Abstract
We sought to determine whether improved cycling performance following ‘Live High-Train Low’ (LHTL) occurs if increases in haemoglobin mass (Hbmass) are prevented via periodic phlebotomy during hypoxic exposure. Eleven, highly trained, female cyclists completed 26 nights of simulated LHTL (16 h day−1, 3000 m). Hbmass was determined in quadruplicate before LHTL and in duplicate weekly thereafter. After 14 nights, cyclists were pair-matched, based on their Hbmass response (ΔHbmass) from baseline, to form a response group (Response, n = 5) in which Hbmass was free to adapt, and a Clamp group (Clamp, n = 6) in which ΔHbmass was negated via weekly phlebotomy. All cyclists were blinded to the blood volume removed. Cycling performance was assessed in duplicate before and after LHTL using a maximal 4-min effort (MMP4min) followed by a ride time to exhaustion test at peak power output (T lim). VO2peak was established during the MMP4min. Following LHTL, Hbmass increased in Response (mean ± SD, 5.5 ± 2.9%). Due to repeated phlebotomy, there was no ΔHbmass in Clamp (−0.4 ± 0.6%). VO2peak increased in Response (3.5 ± 2.3%) but not in Clamp (0.3 ± 2.6%). MMP4min improved in both the groups (Response 4.5 ± 1.1%, Clamp 3.6 ± 1.4%) and was not different between groups (p = 0.58). T lim increased only in Response, with Clamp substantially worse than Response (−37.6%; 90% CL −58.9 to −5.0, p = 0.07). Our novel findings, showing an ~4% increase in MMP4min despite blocking an ~5% increase in Hbmass, suggest that accelerated erythropoiesis is not the sole mechanism by which LHTL improves performance. However, increases in Hbmass appear to influence the aerobic contribution to high-intensity exercise which may be important for subsequent high-intensity efforts.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Maximal aerobic power (VO2max) of elite athletes can be limited by maximal oxygen supply to working muscles (Wagner 1996). Therefore, changes to systemic oxygen transport via alterations to blood volume (BV) and haemoglobin concentration ([Hb]) can have significant implications for VO2max and potentially endurance performance (Gledhill et al. 1999). Erythrocythemia, induced either by red cell infusion or erythropoietin administration, has been shown repeatedly to increase both VO2max and endurance performance (Berglund and Hemmingson 1987; Brien and Simon 1987; Ekblom 1996), whilst conversely, reducing oxygen transport capacity via blood loss or partial blocking of haemoglobin by carbon monoxide has detrimental effects for VO2max and performance (Ekblom and Huot 1972; Kanstrup and Ekblom 1984).
With the benefits of increasing oxygen transport capacity so evident, it is of no surprise that many elite endurance athletes seek to increase their total haemoglobin mass (Hbmass) via hypoxic exposure. An increase in Hbmass in response to both normobaric and hypobaric hypoxia has been documented on many occasions (Clark et al. 2009; Levine and Stray-Gundersen 1997; Robertson et al. 2010), although changes are somewhat lower in magnitude to those possible via blood doping (Gledhill et al. 1999; Schmidt and Prommer 2010). Today, Live high:Train Low (LHTL) (Levine and Stray-Gundersen 1997) has become a popular training strategy for elite endurance athletes due to the widespread paradigm that small gains in Hbmass of approximately 5% arising from hypoxic exposure are responsible for increases in high-intensity endurance performance via increases in VO2max (Levine and Stray-Gundersen 1997, 2005).
The complex response cascade initiated by a lower partial pressure of oxygen in hypoxia may also result in non-haematological adaptations, such as improved muscle efficiency (Saunders et al. 2004) and greater muscle buffering capacity (Gore et al. 2001), which may be equally as important for sea level performance as an increase in Hbmass (Gore et al. 2007; Gore and Hopkins 2005). The relative importance of haematological and non-haematological adaptations to hypoxia for endurance performance has, in the past, been difficult to discern since both may occur concomitantly. However, in many cases, a hypoxia-induced Hbmass response may not be detectable, because the hypoxic dose is insufficient (Levine and Stray-Gundersen 2006), or possibly because the athlete has limited potential for adaptation (Gore et al. 1998). Nevertheless, “worthwhile” performance enhancements are still reported after hypoxic exposure of various durations (Bonetti and Hopkins 2009), leading some researchers to question the sole dependence of performance improvements following LHTL on increases in Hbmass (Gore and Hopkins 2005).
The aim of the present study was to investigate the importance of increases in Hbmass for cycling performance following simulated normobaric LHTL. We employed, a ‘subject-blind’ design, by removing any Hbmass gained throughout the period of hypoxic exposure and, thereby, effectively “clamping” the Hbmass response. If performance gains are still observed despite a Hbmass “clamp”, this would support the concept of a non-haematological component to performance enhancement following LHTL (Gore et al. 2007; Gore and Hopkins 2005).
Methods
Fourteen, highly trained female cyclists were monitored during a 6-week training camp consisting of 26 nights of simulated normobaric LHTL. Cyclists were pair-matched after 14 nights based on their change in Hbmass from baseline and randomly assigned to either a ‘Response’ group in which Hbmass was free to adapt, or a ‘Clamp’ group in which any increase in Hbmass was negated via repeated phlebotomy to ensure Hbmass for each cyclist remained equal to baseline throughout LHTL. Both the groups were trained together near sea-level (Canberra, ~600 m ambient altitude) and were tested under normoxic conditions at the Australian Institute of Sport (AIS) before and after the LHTL period. The study was approved by the AIS Human Ethics committee and all cyclists provided written informed consent before participating.
Study design
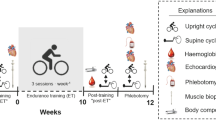
The study design is outlined in Fig. 1. Prior to simulated hypoxic exposure, all cyclists completed baseline testing consisting of: four Hbmass measurements, one incremental-graded exercise test to exhaustion for determination of Peak Power Output (PPO) and two dual-stage cycling performance tests (see “Performance tests” for details). Following baseline testing, the athletes began a 26-night LHTL protocol, sleeping at simulated normobaric altitude of 3,000 m and training at ~600 m. Simulated normobaric hypoxia was created via nitrogen dilution using a purpose-built, five-bedroom facility at the AIS. The cyclists were required to spend at least 14 h day−1 inside the facility and to record their hours of exposure in a log book. All cyclists were supplemented with oral iron daily (305 mg ferrous sulphate), commencing 2 weeks prior to and throughout LHTL in an attempt to establish equal preconditions and to prevent bias arising from iron deficiency. In addition, an iron screen (ferritin) was performed prior to inclusion in the study.
Schematic outline of the study design illustrating the timeline (days) of Live High/Train Low (LHTL) hypoxic exposure (altitude), haemoglobin mass assessments via CO rebreathing (Hbmass), cycling-ergometer performance tests (performance), venous blood collection (blood sample), venous blood withdrawal (blood draw) and daily training volume in km (training volume)
Performance testing was performed again on completion of the LHTL (day 27–30) and involved all athletes completing the full performance testing protocol on two occasions, separated by 1 day (Fig. 1).
Cyclists were assigned to one of two groups based on their Hbmass response. In one group, Hbmass was ‘free’ to respond to hypoxia. In the other group, the Hbmass response was ‘clamped’ by repeatedly removing any Hbmass increases during the hypoxic exposure, such that Hbmass at the end of the simulated LHTL period was equal to baseline (see below for details). The rationale for ‘clamping’ Hbmass throughout the course of LHTL, as opposed to a single blood removal at the end of LHTL, was to reduce any potential benefits that athletes might have from training with a progressively enhanced Hbmass.
Subjects and group allocation
Thirteen out of the 14 cyclists completed the full training camp. One cyclist withdrew after 10 days of LHTL due to reasons unrelated to experimental protocols or requirements. Prior to the start of the study, it was decided that the allocation of cyclists into groups (Response vs. Clamp group) would not occur until after 14 nights of simulated hypoxic exposure, as previous studies (Clark et al. 2009; Robertson et al. 2010) have shown that the time course of the Hbmass response to hypoxia is unlikely to present in a measurable magnitude after only 7 days of exposure. Once the percent difference of Hbmass compared to baseline was calculated (from the mean of duplicate measures each week), cyclists were assigned in a pair-matched manner so that both the groups would include cyclists with a comparable change in Hbmass in response to LHTL after 14 nights. A prerequisite for blood removal in the clamp group was that only cyclists whose Hbmass increase exceeded 2% compared with baseline values at the respective time point were selected for blood removal. This criterion was based on the typical error (TE) for measurement of Hbmass (described below).
One subject lost 3.1 kg (~6%) of body mass and showed a consistent decrease in Hbmass throughout the training camp. She was therefore neither allocated into a ‘group’ nor included in any part of the statistical analysis. Another cyclist disclosed that she experimented with caffeine supplementation prior to her final performance tests. It was not possible to repeat the tests within the study timeframe, thereby contaminating her results with an additional source of variation. Thus, only data from the remaining 11 cyclists were included in further analysis (Response = 5, Clamp = 6). The physical characteristics of the remaining 11 cyclists are outlined in Table 1.
Haematology
In all cyclists, venous haemoglobin concentration (v[Hb]) (g dL−1) and venous haematocrit (vHct) (%) were determined via flow cytometry using a fully automated analyser (ADVIA 120 Haematology Analyzer, Bayer Diagnostics, Tarrytown, USA) while serum ferritin (mg L−1) was measured using ELISA technologies (Dade Behring BN ProSpec, Dade Behring, Inc., Deerfield, IL, USA).
Haemoglobin mass
Hbmass was measured using the optimised carbon monoxide (CO) rebreathing method (Schmidt and Prommer 2005). Briefly, a CO dose of 1.2 ml kg−1 body mass was administered and rebreathed for 2 min through a glass spirometer. Capillary finger tip blood samples (200 μL), for determination of % carboxy-haemoglobin (HbCO) and capillary haemoglobin concentration (c[Hb]), were taken before and 7 min after the initial inhalation of the CO dose. Blood samples were analysed in quintuplet using a CO-oximeter (OSM3, Radiometer, Copenhagen, Denmark). Hbmass was calculated from the mean change in % HbCO as described previously (Schmidt and Prommer 2005). c[Hb] was determined from the mean of five replicates performed on the pre test capillary sample. In addition, two further capillary samples were obtained for measurement of capillary haematocrit (cHct) using microcentrifugation technique (Hawksley England, Micro-Haematocrit Centrifuge. Lancing, Sussex, England). The results of both measurements were averaged.
Hbmass was measured on four consecutive days at the start of the study period, with the mean value of all measures calculated to obtain a ‘true’ baseline value. Thereafter, the weekly individual increase in Hbmass was determined through duplicate measures on consecutive days each week, with the mean of the two measures used to establish changes from baseline for each cyclist (Fig. 1). In an attempt to minimise measurement error associated with the CO rebreathing method, the same experienced researcher conducted all tests and all blood analyses were performed on one OSM3 analyser. The TE for the CO rebreathing method, calculated from the four baseline measures at the start of the study was 2.0% (90% confidence limit: 1.7–2.5%). Multiple measurements reduce the uncertainty of a measurement by a factor of √n, where n is the number of measurements (Hopkins 2000). Therefore, given the TE of 2% at baseline, by performing quadruplicate measures our TE for a change in Hbmass is reduced to ±1.4% ((2.0%/√4) × √2) at a 68% level of confidence, or to ±2.3% (1.4% × 1.645) with 90% confidence. TE calculated from weekly duplicate measures thereafter was: week 1: 2.0% (1.5–3.1%); week 2: 2.2% (1.7–3.4%); week 3: 2.1 (1.6–3.1%); week 4: 1.5% (1.1–2.3%). Similarly, the use of duplicate measures each week reduces the error associated with individual change scores by √2.
Blood volume, red cell volume (RCV) and plasma volume (PV) were calculated using c[Hb], cHct and Hbmass determined during the weekly CO rebreathing tests as described previously (Schumacher et al. 2008). A factor of 0.91 was used to correct for whole body Hct (Chaplin et al. 1953).
Blood removal
In order to obtain a ‘subject-blind’ design, the venepuncture for blood sampling and removal was standardised. More precisely, the duration of the puncture of an antecubital vein from insertion to extraction of the needle was timed to be equal and, importantly, all cyclists were prevented from observing the procedure by placing their arm through a visual screen (opaque curtain) for the entire duration of the procedure. Furthermore, cyclists were not informed of their Hbmass response at any time during the study, nor were they informed of the amount of blood removed at any time point.
Following 20 min of supine rest (to allow plasma volume to stabilize (Ahlgrim et al. 2010)) two tubes [BD Vacutainer EDTA (2 ml) and BD Vacutainer Serum (4 ml), BD Australia, North Ryde, Australia] of venous blood were drawn from all cyclists using a 19 G needle (Wing-Flo, Intermedica GmbH, Mainz, Germany) for determination of v[Hb] (g dL−1), vHct (%) and serum ferritin (μg L−1). Following a single flush with normal saline (3 ml) after the first sample, the needle remained inserted intravenously in all cyclists whilst the EDTA sample was simultaneously analysed on site for individual v[Hb] (ADVIA 120 Hematology Analyzer, Bayer Diagnostics, Tarrytown, USA) in those cyclists selected for the Hbmass removal procedure. If necessary, the amount of whole-blood to be removed was then quantified as follows:
For example, if a cyclist showed a Hbmass increase of 11 g after 14 nights of simulated hypoxia, corresponding to a 2.1% increase from baseline, and on site measured v[Hb] was 13.9 g dL−1, then the blood volume to be removed was calculated to be 79 ml. Blood was removed from the selected cyclists using a three-way-stop-cock (Discofix C-3, B. Braun, Melsungen, Germany) and 50 mL syringes (Original-Perfusor-Spritze OPS 50 mL Luer Lock, B. Braun, Melsungen, Germany) under medical supervision. The volume of blood already removed for analysis (6 ml) was accounted for in the removal process. Cyclists in the Response group received a ‘sham’ withdrawal in which the needle remained inserted inside the vein but no further blood was removed, apart from the initial 6 ml. Plasma volume was not corrected via saline infusion to abide by anti-doping regulations. Instead, cyclists in both the groups were asked to increase their oral fluid intake by 250–500 ml after the procedure.
Performance tests
Cycling performance was assessed in duplicate both before and after simulated LHTL using an electromagnetically braked cycle ergometer (Lode Excalibur Sport, Groningen, The Netherlands). The ergometer was calibrated before, and verified after, the study period using a custom-built dynamic calibration rig described previously (Gardner et al. 2004). On arrival, all cyclists first completed an incremental-graded exercise test to exhaustion on the test ergometer for familiarisation and determination of PPO, beginning at 125 W and increasing 25 W every 3 min until volitional exhaustion. PPO (W) was calculated as follows (Kuipers et al. 1985):
where W L = power output of the last complete workload, t = time (min) for the final incomplete work load, a = workload time increments (min), b = workload power increments (W).
Each performance test consisted of two stages: stage 1 maximal 4-min effort, stage 2 ride time to exhaustion at PPO (T lim). The protocol was designed to discern the effects of LHTL on both a single maximal performance as well as the cyclist’s ability to recover for a subsequent maximal task shortly after (Meeusen et al. 2008). Such a scenario is often present in road cycling races and therefore has high practical relevance. Following a standardised warm up, the cyclists began a 4-min maximal effort. Power output was fixed for the first 2 min at 105% of PPO. In the final 2 min, the linear factor (gearing) of the ergometer was set to elicit 105% of PPO at 98 rpm and cyclists were instructed to complete as much work (kJ) as possible by altering their cadence. If cadence deviated from 98 rpm during the final 2 min, power output was adjusted as follows: power output (W) = rpm2 × linear factor. Maximal mean power elicited over the 4 min (MMP4min) was calculated from the total work performed. Based on the two baseline tests, the TE for MMP4min was 1.4% (90% CL: 1.0–2.0%). The MMP4min was chosen as a measure of performance instead of a traditional VO2max test for two reasons: (1) 4-min efforts are highly important in the sport of cycling, with an individual pursuit in track cycling typically lasting ~4 min and (2) data collected during women’s world cup racing has identified MMP4min as a key determinant of top 20 performances versus non-top 20 (Ebert et al. 2005). Since the 4-min task requires both an aerobic and anaerobic contribution (Craig et al. 1993; Gastin 2001), any non-haematological adaptations to hypoxia may also be important for overall performance.
Oxygen consumption (VO2) during MMP4min was measured continuously using a custom-built automated Douglas bag system with associated in-house software (AIS, Belconnen, Australia) as described previously (Saunders et al. 2004). VO2 values were calculated using standard algorithms for consecutive 30-s periods, with VO2peak determined as the sum of the highest two consecutive measurements. Previous work has shown that VO2peak achieved during a 4 min maximal effort is not significantly different from the VO2max achieved during a graded exercise test (Gore et al. 1998). The maximal accumulated oxygen deficit (MAOD) (Medbo et al. 1988) arising from the 4-min maximal effort was calculated as the difference between estimated oxygen requirements of the work achieved (derived from the power—VO2 regression for each athlete) and the total VO2 measured during the effort (Roberts et al. 2003). The relative aerobic and anaerobic contributions of the effort were calculated as the percentage of measured VO2 compared with the predicted VO2. Blood lactate concentration (Lactate Pro, Akray, Japan) was measured via capillary sampling 1 min following the completion of the effort.
Ten minutes after the completion of the 4-min maximal effort, the cyclists were instructed to ride for as long as possible at PPO (T lim). The rationale for including this novel aspect of the test was to mimic scenarios in road racing in which the duration of an effort is not pre-determined, as well as an attempt to assess the full impact of the LHTL training block on the cyclists’ performance capabilities. Indeed, Meeusen et al. (2008) have suggested that certain aspects of fatigue appear to be better discerned when using a two bout exercise protocol. Cyclists were given a warning when their cadence dropped below 80 rpm and were stopped following the third warning. The cyclists were not able to see elapsed time, with cadence the only feedback available. Based on the two baseline tests, the TE for T lim was 17.0% (90% CL: 12.9–25.8%). During this final part of the performance testing, VO2 was not measured, to ensure that there were no additional sources of distraction for the athletes. Blood lactate concentration was again assessed 1 min after completion of the effort. The typical duration of this effort was 3–5 min and thus the metabolic demands of the task were similar to that of the 4 min maximal test (Craig et al. 1993; Gastin 2001).
Performance testing was completed in duplicate (with one rest day between tests) on all occasions. The ‘best test’ was deemed to be the test in which the highest MMP4min was obtained regardless of the T lim work completed, and all corresponding data were used for analysis.
Training
Cycling training for the entire duration of the camp was prescribed and supervised by the Australian national team coach. Training was reverse-periodised to include a focus on short duration power development at the start of the training block and followed by longer duration rides of low intensity (Table 2). One week following the completion of LHTL, the cyclists competed in a 2-day cycling race, the taper for which was incorporated into the final week of training (Fig. 1). Training was conducted as a single group, with the exception of testing days that were randomly allocated. All training rides were monitored using a calibrated mobile power measuring device fitted to each athlete’s bicycle (SRM Training System, Professional Version, Schoberer Rad Messtechnik, Julich-Weldorf, Germany or PowerTap, Saris Cycling Group, USA). In addition, cyclists were asked to complete a training, illness and injury log on a daily basis, which included perceptual information about the day’s training. Cycling training was quantified using commercially available software (TrainingPeaks WKO + Version 2.2, PeaksWare, Lafayette, Colorado, USA). The software calculates a Training Stress Score™ (TSS) and the associated Intensity Factor™ (IF) using SRM power data (Allen and Coggan 2006). The TSS is analogous to a Banister Training Impulse (TRIMP) (Banister and Calvert 1980) and is a combination of training duration and relative training intensity referenced to a cyclist’s maximum power output for 1 h. The IF is a relative intensity scale with 1.00 reflecting the power output that can be maintained during a maximal 1-hr time trial. The software “normalises” variable power output in an attempt to estimate the intensity of the effort as if it was produced using a constant power output profile.
Statistical analysis
Data were analysed using a contemporary statistical approach (Hopkins et al. 2009) since many conventional approaches can be insensitive to small but practically important changes—performance changes of only 0.5% in magnitude can be important to elite athletes (Hopkins et al. 1999). Measured variables were log transformed prior to analysis in order to reduce bias arising from non-uniformity of error, and back transformed to obtain changes in means and standard deviations (SD) as percents (Hopkins et al. 2009). Mean effects of LHTL in the Clamp versus Response group were estimated via the unequal variances t statistic, using a spreadsheet for standard controlled trials (http://www.sportsci.org/resource/stats/xcontrial.xls), which accounts for the observed difference, and the smallest worthwhile change. The smallest worthwhile change for MMP4min and VO2peak was 1 and 2% for Hbmass whereas, for other physiological parameters (HRpk, Blood Lactate, etc.) the smallest worthwhile change was derived from Cohen’s scale for Effect Sizes in which a small effect size is ≥0.2 (Cohen 1988). The percentage likelihood of the observed differences between the Clamp and Response groups are expressed using the following descriptors: <1%, almost certainly not; 1–5%, very unlikely; 5–25%, unlikely; 25–75%, possibly; 75–95%, likely; 95–99%, very likely; >99%, almost certainly. Effects were deemed substantial if the percentage likelihood that the true value was practically positive (or negative) was >75%. The effect was deemed “unclear” if its confidence interval overlapped the thresholds for both positive and negative change.
Data are expressed as the mean (±SD) unless otherwise stated. Data in graphs are presented as percent changes from the mean of baseline measures (±SD), using the back-transformed values for individuals and groups. Differences between the two groups for baseline and training characteristics were assessed using an unpaired t test, and for these the level of significance was set to p < 0.05.
Results
Simulated hypoxic exposure
The cyclists accumulated a total of (mean ± SD) 423 ± 17 h of exposure and an average of 16.3 ± 0.7 h day−1. There was no substantial difference in duration of exposure between the groups (Response: 16.6 ± 0.4 h day−1; Clamp: 16.0 ± 0.8 h day−1).
Haemoglobin mass, blood volume and blood removal
Hbmass substantially increased with simulated hypoxic exposure in both groups (Fig. 2). After 26 nights of LHTL, mean Hbmass in the Response group was 5.5 ± 2.9% higher than baseline (Fig. 3). In the Clamp group, three cyclists increased Hbmass greater than the 2% threshold after 14 nights, and subsequently had blood removed (Table 3), with further blood removal for two of these cyclists after 26 nights. Blood removal for the remaining three cyclists occurred after 21 nights. The Hbmass of these three cyclists after 26 nights demonstrate our effectiveness in returning Hbmass to baseline (Table 3). The maximum volume of blood removed on any one occasion was 314 ml, with the mean volume removed 180 ml. Individual serial values of Hbmass are displayed in Fig. 2.
Weekly individual and mean (±SD) changes (%) from baseline in Hbmass and blood volume compartments during 26 nights of live high train low simulated hypoxia. Hash symbol denotes blood removed from this individual at this time point. Blood was removed from two subjects after 26 nights of LHTL in order to return Hbmass to baseline values
If the total amount of Hbmass removed is considered, the theoretical additive Hbmass response of the Clamp group was 4.5 ± 2.1% (Fig. 3), and was not substantially different from the response observed in the Response group (p = 0.55). However, at the time of post-LHTL testing (following blood removal), the mean change in Hbmass of the Clamp group compared to baseline was −0.4 ± 0.6%, and was “very likely” lower (−5.6%; 90% CL −8.1 to −3.0, p = 0.01) than the Response group and also well within our TE for the CO rebreathing method.
Mean changes in RCV were consistent with changes in Hbmass (Fig. 2), with the Response group displaying a 6.8 ± 4.1% increase, which was “likely” higher than the 1.1 ± 5.5% increase in the Clamp group (p = 0.08). In the Response group, PV changes mirrored RCV, decreasing by 5.0 ± 4.3% in order to conserve BV, which was not substantially different from baseline following 26 nights of hypoxic exposure (−0.6 ± 3.4%). In the Clamp group, a similar decrease in PV was observed (−7.7 ± 7.3, p = 0.46), however, due to the loss of RCV through phlebotomy, total BV of the Clamp group was “likely” lower (−4.3 ± 6.0%, p = 0.13) compared with baseline, with the mean change “possibly” lower than the Response group (p = 0.22).
VO2peak and cycling performance
Following LHTL, MMP4min increased in all athletes (Fig. 3). Mean increases in the groups were: Response = 4.5 ± 1.1%, Clamp = 3.6 ± 1.4% with “trivial” differences between the groups. (−0.8%; 90% CL −2.2 to 0.6, p = 0.58). Mean VO2peak substantially increased in the Response group (3.5 ± 2.3%) but not in the Clamp group (0.3 ± 2.6%), with the magnitude of change from baseline “likely” lower within the Clamp group (−3.2%; 90% CL −5.7 to 0.5, p = 0.056), (Fig. 3). Cyclists in the Response group rode substantially longer (+28.5 ± 20.8%) at PPO compared with baseline (Fig. 3). In comparison, mean T lim substantially decreased in the Clamp group following LHTL (−19.4 ± 64.5%) and their performance was “likely” worse than the Response group (−37.6%; 90 %CL −58.9 to −5.0, p = 0.07).
MAOD increased substantially in Clamp (18.8 ± 17.6%, p = 0.048) but not in Response (11.6 ± 10.9%, p = 0.076). Similarly, the anaerobic contribution increased substantially in the Clamp group (pre 16.5 ± 3.5%, post 18.9 ± 4.1%) but not in the Response group (pre 14.0 ± 3.6%, post 14.8 ± 2.5%), with the increase observed in the Clamp group “possibly” higher than the Response group (6.5%; 90% CL −7.1 to 22.5, p = 0.41). There were no substantial effect of LHTL on mean post-test lactate concentration following either the MMP4min (Response: 13.0 ± 2.4 vs. 12.9 ± 1.9 mmol L−1; Clamp 14.4 ± 1.0 vs. 14.2 ± 1.2 mmol L−1) or the T lim test (Response: 13.1 ± 2.5 vs. 13.6 ± 1.5 mmol L−1; Clamp 14.6 ± 1.6 vs. 14.6 ± 1.2 mmol L−1); with differences between the groups “unclear.”
Training
Retrospective analysis of the allocated groups (Response vs. Clamp) revealed no substantial differences in training load during LHTL with the exception of the percentage of training time spent above 6 W kg−1 (Table 2).
Discussion
The main finding of the present study is that removal of hypoxia-induced increases in Hbmass during LHTL did not prevent increases in maximal 4-min cycling performance in highly trained female cyclists following 26 nights of simulated hypoxic exposure. Furthermore, enhancements to 4-min cycling performance were not distinguishable between the cyclists whose Hbmass was ‘clamped’ and those in which Hbmass was allowed to increase (~5%). However, the performance of the Clamp group was ~40% worse in a subsequent ride to exhaustion, which reinforces the role of Hbmass for repeated high-intensity efforts.
The relationship between Hbmass and VO2max, is well established with increases in Hbmass directly related to increases in VO2max via enhanced oxygen transport (Gledhill et al. 1999; Schmidt and Prommer 2010). In fact, a 1 g increase in Hbmass results in an ~4 ml min−1 increase in VO2max (Schmidt and Prommer 2010). Therefore, the dominant paradigm which suggests that increases in VO2max secondary to increases in Hbmass following hypoxia are responsible for improvements in sea level performance (Levine and Stray-Gundersen 2005) is intuitively appealing. Indeed, athlete’s who have shown no performance response to altitude training—termed “non-responders” (Levine and Stray-Gundersen 1997), equally did not exhibit changes in red cell volume. However, unlike the classic blood doping studies in which only Hbmass and/or blood volume are manipulated (Berglund and Hemmingson 1987; Brien and Simon 1987; Ekblom 1996), hypoxic exposure may initiate a multitude of responses of which augmented Hbmass is just one (Gore et al. 2007). Therefore, whilst hypoxia-induced increases in Hbmass may partially explain changes in sea level performance; other, non-haematological adaptations may be equally if not more important (Gore and Hopkins 2005). Furthermore, it is postulated that changes in Hbmass may simply indicate an athlete’s ‘adaptive’ state and that other adaptations to hypoxia are occurring independently of changes in Hbmass.
In the present study we attempted to isolate the role of Hbmass on cycling performance following simulated LHTL by repeatedly removing the hypoxia-induced increase in Hbmass. The fundamental aspect of this unique study design was that the EPO and HIF-1 signalling cascades remained ‘active’, with only the end result of erythropoiesis (i.e. increased Hbmass) being blocked, and not the upstream pathways. Using this design, we were able to first, identify which athletes were ‘responding’ haematologically to hypoxia and secondly, remove the Hbmass response without preventing any other adaptive processes associated with the upstream pathway (Sasaki et al. 2000). Previous studies which have documented a performance response without an accompanying increase in Hbmass (Gore et al. 1998) have been criticised for the lack thereof, which has been attributed to either an insufficient hypoxic ‘dose’ (Levine and Stray-Gundersen 2006) or an unsatisfactory state for erythropoietic adaptation in the athlete; e.g. due to illness, inflammation or iron deficiency. It was therefore important in the present study to demonstrate that the athletes were adapting to the hypoxic environment before any blood removal took place. By pair-matching the cyclists based on their Hbmass response (after 14 nights of exposure), we were also able to ensure that both groups displayed a similar haematological response. Due to the error of measurement associated with the CO rebreathing method, and even using duplicate measures, it was not possible to confidently detect changes in Hbmass of <2%. As a result it was not possible to maintain a true Hbmass ‘clamp’ on a daily basis throughout the entire period of exposure, with our study design limited to, at best, weekly blood removals. Therefore, the confounding effect of performing some training sessions with ‘extra’ Hbmass on the subsequent performance tests cannot be dismissed. However, since blood removal was determined on an individual basis, the maximum time frame an athlete in the Clamp group could train with an enhanced Hbmass was limited to 1 week, as opposed to the full exposure period in the Response group. Our data do indicate, however, that we were able to effectively remove the hypoxia-induced increases in Hbmass in the Clamp group at several time-points such that at post-LHTL performance testing, their Hbmass was equivalent to baseline measures. In this way, we were able to assess the importance of ‘extra’ Hbmass, induced by hypoxia, for a cycling-specific performance task.
Role of Hbmass during maximal 4-min cycling performance
Improved 4-minute cycling performance and VO2peak were observed after an adequate dose of LHTL that increased Hbmass by ~5% in the Response group; consistent with the paradigm that increases in sea-level performance following LHTL are primarily mediated by increases in Hbmass (Levine and Stray-Gundersen 2005). However, whilst a strong correlation between the total amount of Hb available and VO2max is evident at sea-level (Kanstrup and Ekblom 1984; Schmidt and Prommer 2010), the relationship between changes in Hbmass and VO2max following LHTL is not clearly defined (Friedmann-Bette 2008; Schmidt and Prommer 2010). Indeed, in the present study, performance improved to a similar extent in the Clamp group without an enhanced Hbmass or VO2peak; raising new questions with respect to the principal role of Hbmass on sea-level performance following LHTL. As often demonstrated in other aspects of physiology, a system is rarely “limited” by solely one component (Schumacher and Roecker 2006), rather adaptive processes serve to compensate for weaker components so that functional capacity is maintained. For example, myoglobin knockout mice are able to somehow compensate for the absence of a key component of the oxygen delivery system; showing indistinguishable exercise capacities to their wild type counterparts during a highly metabolically demanding endurance task (Garry et al. 1998).
Our data indicate that alternate mechanisms for enhanced performance exist following LHTL. Enhanced oxygen transport via increased Hbmass, if available, appears to be the dominant mechanism for increased work during a maximal cycling performance task—as demonstrated by the Response group. However, in its absence, alternative adaptive pathways may be utilised—a scenario demonstrated by our Clamp group, who were able to produce a greater performance without a concomitant increase in VO2peak. Unfortunately, our measurements do not allow us to definitively comment on the mechanisms responsible for the performance improvements in the Clamp group. However, the changes in both MAOD and the relative anaerobic contribution to the task indirectly point towards an increased reliance on anaerobic pathways in this group (Bangsbo et al. 1993). (Roberts et al. 2003) reported increases in both MMP4min and MAOD during an identical cycling performance task in a group of well-trained cyclists following 5, 10, and 15 days of LHTL (8–10 h day−1) at 2,650 m. Whilst haematological changes were not measured in this study, it is unlikely that even the highest ‘dose’ of LHTL used would elicit a sufficient hypoxic dose to induce substantial erythropoietic adaptations (Levine and Stray-Gundersen 2006). Again, the authors were unable to present evidence of the mechanism responsible for the increase in MAOD, but suggest an increased muscle buffering capacity (Mizuno et al. 1990) or changes to lactate transport (Zoll et al. 2006) may explain the hypoxia-induced improvement in performance observed. Further, whilst our postulate of increased anaerobic reliance in the current study is not supported by the blood lactate data (which did not differ between the groups), an improvement in muscle buffering capacity has been documented without a concomitant up-regulation of anaerobic metabolism during intense exercise (Gore et al. 2001). Therefore, it is possible that early performance adaptations to LHTL (when the hypoxic dose is insufficient to initiate remarkable changes in Hbmass) are anaerobic, with aerobic adaptations arising later if hypoxic exposure is continued. Such adaptations would allow for 2–4% increases in a single high-intensity cycling performance task, independent of Hbmass.
Role of Hbmass during ride time to exhaustion (T lim)
The contrasting results of the two groups during the second part of the performance test, may offer further insight into the energy systems utilised by the athletes in the preceding task. The striking increase T lim exhibited by the Response group supports a preferential role of aerobic metabolism during multiple maximal efforts. These data are consistent with previous work showing an increase in performance following induced erythrocythemia (Berglund and Hemmingson 1987; Brien and Simon 1987; Gledhill et al. 1999). The increased Hbmass of the Response group not only served to enhance oxygen transport during the maximal effort (as demonstrated by the increased VO2peak), but may also have contributed to improved rates of recovery in terms of lactate and metabolite clearance following the effort (Brosnan et al. 2000; Kanstrup and Ekblom 1984). In contrast, whilst the Clamp group was able to draw on alternate mechanisms to produce a similar single effort to the Response group in the MMP4min test, when asked to repeat a maximal effort, they displayed a marked amount of fatigue and were unable to reach even their pre-LHTL standards. Kanstrup reported similar findings after ‘blood loss’ with both VO2max and performance time decreased following an induced decrease in both [Hb] and blood volume (Kanstrup and Ekblom 1984). (Gledhill 1999) has suggested that “improvements to aerobic performance may be attributed in part to physiological alterations related to increase in [Hb], e.g. augmented buffering”, therefore the role of a modest (~5%) increase in Hbmass upon sea level performances post-LHTL, may also be related to recovery processes associated with the effort, as opposed to defining the effort itself. The disparate efforts of the Clamp group in the two all-out performance tests suggest that, in the absence of an enhanced Hbmass, the 10-min recovery was inadequate to dissipate the residual fatigue arising from the elevated anaerobic contribution of the prior maximal effort. However, it is important to note the unique design of the performance tests, in which the two tasks were juxtaposed. It is highly likely that the preceding MMP4min effort influenced the athlete’s capabilities in the subsequent T lim test, and therefore the results of a single ride time to exhaustion may be somewhat different. In summary, the increases in Hbmass and VO2max of the Response group allowed the athletes to adopt a more favourable metabolic strategy during the performance tests, which meant they were more capable of producing repeated maximal efforts. In a cycling road-race context, where multiple all out efforts are required, our results highlight the clear benefit of a modestly increased Hbmass after LHTL.
Limitations
It must be acknowledged that the design employed for blood removal may have exerted confounding effects that we were unable to capture. A single blood removal at the end of simulated LHTL may have minimised such effects; however, the impact of a larger blood removal on total blood volume would have been difficult to manage since reinfusion of plasma was not possible due to anti-doping regulations. Plasma losses associated with the blood volumes removed in the present study are typically recovered within 24 h (http://www.givelife2.org/donor/faq.asp#3) and thus acute alterations to blood volume should not have affected performance testing in the following days. However, whilst blood removal was effective in clamping the erythropoietic response, when combined with the reduction in plasma volume induced by hypoxia (which serves to increase [Hb] in order to maintain oxygen supply (Schmidt and Prommer 2010)), total blood volume of the Clamp group was in fact reduced compared to baseline. The effect of mild hypovolemia on performance, therefore, cannot be discounted (Gledhill et al. 1999). It may also be speculated that dehydration induced from living in normobaric hypoxia may have further confounded plasma volume and influenced performance. Whilst hydration status on the morning of performance testing was not determined, individual body mass changes did not indicate that any athletes were dehydrated, with both groups showing a modest 0.4 kg decrease in body mass following LHTL.
The central and neural adaptations to the LHTL period and the subsequent effect on performance must also be considered (Millet et al. 2010), especially with regard to the Clamp group. Despite the fact that all training was conducted as a group, the Clamp group spent a significantly greater percentage of training time at the highest training intensity (>6 W kg−1) and thus the neural effects of training may have been greater in this group, therein contributing to the improved performance at sea level (Friedmann-Bette 2008).
The study is limited by the absence of a classic control group, however, the positive effects of LHTL compared to sea level training have been demonstrated previously (Levine and Stray-Gundersen 1997), and thus in the present study, the Response group serve as a control group of sorts. The small sample size of our groups—due to the elite population studied, is also a limitation, but our design is strengthened by the multiple measurements performed. Furthermore, whilst follow up performance testing in the weeks following cessation of LHTL may have provided further insight into the benefits for each group, this was not possible due to the training and competition demands of the cyclists. Lastly, whilst our measurements do not allow us to discern the mechanisms responsible for the improved performance in the Clamp group, our data provides important information for athletes engaged in normobaric LHTL about the contribution of Hbmass for subsequent performance improvement, which may assist in the planning and implementation of hypoxic training.
Conclusion
Improved 4-min performance and VO2peak were observed after an adequate dose of simulated normobaric LHTL that increased Hbmass by ~5%. However, 4-min performance improved to a similar extent in a matched group without an enhanced Hbmass or VO2peak. On one hand, Hbmass remains an important factor for overall performance following LHTL, since only the group who increased Hbmass was able to improve performance in a ride time to exhaustion test following a maximal 4-min effort. Nevertheless, our novel findings contest the widespread paradigm that modest increases in Hbmass are a prerequisite for enhanced performance following LHTL and suggest that accelerated erythropoiesis is not the sole mechanism by which LHTL improves performance. Future research should therefore focus further on the exploration of the non-haematological mechanisms that determine enhanced performances following LHTL, or incorporate performance tests of sufficient duration (e.g. 30 min) to have an over-whelming dependence on aerobic metabolism.
Ethical standards The experiments in this manuscript comply with current Australian Law.
References
Ahlgrim C, Pottgiesser T, Robinson N, Sottas PE, Ruecker G, Schumacher YO (2010) Are 10 min of seating enough to guarantee stable haemoglobin and haematocrit readings for the athlete’s biological passport? Int J Lab Hematol 32(5):506–511
Allen H, Coggan A (2006) Training and racing with a power meter. Velo Press, Boulder
Bangsbo J, Michalsik L, Petersen A (1993) Accumulated O2 deficit during intense exercise and muscle characteristics of elite athletes. Int J Sports Med 14:207–213
Banister EW, Calvert TW (1980) Planning for future performance: implications for long term training. Can J Appl Sport Sci 5:170–176
Berglund B, Hemmingson P (1987) Effect of reinfusion of autologous blood on exercise performance in cross-country skiers. Int J Sports Med 8:231–233
Bonetti DL, Hopkins WG (2009) Sea-level exercise performance following adaptation to hypoxia: a meta-analysis. Sports Med 39:107–127
Brien AJ, Simon TL (1987) The effects of red blood cell infusion on 10-km race time. JAMA 257:2761–2765
Brosnan MJ, Martin DT, Hahn AG, Gore CJ, Hawley JA (2000) Impaired interval exercise responses in elite female cyclists at moderate simulated altitude. J Appl Physiol 89:1819–1824
Chaplin H Jr, Mollison PL, Vetter H (1953) The body/venous hematocrit ratio: its constancy over a wide hematocrit range. J Clin Invest 32:1309–1316
Clark SA, Quod MJ, Clark MA, Martin DT, Saunders PU, Gore CJ (2009) Time course of haemoglobin mass during 21 days live high:train low simulated altitude. Eur J Appl Physiol 106:399–406
Cohen J (1988) Statistical power analysis for the behavioural sciences. Lawrence Erlbaum Associates, Hillsdale
Craig NP, Norton KI, Bourdon PC, Woolford SM, Stanef T, Squires B, Olds TS, Conyers RA, Walsh CB (1993) Aerobic and anaerobic indices contributing to track endurance cycling performance. Eur J Appl Physiol Occup Physiol 67:150–158
Ebert TR, Martin DT, McDonald W, Victor J, Plummer J, Withers RT (2005) Power output during women’s World Cup road cycle racing. Eur J Appl Physiol 95:529–536
Ekblom B (1996) Blood doping and erythropoietin. The effects of variation in hemoglobin concentration and other related factors on physical performance. Am J Sports Med 24:S40–S42
Ekblom B, Huot R (1972) Response to submaximal and maximal exercise at different levels of carboxyhemoglobin. Acta Physiol Scand 86:474–482
Friedmann-Bette B (2008) Classical altitude training. Scand J Med Sci Sports 18(Suppl 1):11–20
Gardner AS, Stephens S, Martin DT, Lawton E, Lee H, Jenkins D (2004) Accuracy of SRM and power tap power monitoring systems for bicycling. Med Sci Sports Exerc 36:1252–1258
Garry DJ, Ordway GA, Lorenz JN, Radford NB, Chin ER, Grange RW, Bassel-Duby R, Williams RS (1998) Mice without myoglobin. Nature 395:905–908
Gastin PB (2001) Energy system interaction and relative contribution during maximal exercise. Sports Med 31:725–741
Gledhill N, Warburton D, Jamnik V (1999) Haemoglobin, blood volume, cardiac function, and aerobic power. Can J Appl Physiol 24:54–65
Gore CJ, Hopkins WG (2005) Counterpoint: positive effects of intermittent hypoxia (live high:train low) on exercise performance are not mediated primarily by augmented red cell volume. J Appl Physiol 99:2055–2057 discussion 2057–2058
Gore CJ, Hahn A, Rice A, Bourdon P, Lawrence S, Walsh C, Stanef T, Barnes P, Parisotto R, Martin D, Pyne D (1998) Altitude training at 2690 m does not increase total haemoglobin mass or sea level VO2max in world champion track cyclists. J Sci Med Sport 1:156–170
Gore CJ, Hahn AG, Aughey RJ, Martin DT, Ashenden MJ, Clark SA, Garnham AP, Roberts AD, Slater GJ, McKenna MJ (2001) Live high:train low increases muscle buffer capacity and submaximal cycling efficiency. Acta Physiol Scand 173:275–286
Gore CJ, Clark SA, Saunders PU (2007) Nonhematological mechanisms of improved sea-level performance after hypoxic exposure. Med Sci Sports Exerc 39:1600–1609
Hopkins WG (2000) Measures of reliability in sports medicine and science. Sports Med 30:1–15
Hopkins WG, Hawley JA, Burke LM (1999) Design and analysis of research on sport performance enhancement. Med Sci Sports Exerc 31:472–485
Hopkins WG, Marshall SW, Batterham AM, Hanin J (2009) Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41:3–13
Kanstrup IL, Ekblom B (1984) Blood volume and hemoglobin concentration as determinants of maximal aerobic power. Med Sci Sports Exerc 16:256–262
Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G (1985) Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med 6:197–201
Levine BD, Stray-Gundersen J (1997) “Living high-training low”: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol 83:102–112
Levine BD, Stray-Gundersen J (2005) Point: positive effects of intermittent hypoxia (live high:train low) on exercise performance are mediated primarily by augmented red cell volume. J Appl Physiol 99:2053–2055
Levine BD, Stray-Gundersen J (2006) Dose-response of altitude training: how much altitude is enough? Adv Exp Med Biol 588:233–247
Medbo JI, Mohn AC, Tabata I, Bahr R, Vaage O, Sejersted OM (1988) Anaerobic capacity determined by maximal accumulated O2 deficit. J Appl Physiol 64:50–60
Meeusen R, Nederhof E, Buyse L, Roelands B, De Schutter G, Piacentini MF (2008) Diagnosing overtraining in athletes using the two bout exercise protocol. Br J Sports Med 44(9):642–648
Millet GP, Roels B, Schmitt L, Woorons X, Richalet JP (2010) Combining hypoxic methods for peak performance. Sports Med 40:1–25
Mizuno M, Juel C, Bro-Rasmussen T, Mygind E, Schibye B, Rasmussen B, Saltin B (1990) Limb skeletal muscle adaptation in athletes after training at altitude. J Appl Physiol 68:496–502
Roberts AD, Clark SA, Townsend NE, Anderson ME, Gore CJ, Hahn AG (2003) Changes in performance, maximal oxygen uptake and maximal accumulated oxygen deficit after 5, 10 and 15 days of live high:train low altitude exposure. Eur J Appl Physiol 88:390–395
Robertson EY, Saunders PU, Pyne DB, Aughey RJ, Anson JM, Gore CJ (2010) Reproducibility of performance changes to simulated live high/train low altitude. Med Sci Sports Exerc 42:394–401
Sasaki R, Masuda S, Nagao M (2000) Erythropoietin: multiple physiological functions and regulation of biosynthesis. Biosci Biotechnol Biochem 64:1775–1793
Saunders PU, Telford RD, Pyne DB, Cunningham RB, Gore CJ, Hahn AG, Hawley JA (2004) Improved running economy in elite runners after 20 days of simulated moderate-altitude exposure. J Appl Physiol 96:931–937
Schmidt W, Prommer N (2005) The optimised CO-rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol 95:486–495
Schmidt W, Prommer N (2010) Impact of alterations in total hemoglobin mass on VO 2max. Exerc Sport Sci Rev 38:68–75
Schumacher YO, Roecker K (2006) Comment on point: counterpoint “in health and in a normoxic environment, VO2max is/is not limited primarily by cardiac output and locomotor muscle blood flow”. Vol 100: 744–8, 2006. Discrete, well-developed components may be able to compensate for weaker ones. J Appl Physiol 100:1086–1087
Schumacher YO, Pottgiesser T, Ahlgrim C, Ruthardt S, Dickhuth HH, Roecker K (2008) Haemoglobin mass in cyclists during stage racing. Int J Sports Med 29:372–378
Wagner PD (1996) Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58:21–50
Zoll J, Ponsot E, Dufour S, Doutreleau S, Ventura-Clapier R, Vogt M, Hoppeler H, Richard R, Fluck M (2006) Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J Appl Physiol 100:1258–1266
Acknowledgments
The authors gratefully acknowledge the work and support of the staff and students of the AIS Physiology Department, particularly the work of Marilyn Dickson, Nicole Thomas, and Jamie Plowman. The authors would also like to thank Amanda Spratt, Dave McPartland and Beth Duryea for their valuable assistance in the conduct of the study and the riders for their efforts during training and testing. This study was funded by a General and Collaborative Research Grant awarded by the Performance Research Centre at the Australian Institute of Sport. Grants and financial support were received from Applied Research Centre at the Australian Institute of Sport and the University Hospital of Freiburg.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan Ward.
Rights and permissions
About this article
Cite this article
Garvican, L.A., Pottgiesser, T., Martin, D.T. et al. The contribution of haemoglobin mass to increases in cycling performance induced by simulated LHTL. Eur J Appl Physiol 111, 1089–1101 (2011). https://doi.org/10.1007/s00421-010-1732-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1732-z