measured before the contraction (traditional method, open circles) and active force measured in the alternative method (green squares) is shown. Vertical dotted blue line represents active force measured in the alternative method. Realistic myofilament overlap and actin–myosin spacing is shown in c for human sarcomeres at 2.0, 2.8 and 3.4 µm. These models of myofilaments were created by John Holash using images imported from the protein data bank. Perspective of the myofilaments is from near on the left to further away on the right. This perspective explains the narrowing of the image to the right. The dark object at the near end is the M-line and the dark object at the far end is the Z-disk. (Color figure online)
There were several key messages of the 1999 review of the length dependence of muscle contraction (Rassier et al. 1999). These key messages are summarized below. The suggested convention of using force–length relationship to refer to maximally activated muscle and the length dependence of contractile force when activation is submaximal will be retained in this paper. The force–length relationship is also known as the length–tension relationship. Here, force–length relationship is used because this term is consistent with other terms used to refer to fundamental muscle properties: force–velocity relationship, force–pCa2+ relationship and force–frequency relationship. In each case, the force generated by the muscle, the dependent variable is dependent on the second term in the expression, the independent variable.
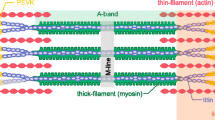
The sliding filament and the cross-bridge theories of skeletal muscle contraction can explain why maximal isometric active force decreases from the plateau of the force–length relationship when contractions occur with longer or shorter sarcomere length than at the plateau. These theories describe the relationship between the contractile filaments, actin and myosin and the distribution of myosin heads that form cross-bridges, use energy and generate force. Assuming a constant force per cross-bridge, the active force of an isometric contraction is dependent on the number of myosin heads that can interact with binding sites on actin and contribute to force generation. The number of possible interactions decreases as muscle sarcomere length is increased from the plateau of the force–length relationship until no interactions are possible at a length corresponding to the length where no filament overlap exists. As length decreases from the plateau, force decreases due to possible interference from thin filaments projecting into the opposite side of the sarcomere (see Fig. 1c) and pressing of the myosin filament against the z-disk. Interfilament distance also increases at shorter lengths (see Fig. 1c). This increase in interfilament distance associated with short sarcomere length may also contribute to the lower force on the ascending limb of the force–length relationship, and this is thought to be due to lower force per cross-bridge (Bagni et al. 1990), but could also relate to fewer cross-bridges forming, due to the distance between myofilaments.
Skeletal muscle has several properties that change as a result of prior activation. Fatigue and activity-dependent potentiation are two of these history-dependent properties and these each have a length dependence. The force exerted at a given length is also history dependent during a single contraction (Abbott and Aubert 1952; Bullimore et al. 2007). When muscle is activated then stretched to a longer length, the active force is greater than the force observed when the muscle is activated at the longer length. This property of muscle is called stretch induced enhancement of force. In contrast, when muscle is activated then allowed to shorten, the active force is less than that observed for an isometric contraction at the final length. This is called shortening-induced depression. The sliding filament theory and cross-bridge theory cannot explain stretch-induced enhancement or shortening-induced depression of active force (Rassier et al. 1999). Stretch-induced enhancement may be dependent on passive properties of titin, which has increased stiffness in the presence of elevated Ca2+ (Labeit et al. 2003).
When activation is submaximal, there is a shift of optimal length to longer sarcomere lengths due to length-dependent increase in Ca2+ sensitivity. When the level of activation is controlled by constant frequency stimulation in an intact muscle preparation or by constant [Ca2+] in a skinned fiber preparation, the sarcomere length which yields the highest peak force is longer than the length at which maximal overlap of the thick and thin filaments occurs. This property of striated muscle is referred to as length-dependent activation. The mechanism for length-dependent activation is thought to be related to proximity of the myosin heads with the actin filaments. As length is increased, the myofilaments become closer together (see Fig. 1c) and this proximity increases the probability of actin myosin interaction.
Staircase, post-tetanic potentiation and post-activation potentiation are three similar forms of history-dependent properties of muscle (MacIntosh and Rassier 2002; MacIntosh and Bryan 2002). Staircase is potentiation during repeated low frequency activation. Post-tetanic potentiation is enhanced active force of twitch or submaximal contractions following a tetanic contraction and post-activation potentiation is enhanced active force of twitch or submaximal contractions following a voluntary contraction. Prior activation of muscle results in activation of myosin light chain kinase, an enzyme that phosphorylates the regulatory light chains of myosin (Grange et al. 1993). Phosphorylation of the regulatory light chains results in increased mobility of the myosin head, bringing it closer to the binding site on actin (Levine et al. 1996). On subsequent activation, the myosin heads have a greater probability of binding than when they were lying close against the thick filament backbone, as they do in the nonphosphorylated condition. This advantage results in more force during submaximal activation than would otherwise occur. More force at a given submaximal [Ca2+] is referred to as increased Ca2+ sensitivity (MacIntosh 2003). The relative enhancement of force at a given [Ca2+] is greater at short sarcomere lengths, giving activity-dependent potentiation a length dependence (Rassier et al. 1997; MacIntosh and Rassier 2002). This length dependence is thought to occur because at long lengths the myofilaments are already closer together and the proximity of the myofilaments already increases Ca2+ sensitivity (MacIntosh 2003).
The force–length relationship is often represented graphically, showing active force as a continuous line. The descending limb of the force–length relationship has been interpreted as an unstable, softening material (Allinger et al. 1996). However, the force–length relationship should not be thought of as a continuous property. Each point on the relationship represents a single fixed-end or isometric contraction where force rises, may be held briefly, then decreases. There is no tendency for muscle to continuously stretch during contraction as would be expected for a softening material. In fact, in the case of a fixed-end contraction, the muscle fibers can shorten at the expense of tendon elongation.
The number of sarcomeres along the length of a muscle varies between individuals, for a given muscle, in a functional manner. Athletes who compete in an upright position (runners) apparently have more sarcomeres in series in their rectus femoris muscle (Herzog et al. 1991) than athletes who compete in a more bent over position (cyclists and speed skaters). The number of sarcomeres in series can be reduced when a muscle is immobilized for prolonged periods of time (Spector et al. 1982). The variability of number of sarcomeres in series dictates that the moment–joint angle relationship is not necessarily similar between individuals.
The properties of muscle described above and the then-current understanding of them were well described in the review by Rassier et al. (1999) and that material will not be further described here. This review will provide an update, based on studies published since that review was written. Recent work has enhanced our understanding of the force–length relationship and the length dependence of contraction. Much of the work presented here will consider submaximal contractions because these are more common in every-day life than maximal contractions. The following topics will be covered here: (1) the functional range of sarcomere lengths in vivo; (2) passive tension change during muscle contraction; (3) a new way to calculate active force; (4) the length dependence of fatigue; and (5) length dependence of activation. This recent work improves our understanding of how sarcomere length impacts the force of contraction but also reveals inadequacies in our knowledge that need to be addressed by additional research.
The functional range of sarcomere lengths in vivo in humans
The force–length relationship is typically measured in muscle in vitro where the range of sarcomere lengths can be evaluated over the full force–length relationship (see Fig. 1a). The full range of sarcomere lengths is dependent on filament lengths. Human actin filaments are slightly longer than the familiar frog actin filaments. Lieber (1994) reports 1.3 µm and Walker and Schrodt (1974) report 1.27 µm for human muscle thin filament length. The human Z-disk width is also slightly greater than frog Z-disk width, but the above estimates of thin filament length takes this into consideration; 1.3 is half the distance from the end of the thin filaments near the middle of one sarcomere to the middle of the neighboring sarcomere. Sarcomere length at optimum should be 2.6–2.8 µm. This estimate uses 1.3 µm for two thin filament lengths, including 0.1 µm for Z-disk width (half of a Z-disk is at each end of the sarcomere) and 0.2 µm for the bare zone in the middle of the thick filament. At the short end of the plateau the thin filaments from each end of the sarcomere would just meet in the middle of the M-band. At the long end of the plateau the thin filaments would be pulled apart to expose the bare region of the thick filament. This range of sarcomere lengths would allow the greatest overlap of thick and thin filaments, of human muscle, with the maximum possible interaction between myosin heads and actin binding sites. The myofilament arrangement expected for human muscle is presented in Fig. 1c for sample sarcomere lengths.
The functional range of sarcomere length in vivo, the actual range over which muscle sarcomere length can vary, is dependent on several factors: absolute muscle length, number of sarcomeres, tendon length and stiffness, moment arm length and joint range of motion. The functional range should be less than the theoretical range, otherwise active force would be zero at some joint position. However, the fact that there are more than one muscle acting across a joint and these muscles may not be functioning at the same sarcomere length means that it is possible that one muscle may be stretched to a length where no active force is generated. This would actually broaden the functional moment-joint angle relationship (Gerritsen et al. 1998). Other factors that could broaden the functional moment–joint angle relationship relative to the theoretical relationship include depression of maximal voluntary force near optimal length and nonuniformity of sarcomere length. It has long been known that isolated muscle demonstrates a widening of the force–length relationship for fixed-end contractions (ter Keurs et al. 1978).
Actual measurements of sarcomere lengths for human muscle in vivo are rare. Laser diffraction has been used to measure sarcomere length over a range of joint positions in wrist muscles (Lieber et al. 1994). Estimates of sarcomere length can also be obtained from cadaver material (Herzog et al. 1992) and from measurement of the moment–joint angle relationship in conjunction with ultrasound imaging (Maganaris 2001; Kawakami and Fukunaga 2006). Values obtained by these techniques are presented in Table 1.
Clearly, different muscles function over different ranges of sarcomere lengths. It is often reported that muscles operate during maximal contraction at or near the plateau of the force–length relationship. In this case, it would be expected that muscles with a compliant tendon would have relatively long sarcomere length at rest, and shorten to the optimal length or to the ascending limb of the force–length relationship during maximal effort. In contrast, muscles with a particularly stiff tendon will shorten less during a fixed-end contraction, so they might begin at a shorter sarcomere length in the stretched passive position. However, this conjecture appears not to be the case, as can be seen in Fig. 2. Considering the limited number of direct measures of sarcomere length in vivo, this is an area that requires further research.
Theoretical force–length relationship for human muscle, showing reported range of resting and active sarcomere lengths for medial gastrocnemius muscle (red; Kawakami and Fukunaga 2006) and extensor carpi radialis (green; Lieber 1994). For each muscle, the solid line represents the sarcomere length from rest to full contraction at the long end of the range of observed sarcomere lengths. The dotted line represents the sarcomere length from rest to full contraction at the short end of the range of observed sarcomere lengths. (Color figure online)
It has been reported that human flexor carpus ulnaris functions at relatively long sarcomere lengths, in the range 3.2–4.2 µm (Lieber et al. 1996). Sarcomere length of human extensor carpus radialis apparently has a more restricted length range from 2.6 to 3.4 µm at rest, corresponding to passive movement through the range of motion. These authors also estimated shortening during fixed-end contraction could account for a change in sarcomere length of less than 0.15 µm, yielding a full operating sarcomere length range of 2.45–3.4 µm. This range is predominantly on the plateau and descending limb of the force–length relationship (see Fig. 2).
Sarcomere length can also be estimated from measures of the joint angle, moment relationship in vivo. Such measures indicate that many of the muscles of the human body operate during maximal fixed-end contraction at the plateau or on the ascending limb of the force–length relationship. For example, Maganaris (2003) evaluated the force–fascicle length relationship of the medial gastrocnemius muscle during maximal voluntary plantarflexion at a variety of ankle joint angles (20° dorsiflexion to 30° plantarflexion, measured from a neutral position). The resulting force–fascicle length relationship was linear, increasing force with increasing length. It can be assumed that sarcomere lengths were no longer than that length expected on the short side of the plateau of the relationship (2.60 µm). The measured fascicle length at the shortest position was 75.8% of that at the longest length so it can be estimated that the short sarcomere length would be no longer than 1.97 µm.
These measurements, reported by Maganaris (2003) were taken with the knee extended, so considering the gastrocnemius is a two-joint muscle, the lengths would represent the long end of the active range of motion; passive lengths were not given, but would be longer than the lengths measured during activation.
The Achilles tendon is somewhat compliant (Fletcher et al. 2010), so it can be imagined that muscle fascicle length change occurs during these maximal effort contractions. Achilles tendon compliance was confirmed by the report of Wakahara et al. (2007) in which fascicle length of the medial gastrocnemius muscle during maximal effort plantarflexion yielded an estimated sarcomere length range of 1.6–3.4 µm. Considering that passive length was 66 mm before the slow shortening contraction with the knee extended, and maximum force during slow shortening was reached at 40 mm, sarcomere length at rest must have been greater than 4.3 µm, the theoretical sarcomere length at which active force is zero. The only way this could happen would be if another muscle (synergist) was at a shorter sarcomere length at this joint position.
Ichinose et al. (1997) give a more complete view of the force–length relationship for the vastus lateralis muscle. Measurements of fascicle length were obtained by ultrasound imaging at rest and during submaximal as well as maximal contractions initiated at several knee joint angles. At rest, fascicle length ranged from 88 to 117 mm across the range of motion. During maximal contraction, fascicle length ranged from 56 to 95 mm, indicating considerable shortening during these fixed-end contractions. Considering that several muscles contribute to knee extensor moment, the true force–length relationship for the vastus lateralis cannot be known. However, if it is assumed that the shape of the relationship between length and force exerted by the vastus is similar to that observed for all agonists, then conclusions can be made. The highest active force occurred at 70° of flexion and the fascicle length at the peak of this contraction was about 88 mm. If this position is assumed to be at the middle of the plateau of the theoretical force–length relationship, then it can be estimated that passive lengths correspond to 2.64–3.51 µm and during maximal voluntary contraction sarcomere length varied from 1.68–2.85 µm. This range begins at a sarcomere length that should represent close to the shortest length that would be expected and straddles the optimal sarcomere length range.
Ichinose et al. (1997) also evaluated submaximal contractions. They asked their subjects to target 10, 30, 50 and 80% of the peak force achieved from each starting joint angle. The fascicle length at which the highest force for a given apparent level of activation occurred was progressively longer as the level of activation decreased. This observation is similar to that illustrated in the force–length relationship in Fig. 3 where 30, 50 and 70% of maximum force is illustrated for three initial positions. It can be seen that regardless of the percent of peak force targeted, the joint angle with the highest force will always be from the same initial length as that which gave the highest maximal force. The lower the force (smaller percent of maximum), the longer the fascicles will be at this joint angle. This shift of the apparent optimal length to longer fascicle lengths was reported by Ichinose et al. (1997) and was attributed to length-dependent activation. However, considering that they assumed that constant level of activation could be achieved during submaximal activation by targeting a predetermined percent of maximal force, it was a forgone conclusion that whatever initial length gave the highest force would also yield the highest force at any given percent of maximum. Their result for submaximal contractions was totally anticipated.
The theoretical force–length relationship is shown for maximal effort contractions and three sample contractions are shown with initial sarcomere length short (blue), optimal (brown) and long (grey). Submaximal contractions at 30, 50 and 70% of maximum are shown for the three initial lengths. The dashed lines join the common percent of maximum contractions, illustrating that the “optimal” length will follow the path of the sarcomere length change for the initial length that gave the largest peak active force. (Color figure online)
The relevant question is, does targeting a fixed percent of maximum at each initial joint position require the same level of activation for all lengths? This seems unlikely. Arampatzis et al. (2007) demonstrated that percent of maximal isometric force does not translate to a similar level of activation at all joint angles.
Rack and Westbury (1969) addressed this issue of submaximal activation by considering the force–frequency relationship at several lengths. Summation of tetanic contractions occurs differently across the lengths because the twitch time-course varies across lengths. Twitch contraction time is brief at short lengths and increases as length is increased (Rassier and MacIntosh 2002). This difference in time-course of contraction will result in enhanced summation at long lengths and a length dependence of the force–frequency relationship. Rack and Westbury reported a shift to longer lengths for optimal length as frequency of activation was decreased.
One of the problems with most of the studies mentioned above is that they have used the traditional method for calculation of active force. This method of calculation will yield errors when passive force is substantial, which may have been the case for measures on the Achilles tendon, but would not have been a problem for the quadriceps muscle which can move through the full range of motion without substantial passive force. Calculation of active force may be performed incorrectly in many published studies. This issue will now be addressed before we return to a discussion of the length dependence of activation.
Passive force decreases during contraction
Quantification of the force–length relationship is not as easy as the description above would imply. Usually, there is a series elastic structure that stretches as muscle active force is developed, causing the fascicle and therefore sarcomere length to change during contraction. This shortening is evident as a fascicle length change in Fig. 3. Ichinose et al. (1997) report length changes that correspond to 20–36% of initial length. Furthermore, calculation of the active force requires subtraction of the passive force and the passive force subtracted is often not measured at the fascicle length at which peak force occurred. Active force is defined here as the force generated by active, energy requiring processes in the muscle. Passive force is evident when muscle is stretched without activation and therefore without the engagement of active processes. The interaction of passive and active force is not always obvious. More about this problem will be presented below, but first some background will be discussed.
Historically, the force–length properties of muscle were studied in isolated muscle, clamping the tendon very close to the fiber or muscle (Levin and Wyman 1927; Jewell and Wilkie 1958; Edman and Kiessling 1971), leaving very little tendon in series with the contractile tissue. Tendon is compliant, so this exclusion allowed the study of reasonably isometric contractions, even without feedback to control segment or sarcomere length, a technique introduced by Gordon et al. (1966).
Passive force is not usually represented on the force–length relationship (see Figs. 1a, 2, 3), and when it is, it is presented alone or with active force illustrated at the same initial length, erroneously suggesting that there was no shortening during the contraction. The problem with this method of presenting the force–length relationship is that passive force very likely changes during contraction as fascicles shorten against a compliant tendon, as was seen in Fig. 1b. This change in passive force happens because in an intact whole muscle, passive resistance to stretch is a function of the connective tissue between the myofibers. As the fascicles shorten during a contraction, stretching the natural in-series structures, this passive force must decrease. A more appropriate way of presenting the force–length relationship is illustrated in Fig. 1b. Here, the length range of the passive force is different from the length range of the active muscle.
The approach to study the force–length relationship with minimal tendon or with feedback to control segment or sarcomere length is a valuable approach that allows understanding of the underlying mechanisms contributing to this fundamental property of muscle. However, it is not the same as what goes on in vivo, obscuring our understanding of how muscle functions in reality, with an intact and compliant tendon. A key problem arises when it is of interest to isolate the active force of contraction in vivo. Active force is typically calculated by subtracting a passive force from the total force. Traditionally, the passive force that has been subtracted was measured at the initial length for the contraction. This may not be the passive force still acting at the peak of force development.
Jewell and Wilkie (1958) evaluated the magnitude of shortening of muscle fascicles during fixed-end contractions and concluded that minimal fiber shortening actually occurred. Under this circumstance, it is appropriate to subtract the passive force measured at the initial length at which the contraction was performed, to calculate active force. This method is referred to here as the traditional method of calculating active force. The preparation used by Jewell and Wilkie was a parallel fibred muscle with little or no tendon, and extreme caution was taken to ensure that the measurement system was quite stiff. There was very little opportunity for fascicle length, and therefore sarcomere length, to change during contraction. Under this condition, it seems appropriate to estimate active force in the traditional way.
Alternative calculation of active force
However, when the muscle preparation has substantial tendon, as it often does in vivo, then it can be expected that during a contraction where the ends are fixed, the fascicles will shorten as the tendon elongates and force develops (MacIntosh and MacNaughton 2005). Muscle–tendon length does not change, but fascicle and therefore sarcomere length does change as the tendon stretches with development of force. This change in sarcomere length will change the passive force expressed across the parallel elastic elements: titin, membrane, and connective tissue. This revelation makes it apparent that active force is not as easy to calculate as was once thought. Furthermore, there are only a few examples of publications where the appropriate passive force was subtracted (Bobbert et al. 1990; Brown et al. 1999; Scott et al. 1996; ter Keurs et al. 1980; MacNaughton et al. 2006). For this reason, we have a limited understanding of the true active force at lengths where passive force is substantial. This method to calculate active force is referred to as the alternative method and will only affect values for active force at initial sarcomere lengths where passive force is evident, and fascicle or fibre shortening occurs during the development of active force. It is nevertheless important to consider evidence that passive force is changing during a muscle contraction.
In 2005, MacIntosh and MacNaughton demonstrated that passive force must decrease during an isometric, fixed end, contraction. The evidence for this conclusion was obtained from observation of repeated fixed-end contractions of the rat medial gastrocnemius muscle in situ. The muscle was initially stretched to a length that resulted in substantial passive force, and the muscle was indirectly stimulated with supramaximal doublet (5 ms delay) pulses every 30 s; intervals for which neither potentiation nor fatigue would be expected. Similar stimulation at a short length (very low passive force) resulted in repeat contractions of the same amplitude, with no change in passive force.
When held at a long initial length, the passive force decreased over time (stress relaxation), but total force during sequential contractions did not change and, of critical importance, initial fascicle length did not change. When active force was calculated in the traditional way then it appeared that active force was increasing over the course of these contractions. However, when the passive force assumed to be present at the peak of total force was subtracted, active force remained constant. Further evidence favoring this alternative method of calculating active force was obtained by repeat determination of the force–length relationship prior to and following the period of stress relaxation. In the second case, passive force had decreased, total force at each length remained constant and fascicle length at a given whole muscle length was not different. When calculated in the alternative manner, active force did not change, but traditional active force was increased for contractions at long lengths. The alternative method of calculating active force gives the correct active force.
The different ways of calculating active force (traditional and alternative) will give the same active force in three conditions: when the isometric contraction is initiated at a length where there is no passive force; when there is essentially no series elastic structure; and when segment or sarcomere length feedback is used to control length. However, when the fixed-end contraction is initiated at a length where passive force is present, and fascicles shorten during the contraction, the two methods will result in different estimates of active force. Considering that until 2005, almost everyone accepted the traditional method of calculating active force, there are quite likely several published reports related to in situ or in vivo force–length relationship that will need to be reconsidered.
The alternative method of calculating the active force–length relationship shown in Fig. 1b would yield an accurate estimate of active force regardless of the preparation in use. The only assumption is that the passive force at any fascicle or sarcomere length can be determined by passive stretching the muscle through the range of length change. It is necessary then to know the fascicle or sarcomere length at the peak of the contraction to know what passive force to subtract. It is important to realize that passive force can change over time (MacNaughton and MacIntosh 2006), so it may be necessary to frequently determine the passive force–length relationship. The realization that passive force changes during repeated contractions has helped us to gain an understanding of the length dependence of fatigue.
The length dependence of fatigue
The research literature relating muscle length and muscle fatigue is rather confusing and until recently, no good explanation could be found for the curious results. In general, it has been reported that fatigue was greater when repeated contractions were elicited at long length than when they were at a short length. This, by itself was not difficult to understand; stronger contractions at the longer length might be expected to cause greater fatigue. However, repeated contractions at any length has been reported to result in greater fatigue when assessed at short length than when measured at long length. In fact there have been reports of substantial fatigue when measured at short lengths while fatigue was absent when measured at long lengths, similar to the traditional measures presented in Fig. 4.

Source: MacNaughton et al. 2007 with permission of the author. Muscle length is presented as mm difference from the original optimal length determined with double pulse stimulation at high frequency. Optimal length for 50 Hz contractions occurs at a length longer than the optimum for double-pulse stimulation at 200 Hz
The length dependence of active force for 50 Hz (submaximal) contractions of rat medial gastrocnemius muscle prior to and after repetitive contractions resulting in fatigue. Pre refers to before fatigue and post refers to after fatigue. Active force (mean ± SEM) was calculated in the traditional (solid symbols) or alternative (open symbols) manner. With the traditional method, optimal length is shifted to the left and there appears to be no fatigue at the longest tested length. However, with the alternative method, active force decreases in a nearly parallel fashion across lengths.
Much of the discrepancy in reports of fatigue can be explained by the difficulty of measuring active force at lengths where substantial passive force exists. This difficulty is avoided in most studies by ignoring the issue; active force is calculated in the traditional manner. However, there is a problem with this approach when the muscle under study has substantial in-series elasticity as is the case for many muscles in vivo. The problem is only evident at long sarcomere lengths where passive force is substantial, and this problem relates to the issue that passive force does change over time, particularly as a result of a series of repeated contractions, like those that would result in fatigue.
Using the alternative method to calculate active force, MacNaughton and MacIntosh (2006) showed that fatigue is nearly uniform across all lengths when contractions are conducted at one length then measured across a broad range of lengths (see Fig. 4). This realization that the length dependence of fatigue was a consequence of miscalculation of the active force should be considered a warning that other properties of muscle may also be misunderstood due to inappropriate consideration of passive force.
It should be noted, however, that even when active force is calculated using the alternative method, there is still a length dependence of fatigue. When fatigue results in a parallel shift downwards in active force, the percent change is actually greater at short muscle lengths where the initial force was less (see Fig. 5). MacNaughton et al. (2007) demonstrated that this greater relative depression of active force at a short length also occurs with exposure to dantrolene (see Fig. 5), a drug that inhibits stimulation-induced release of Ca2+ from the terminal cisternae. A greater relative depression of active force at shorter lengths is essentially the same as a slight shift to the right for the optimal length. This is in association with a depression in activation. This emphasized fatigue effect at short lengths is associated with the length dependence of activation and there is no need to pursue additional mechanisms to explain this length dependence of fatigue.

Source: MacNaughton et al. 2007 with permission of the author
Active force of rat medial gastrocnemius muscle after fatigue or after dantrolene injection, measured in the alternative manner and expressed as percent of prefatigue or predantrolene. Length is measured the same way as open symbols in Fig. 4. Fatigue and dantrolene have a similar effect, greater relative depression of force at short length, demonstrating that much of the length dependence of fatigue can be explained by a mechanism similar to that of dantrolene, simply reduced Ca2+ release. A lower average [Ca2+] during the contraction will enhance the contraction in proportion to sarcomere length.
Length dependence of activation
In 1969, Rack and Westbury published the results of a study in which submaximal contractions were evaluated for the length dependence of active force. They used a very clever distributed stimulation to activate all motor units at the same frequency, but in a pseudo-asynchronous manner. To achieve this, they divided the ventral roots to allow independent activation of groups of motor units. By activating these groups at the same frequency, but at different times a smooth contraction was obtained at a variety of submaximal stimulation frequencies. They reported an interesting observation: optimal length for submaximal contractions occurs at longer muscle lengths when frequency of activation is lower. This shift to longer lengths of the apparent optimal length for submaximal contractions has been referred to as length-dependent activation and is evident in skinned fibers as well (Stephenson and Williams 1982; Yang et al. 1998; Martyn and Gordon 1988).
It has generally been considered that length dependence of activation in skeletal muscle is due to increased Ca2+ sensitivity at long sarcomere lengths (Stephenson and Williams 1982). To understand this, it is important to realize that most cases of altered Ca2+ sensitivity relate to proximity of the myosin heads to the thin filaments (MacIntosh 2003). Stretching the sarcomere to long lengths brings the myofilaments closer together (see Fig. 1c). Confirming this theory, Yang et al. (1998) showed that skinned fibers demonstrated increased Ca2+ sensitivity when myofilaments were compressed with dextran without changing the sarcomere length. Dextran imposes an osmotic pressure on the myofilament lattice, causing the movement of fluid out of the lattice, bringing the myofilaments closer together. These authors also demonstrated that regulatory light chain phosphorylation increased Ca2+ sensitivity at short sarcomere lengths, but only when the myofilament matrix had not been compressed with dextran. It is thought that phosphorylation of the regulatory light chains increases Ca2+ sensitivity by allowing more mobility of the myosin heads, permitting them to transition to a position closer to the actin filaments. This observation confirms that increased Ca2+ sensitivity is a property of proximity of the myosin heads with the actin filaments, and that mechanisms are not additive. This means that submaximal contractions of intact muscle with regulatory light chain phosphorylation should not demonstrate length dependence of activation. This possibility needs to be evaluated.
Length dependence of activation refers to the fact that peak isometric force occurs at a length longer than the length where overlap of the myofilaments is optimal. As length increases beyond the length associated with optimal overlap, the increase in probability of cross-bridge formation due to proximity of myosin heads to the thin filament must be more effective than the decreased probability due to fewer myosin heads in the overlap region. This length dependence of activation can only be effective up to about 125–140% of optimal length. Beyond this length, the decreasing overlap dominates the influence of active force.
Recent work has raised some interesting questions related to this theory of length-dependent activation. Holt and Azizi (2014) challenged the theory by testing contractions with maximal activation but activation of fewer than all of the motor units. Remarkably, their results show that maximal activation of fewer motor units results in a shift of the optimal length to longer lengths (see Fig. 6). This shift was not expected, since the motor units were maximally activated. Of particular interest, Holt and Azizi reported a substantially larger shift to the right of the optimal length than previously reported, to 1.6 times optimal length; considerably more than the shift reported by Rack and Westbury which was only 1.25 times optimal length.
Simulated experiment, similar to the data from Holt and Aziz. A theoretical frog muscle force–length relationship is shown (similar to Fig. 1a) and superimposed are values for contractions with maximal (blue dots) and submaximal activation with twitch contractions (green) or progressively fewer motor units (brown) activated with high frequency stimulation. The motor units were not submaximally stimulated, except for the twitch contractions, yet optimal length shifted to longer lengths when force was less. In the case of Holt and Azizi (2014), the optimal length for the lowest force contractions was 1.6 time longer than optimal length for maximal contractions. This would correspond to a sarcomere length of 3.36 µm if the optimum for maximal contractions occurred at 2.1 µm. (Color figure online)
It is thought that there is a sarcomere length beyond which the impact of decline in overlap exceeds the improvement due to proximity of the myofilaments. Yang et al. (1998) showed that phosphorylation of the regulatory light chains had no effect at a sarcomere length of 3.2 µm. This observation suggests that the limit of benefit from proximity of myofilaments is likely at or shorter than 3.2 µm. For rabbit muscle with an optimal length of 2.32 µm, the maximum benefit would occur at no longer than 1.4 times optimal length.
Holt and Azizi (2014) interpret their results as evidence that it is less than maximal force that is important for the shift of optimal length referred to as length-dependent activation, not submaximal activation of individual fibers nor proximity of the myofilaments. One problem with this work is that they used the traditional method of calculating active force. It is known that optimal length is underestimated for strong contractions when the traditional method of calculating active force is used (see Fig. 3). The muscle they studied was the frog plantaris muscle. This muscle has a substantial passive force within the length range evaluated (Azizi and Roberts 2010). The plantaris muscle also has a very compliant tendon (Azizi and Roberts 2010).
Fontana and Herzog (2016) determined the force–length relationship for vastus lateralis muscle, using the approach by Ichinose where force was targeted at fixed percent of maximum for each initial length. Their results were quite similar to those presented by Ichinose where the peak of the relationship between length and force shifted to the right for submaximal activation, but the maximum always occurred with the same initial length. However, when they normalize force across the lengths to a common percent of maximum electromyographic signal, to obtain a similar level of activation across the length range, a different result was obtained. All curves previously showing the length dependence of submaximal force now lined up with the optimum at the same fascicle length. This is certainly an interesting observation, but it is not clear that this method can assure a similar level of activation. The same fascicle length in the latter case was associated with different joint positions, because the variation in force resulted in variations in tendon length and therefore muscle–tendon unit length. It is also possible that this similar optimal fascicle length relates to persistent potentiation (see above regarding the length dependence of activity-dependent potentiation). Fontana and Herzog only allowed 2 min rest between sequential contractions, so activity-dependent potentiation which persists for several minutes would still be present, and might mask length dependence of activation.
It has been shown that the traditional method of calculating active force results in an underestimation of the true optimal length by 1–2 mm in the rat medial gastrocnemius muscle (MacIntosh and MacNaughton 2005). Although the rat medial gastrocnemius muscle is about 30 mm long, individual fascicles are just 12–15 mm, so this 1–2 mm represents a substantial change in optimal length. When active force is calculated in the alternative manner, there still appears to be a length dependence of activation, but the result is relatively small (see Fig. 7). This discrepancy between traditional and alternative method of calculating active force is affected by compliance of the in-series structures and the measurement system, as well as the magnitude of passive force. High frequency doublet stimulation resulted in an optimal length very similar to the length anticipated with longer duration tetanic stimulation (Rassier and MacIntosh 2002). However, 50 Hz stimulation and twitch contractions resulted in slightly longer optimal length, in spite of the fact that 50 Hz stimulation resulted in greater active force than doublet stimulation. Considering that the work I am quoting was not intended to address this question, it is clear that additional work is needed. Length-dependent activation is real, but clearly there is interaction with other mechanisms that change Ca2+ sensitivity and active force must be calculated in the alternative manner to allow quantification of the impact of this mechanism on the length dependence of active force.

Source: MacNaughton et al. 2007 with permission of the author. (Color figure online)
Length dependence of force for submaximal contractions. Stimulation was 50 Hz, for 300 ms (green), 200 Hz double pulse stimulation (pink) and single stimulus (blue). Active force was about, 50% of maximum fused tetanic force with 50 Hz stimulation. Double pulse stimulation at 200 Hz yields an optimal length clearly shorter than for the higher force 50 Hz Contractions. Twitch contractions gave the lowest force and an optimal length not much different from the 50 Hz contractions.
Conclusions
Considerably more work has been completed evaluating the force–length relationship of human muscle in vivo than was the case at the time of the last major review of this topic (Rassier et al. 1999). This new research has contributed to our understanding of the sarcomere length over which our muscles operate, but has raised further questions regarding the length dependence of submaximal contractions.
The realization that passive force changes during contraction in vivo has on the one hand allowed a better understanding of the length dependence of fatigue, but has also raised questions about the length dependence of submaximal contractions. Clearly additional work is needed to address the issue of length dependence of activation and in vivo contractions.
To summarize, the key observations reviewed here are the following. (1) Human skeletal muscle seems to function at a sarcomere length that allows the highest active force contribution, but in many cases length of the fascicles changes substantially during maximal contractions. (2) Passive force changes during contraction because tendon elongates and fascicles shorten during contraction. (3) Active force can be calculated in spite of the changing passive force, but there are published examples where active force has not been calculated correctly. (4) There is a persistent length dependence of fatigue that probably associates with length dependence of activation. (5) Length dependence of activation may explain some of the increase in optimal length for submaximal contractions, but use of the traditional method of calculating active force probably makes some attempts to study length-dependent activation incorrect.
Abbreviations
- %:
-
Percent
- Ca2+ :
-
Calcium ion
- mM:
-
Millimolar (millimoles per liter)
- Nm:
-
Nanometer
- µm:
-
Micrometer
References
Abbott BC, Aubert XM (1952) The force exerted by active striated muscle during and after change of length. J Physiol 117:77–86
Allinger TL, Epstein M, Herzog W (1996) Stability of muscle fibers on the descending limb of the force–length relation. A theoretical consideration. J Biomech 29:627–633
Arampatzis A, Mademli L, De Monte G, Walsh M (2007) Changes in fascicle length from rest to maximal voluntary contraction affect the assessment of voluntary activation. J Biomech 40(14):3193–3200
Azizi E, Roberts TJ (2010) Muscle performance during frog jumping: influence of elasticity on muscle operating lengths. Proc R Soc B Biol Sci 277(1687):1523–1530. doi:10.1098/rspb.2009.2051
Bagni MA, Cecchi G, Colomo F (1990) Myofilament spacing and force generation in intact frog muscle fibres. J Physiol 430:61–75
Blix M (1891) Die lange und die spannung des muskels. Skandinavische Archiv fur Physiologie 3 295–318
Blix M (1892) Die lange und die spannung des muskels. Skandinavische Archiv fur Physiologie 4:10
Blix M (1894) Die lange und die spannung des muskels. Skandinavische Archiv fur Physiologie 5:57
Bobbert MF, Ettema GC, Huijing PA (1990) The force–length relationship of a muscle-tendon complex: experimental results and model calculations. Eur J Appl Physiol Occup Physiol 61(3–4):323–329
Brown IE, Cheng EJ, Loeb GE (1999) Measured and modeled properties of mammalian skeletal muscle. II. The effects of stimulus frequency on force–length and force–velocity relationships. J Muscle Res Cell Motil 20(7):627–643
Bullimore SR, Leonard TR, Rassier DE, Herzog W (2007) History-dependence of isometric muscle force: effect of prior stretch or shortening amplitude. J Biomech 40(7):1518–1524
Edman KAP, Kiessling A (1971) The time course of the active state in relation to sarcomere length and movement studied in single skeletal muscle fibres of the frog. Acta Physiol Scand 81:182–196
Fletcher JR, Esau SP, MacIntosh BR (2010) Changes in tendon stiffness and running economy in highly trained distance runners. Eur J Appl Physiol 110:1037–1046
Fontana HdB, Herzog W (2016) Vastus lateralis maximum force-generating potential occurs at optimal fascicle length regardless of activation level. Eur J Appl Physiol 116:1267–1277. doi:10.1007/s00421-016-3381-3
Gerritsen KGM, van den Bogert AJ, Hulliger M, Zernicke RF (1998) Intrinsic muscle properties facilitate locomotor control—a computer simulation study. Motor Control 2(3):206
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184(1):170–192
Grange RW, Vandenboom R, Houston ME (1993) Physiological significance of myosin phosphorylation in skeletal muscle. Can J Appl Physiol 18(3):229–242
Herzog W, Guimaraes AC, Anton MG, Carter-Erdman KA (1991) Moment-length relations of rectus femoris muscles of speed skaters/cyclists and runners. Med Sci Sports Exerc 23(11):1289–1296
Herzog W, Abrahamse SK, ter Keurs HEDJ (1990) Theoretical determination of force-length relations of intact human skeletal muscles using the cross-bridge model. Pflugers Archiv 416(1-2):113-119
Herzog W, Kamal S, Clarke HD (1992) Myofilament lengths of cat skeletal muscle: theoretical considerations and functional implications. J Biomech 25:945–948
Hill AV (1953) The mechanics of active muscle. Proc R Soc Lond Ser B Biol Sci 141:104–117
Holt NC, Azizi E (2014) What drives activation-dependent shifts in the force–length relationship. Biol Lett 10:4. doi:10.1098/rsbl.2014.0651
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Ichinose Y, Kawakami Y, Ito M, Fukunaga T (1997) Estimation of active force–length characteristics of human vastus lateralis muscle. Acta Anat 159(0001–5180):78–83
Jewell BR, Wilkie DR (1958) An analysis of the mechanical components in frog’s striated muscle. J Physiol 143:515–540
Kawakami Y, Fukunaga T (2006) New insights into in vivo human skeletal muscle function. Exerc Sport Sci Rev 34(1):16–21
Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H (2003) Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci 100(23):13716–13721
Levin A, Wyman J (1927) The viscous elastic properties of muscle. Proc R Soc Lond Ser B Biol Sci 101:218–243
Levine RJC, Kensler RW, Yang ZH, Stull JT, Sweeney HL (1996) Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys J 71:898–907
Lieber RL, Loren GJ, Friden J (1994) In vivo measurement of human wrist extensor muscle sarcomere length changes. J Neurophysiol 71:874–881
Lieber RL, Ponten E, Burkholder TJ, Friden J (1996) Sarcomere length changes after flexor carpi ulnaris to extensor digitorum communis tendon transfer. J Hand Surg [Am] 21A:612–618
MacIntosh BR (2003) Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol Sci 18:222–225
MacIntosh BR, Bryan SN (2002) Potentiation of shortening and velocity of shortening during repeated isotonic tetanic contractions. Pflügers Archiv 443:804–812
MacIntosh BR, MacNaughton MB (2005) The length dependence of muscle active force: considerations for parallel elastic properties. J Appl Physiol 98(5):1666–1673
MacIntosh BR, Rassier DE (2002) What is fatigue? Can J Appl Physiol 27(1):42–55
MacNaughton MB, MacIntosh BR (2006) Reports of the length dependence of fatigue are greatly exaggerated. J Appl Physiol 101:23–29
MacNaughton MB, Campbell JJ, MacIntosh BR (2007) Dantrolene, like fatigue, has a length-dependent effect on submaximal force–length relationships of rat gastrocnemius muscle. Acta Physiol (Oxf) 189(3):271–278
Maganaris CN (2001) Force–length characteristics of in vivo human skeletal muscle. Acta Physiol Scand 172(4):279–285
Maganaris CN (2003) Force–length characteristics of the in vivo human gastrocnemius muscle. Clin Anat 16(3):215–223
Martyn DA, Gordon AM (1988) Length and myofilament spacing-dependent changes in calcium sensitivity of skeletal fibres: effects of pH and ionic strength. J Muscle Res Cell Motil 9(5):428–445
Rack PMH, Westbury DR (1969) The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol 204(2):443–460
Ramsey RW, Street SF (1940) The isometric length-tension diagram of isolated skeletal muscle fibers of the frog. J Cell Comp Physiol 15(1):11–34. doi:10.1002/jcp.1030150103
Rassier DE, MacIntosh BR (2002) Length-dependent twitch contractile characteristics of skeletal muscle. Can J Physiol Pharmacol 80(10):993–1000. doi:10.1139/Y02-127
Rassier DE, Tubman LA, MacIntosh BR (1997) Length-dependent potentiation and myosin light chain phosphorylation in rat gastrocnemius muscle. Am J Physiol Cell Physiol 273(1 Pt 1):C198–C204
Rassier DE, MacIntosh BR, Herzog W (1999) Length dependence of active force production in skeletal muscle. J Appl Physiol 86(5):1445–1457
Scott SH, Brown IE, Loeb GE (1996) Mechanics of feline soleus: I. Effect of fascicle length and velocity on force output. J Muscle Res Cell Motil 17(2):207–219. doi:10.1007/BF00124243
Spector SA, Simard CP, Fournier M, Sternlicht E, Edgerton VR (1982) Architectural alterations of rat hind-limb skeletal muscles immobilized at different lengths. Exp Neurol 76(1):94–110. doi:10.1016/0014-4886(82)90104-2
Stephenson DG, Williams DA (1982) Effects of sarcomere length on the force–pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol 333:637–653
ter Keurs HEDJ, Iwazumi T, Pollack GH (1978) The sarcomere length-tension relation in skeletal muscle. J Gen Physiol 72:565–592
ter Keurs HEDJ, Rijnsburger WH, van Heuningen R, Nagelsmit MJ (1980) Tension development and sarcomere length in rat cardiac trabeculae: evidence of length-dependent activation. Circ Res 46:703–714
Walker SM, Schrodt GR (1974) I segment lengths and thin filament periods in skeletal muscle fibers of the rhesus monkey and the human. Anat Rec 178(1):63-81
Wakahara T, Kanehisa H, Kawakami Y, Fukunaga T (2007) Fascicle behavior of medial gastrocnemius muscle in extended and flexed knee positions. J Biomech 40(10):2291–2298. doi:10.1016/j.jbiomech.2006.10.006
Yang Z, Stull JT, Levine RJ, Sweeney HL (1998) Changes in interfilament spacing mimic the effects of myosin regulatory light chain phosphorylation in rabbit psoas fibers. J Struct Biol 122:139–148
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Nigel A. S. Taylor.
Rights and permissions
About this article
Cite this article
MacIntosh, B.R. Recent developments in understanding the length dependence of contractile response of skeletal muscle. Eur J Appl Physiol 117, 1059–1071 (2017). https://doi.org/10.1007/s00421-017-3591-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3591-3