Abstract
This study sought to compare the respective effects of resistance or aerobic exercise of higher or lower intensities on the acute plasma interleukin-6 (IL-6) and C-reactive protein (CRP) response in a sedentary, middle-aged, disease-free cohort. Following baseline testing, and in a randomized cross-over design, 12 sedentary males completed four exercise protocols, including 40 min of moderate–vigorous (M-VA) or low-intensity (LA) aerobic exercise on a cycle ergometer; and a moderate–vigorous (M-VR) or low-intensity (LR) full-body resistance session matched for protocol duration. Venous blood was obtained pre-, post-, 3 h post and 24 h post-exercise and analysed for IL-6, CRP, leukocyte count, myoglobin, creatine kinase (CK), and cortisol. Diet and physical activity were standardized 24 h before and after exercise. Results indicated an elevated CRP response in the M-VR protocol in comparison to the low-intensity protocols (P < 0.05); however, no changes were evident between the moderate–vigorous intensity protocols. The moderate–vigorous intensity protocols induced significant increases of IL-6, cortisol, and leukocytes in comparison to the low-intensity protocols (P < 0.05). However, the IL-6 response showed no significant differences between the moderate–vigorous intensity protocols, despite the M-VR protocol inducing the largest response of markers indicative of muscle damage (CK, myoglobin, and neutrophil count) (P < 0.05). Hence, indicating a disassociation between the IL-6 response and markers of muscle damage within the respective exercise bouts. The highest IL-6 response was evident in the moderate–vigorous intensity protocols immediately post-exercise. Moreover, the exercise modality did not seem to influence the acute IL-6 and CRP response, with the main determinant of the IL-6 response being exercise intensity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence suggests interleukin-6 (IL-6) and C-reactive protein (CRP) as two sensitive physiological markers of chronic systemic inflammation, which have demonstrated predictive ability regarding the development of cardiovascular disease (CVD) and non-insulin dependent diabetes mellitus (NIDDM) in initially disease-free, sedentary populations (Pradhan et al. 2001; Ridker et al. 2000). Accordingly, elevations in resting concentrations of CRP and IL-6 have been shown to be accurate prognostic predictors of all-cause mortality and associated NIDDM and CVD (Koenig et al. 2008; Luc et al. 2003; Spranger et al. 2003). Further, exercise training is reported as an intervention capable of reducing adipose tissue (Donges et al. 2010; Okita et al. 2004), increasing lean muscle mass and decreasing resting concentrations of IL-6 and CRP (Donges et al. 2010; Panagiotakos et al. 2004; Stewart et al. 2007). In turn, these chronic adaptations may result in a reduced likelihood of disease development (Berg and Scherer 2005; Stewart et al. 2007). However, to date few studies report the acute exercise-induced IL-6 and CRP responses that may pre-empt the reported chronic adaptations (Stewart et al. 2007; Panagiotakos et al. 2004; Donges et al. 2010; Kohut et al. 2006; Dekker et al. 2007; Koenig et al. 2008; Nicklas et al. 2008).
Consistent with cytokine production and disease development, there is a direct relationship between basal cytokine values with adiposity and measures associated with body composition (Berg and Scherer 2005; Cottam et al. 2004; Festa et al. 2001). As evidence, physically active individuals, with lower fat mass, are reported to have lower plasma concentrations of IL-6 and CRP in comparison to age and gender-matched inactive groups (Reuben et al. 2003). Accordingly, the reduction of systemic inflammatory markers following exercise training may be viewed as a protective intervention capable of preventing or slowing disease development in non-diseased populations (Pedersen and Febbraio 2008).
Furthermore, exercise training studies have reported equivocal results (decreases or no changes) in baseline levels of IL-6 and CRP (Stewart et al. 2007; Panagiotakos et al. 2004; Donges et al. 2010; Kohut et al. 2006; Dekker et al. 2007; Koenig et al. 2008; Nicklas et al. 2008). Factors that could possibly explain the disparity of results for inflammatory markers include differences in modes, intensities, and duration of the acute exercise stimuli (Nindl et al. 2009). However, few studies report the acute exercise-induced responses that may explain the training-induced reductions in chronic systemic inflammation in sedentary populations. Thus, research into the acute inflammatory response resulting from differing exercise modes and intensities is warranted.
Acute exercise may be an impetus for the inhibition of chronic disease development via the inducement of post-exercise inflammatory responses (Kramer and Goodyear 2007). Accordingly, it has been demonstrated that plasma IL-6 concentration increases during differing bouts of acute exercise (Pedersen and Febbraio 2008; Fischer 2006; Febbraio and Pedersen 2002). Pedersen and Febbraio (2008) report IL-6 as having anti-inflammatory capacities and as the earliest and most marked post-exercise inflammatory respondent; stimulating the appearance of anti-inflammatory cytokines including IL-10, IL-1ra, and soluble tumour necrosis factor receptors, whilst also occurring in a tumour necrosis factor-alpha independent manner (Kramer and Goodyear 2007; Petersen and Pedersen 2005; Fischer 2006). Such anti-inflammatory contributions during acute exercise have shown to be associated with enhanced metabolic function in both NIDDM and normo-glycaemic subjects (Kramer and Goodyear 2007; Pedersen and Febbraio 2008; Petersen and Pedersen 2005). However, to date few studies report the acute exercise-induced IL-6 and CRP responses across different exercise modes and intensities within a sedentary, middle-aged cohort.
Therefore, the aim of this study is to investigate the acute exercise-induced plasma IL-6, CRP and total leukocyte count to different modes (resistance or aerobic) and intensities (higher or lower), within a middle aged, sedentary and disease-free subject population. Based on previous research, it was hypothesized that both the higher-intensity aerobic and resistance protocols would be associated with the greatest increase in CRP, IL-6 and total leukocyte count in comparison to the lower intensity protocols.
Methods
Subjects
The study population comprised of 12 sedentary male subjects, who were perceived to be in a healthy, non-diseased state. Mean ± SEM values for age, body mass, and height were 46.2 ± 1.1 years, 93.9 ± 1.7 kg, and 178.8 ± 0.02 cm, respectively. Subject recruitment ensured a sample population group representative of a sedentary lifestyle, but not clinically diagnosed with any pre-existing CVD or metabolic disorders. Subjects were included in the study if in the preceding 6 months they were not involved in more than one regular exercise (aerobic or resistance) session per week for longer than 20 min. Those excluded from the study included: subjects with immunological irregularities, smokers (<2 years cessation); those suffering from recurrent or recent influenza illness (including flu shot recipients); recent surgical patients; those on cholesterol lowering, anti-inflammatory, or any other medication reported to affect the inflammatory response; rheumatoid arthritis patients; those with known or recent periodontal disease; and other conditions associated with a systemic inflammatory response. Prior to the subject’s involvement, the study was approved by the Research in Human Ethics Committee of the University. All subjects provided verbal and written consent prior to commencing testing procedures.
Overview
Prior to involvement in the exercise sessions, subjects were required to obtain clearance and approval from a medical practitioner who was informed of the above exclusion criteria. Subjects refrained from any physical activity or exercise for 72 h prior to testing and the consumption of alcohol and caffeine 24 h prior to each data collection session. Additionally, subjects were asked to inform the study investigators of any illness prior to a prospective testing session. Accordingly, acute fluctuations of systemic inflammatory markers and muscle damage markers were minimized prior to venous collection. Following an overnight fast (10–12 h), subjects reported to the laboratory between 0630 and 0930 hours for baseline testing procedures. Baseline testing was also used as an information and familiarization session, explaining all details of the study and testing procedures. Subjects completed a PAR-Q, involving documentation of past and current health information obtained from their medical practitioner, ensuring that subjects were free from known CVD, NIDDM, and immunological irregularities. Finally, subjects completed a baseline testing session, involving measurement of body composition, aerobic fitness, and muscular strength.
Following the collection of baseline measures, subjects completed four exercise protocols in a randomized cross-over design, each separated by a 7 day recovery period. The four different protocols included low-intensity (LA) or moderate–vigorous intensity (M-VA) aerobic exercise, and low-intensity (LR) or moderate–vigorous intensity (M-VR) resistance exercise. All aerobic and resistance sessions were matched for session duration, which was standardized at 40 min. Exercise protocols were designed relative to a standard exercise session prescribed for sedentary individuals, ensuring successful completion of both protocols without complications (Pollock et al. 1998). Diet was standardized 24 h before and after each exercise intervention and venous blood was collected, prior to exercise (pre), immediately post-exercise (post), 3 h post, and 24 h post from all subjects during all exercise sessions. Baseline and exercise testing sessions were standardized to the same time of day, and all sessions were conducted by the same research team in a climate-controlled (20–22°C, 30–35% relative humidity) laboratory and gymnasium.
Measures
On arrival at baseline testing, anthropometric measures were obtained, including height (stadiometer: Custom CSU, Australia), body mass on calibrated scales (HW 150 K, A & D, Australia), waist and hip girths (steel tape, EC P3 metric graduation, Australia), and a supine whole body dual-energy X-ray absorptiometry (DXA) scan for analysis of whole body and regional body composition (XR800, Norland, Cooper Surgical Company, USA). Scanning resolution was set at 6.5 × 13.0 mm, and scanning speed was set at 130 mm s−1. The whole body and lower body scan was analysed (Illuminatus DXA, ver. 4.2.0, USA), and total body lean mass (TB-LM), total body fat mass (TB-FM), and lower body lean mass (LB-LM) (Kim et al. 2002) quantities were calculated and reported in kg and percentage.
Baseline testing further involved measures of aerobic fitness and muscular strength. Aerobic fitness measures were obtained via a graded exercise test (GXT) to determine VO2max and maximal aerobic power output. Pulmonary gas exchange was measured by determining O2 and CO2 concentrations and ventilation to calculate VO2 consumption using a metabolic gas analysis system (ParvoMedics, True2400, East Sandy, Utah, USA). Prior to each session, the ventilometer was calibrated using a three litre syringe, while gas analysers were calibrated for fractional gas concentration with a gravimetric gas mixture of known concentrations (CO2, 4.1 ± 0.08%; O2, 15.7 ± 0.2%), in accordance with the manufacturer’s instructions. The GXT was performed on an electronically braked cycle ergometer (LODE Excalibur Sport, LODE BV, Groningen, The Netherlands). The test incorporated an incremental step protocol consisting of 1 min stages for each step of the protocol, commencing at 25 W with increments of 25 W each minute. Rate of perceived exertion (RPE) (Modified Borg CR10 scale) and heart rate (Vantage NV, Polar, Finland) were recorded each minute throughout the protocol, and subjects exercised until volitional exhaustion or upon attainment of age-predicted maximum heart rate.
Following a 30 min recovery period, muscular strength measures were conducted, including a three repetition maximum (3RM) testing procedure, to identify upper-body (chest press, shoulder press, lat pull-down) and lower body (leg press, leg curl, leg extension, static lunge) strength. Subjects were instructed and familiarized with the appropriate operation of the pulley weight machines (Panatta Sport, Apiro, Italy). During strength assessment, subjects were required to attempt ascending resistances, separated by a 2–3 min recovery period until the determination of upper- and lower-body 3RM; usually requiring 2–3 attempts (2–3 sets). All strength values during baseline testing were converted from 3RM to 1RM (Baechle and Earle 2000).
Exercise protocols
Physical activity and dietary standardization
Throughout all data collection sessions subjects standardized and replicated the same diet and physical activity patterns. Specific guidelines were provided on a recommended dietary intake for each meal, requiring subjects to replicate for each testing session. As part of dietary standardization, subjects consumed 50 g of a standardized protein sports drink supplement (Sustagen® Sport Chocolate, Mead Johnson Nutritionals, Nestle; consisting of 18.9 g Protein and 41.8 g CHO) within 300 mL of light milk for breakfast 2 h before all exercise sessions, and 2 h before the 24 h post-exercise blood samples. During each exercise protocol and 3 h after all testing sessions, subjects remained fasted and consumed water ab libitum until a 3 h post-exercise blood sample was obtained. Water consumption was standardized for each subject during the respective testing sessions through the consumption of a set volume (300 mL). Following the collection of the 3 h post-exercise blood sample, subjects were provided with carbohydrate-rich food (2 muesli bars; 40.4 g CHO and 3.2 g protein) and drink (500 mL Powerade, The Coca-Cola Company; 38.0 g CHO). Subjects were provided with an exercise and physical activity diary, requiring the entry of any physical activity within this time period, noting duration, intensity and type of activity. Further, subjects were required to record all fluid and food ingestion before and after each session noting, time, food type/s and serving quantity. Subjects subsequently replicated their meals for the following exercise testing protocols. Diaries were inspected by the research team to ensure compliance with dietary and physical activity requirements.
Resistance protocols
Both resistance exercise protocols involved pulley-weight machine exercises which were performed for the duration of 40 min. All exercises were performed as 3 sets of 10 repetitions, involving a 90 s recovery period between each set. The LR and M-VR protocols were performed at 60 or 80% of 1RM, respectively (Pollock et al. 1998; Howley 2001; Ratamess et al. 2009). Exercises involved in the protocol included chest press, shoulder press, lat pull-down, leg press, leg curl, leg extension, and static lunge. Subjects wore a heart rate monitor (Vantage NV, Polar, Finland) and heart rate was recorded prior to and following each set and a mean heart rate was obtained for the session. Following exercise (10-min post), subjects provided an overall sessional rate of perceived exertion for the whole protocol, recorded as an RPE value.
Aerobic protocols
Both aerobic exercise protocols involved cycling exercise on Monark stationary cycle ergometers (Monark 828E, Varburg, Sweden) which incorporated a flywheel and a manually adjustable surrounding belt. The pedalling resistance was converted from watts (obtained from baseline testing) to be adjusted to a fixed intensity (kiloponds) for the duration of the 40 min aerobic protocol. The M-VA protocol involved a fixed intensity at 50% of the pre-determined maximal aerobic workload, while the LA involved a fixed intensity of 30% of maximal aerobic workload (Ekkekakis and Petruzzello 1999). Heart rate was continuously monitored throughout all sessions and a mean heart rate value was calculated post-exercise. Additionally, a sessional RPE value was recorded approximately 10-min post-exercise.
Venous blood sampling
During baseline testing 4 mL of blood was collected in an EDTA tube for analysis of HbA1c and 5 mL collected in a serum separator tube (SST) for analysis of all cholesterol values. During all testing sessions and at each time point a total of 28 mL of blood was collected. Specifically, 8 mL of blood was collected in EDTA tubes of which 4 mL was analysed as whole blood for total leukocyte count and the remaining 4 mL analysed for high sensitivity (hs)-IL-6. The remaining 20 mL of blood was collected in SST and used for analysis of CRP, cortisol, myoglobin and CK. All venous blood samples were collected from a medial antecubital vein. Serum or plasma was then collected following centrifugation at 3,500 rpm for 15 min at 4°C. Aliquots were immediately frozen at −80 and −20°C for EDTA and SST, respectively. For the analysis of leukocytes, whole blood was stored at room temperature in EDTA tubes for a maximum of 6 h post collection, until further analysis.
Blood analysis
Blood samples were analysed for total cholesterol, high-density lipoprotein (HDL), triglycerides, cortisol, glycosylated haemoglobin (HbA1c), CK, total and fractional leukocyte count, myoglobin, hs-CRP, and hs-IL-6. All biochemistry variables were analysed according to manufacturer’s instructions provided in the respective assay kits (Dade Behring Dimension Xpand, Siemens healthcare Diagnostics, Sydney, Australia). Total cholesterol was assayed using an enzymatic method and polychromatic endpoint technique measurement. HDL cholesterol was measured using accelerator selective detergent methodology. Triglycerides were analysed using an enzymatic method and biochromatic endpoint technique measurement. Low-density lipoprotein (LDL) and very low-density (VLDL) cholesterol was calculated using the Friedwald Equation, which incorporates total cholesterol, HDL cholesterol and triglyceride measures. HbA1c was measured using automated high-performance liquid chromatography (HPLC) methodology (Bio-Rad Variant, Sydney, Australia). CK concentrations were determined using an enzymatic method and biochromatic rate technique. Leukocyte count was determined by a cell counter (Cell-Dyn 3200, Abbott Laboratories, IL, USA). Analysis of biochemistry variables, CK and leukocyte count showed intra and inter-assay coefficients of variation were between 2.0–5.0, 2.1 and 2.8%, respectively. Cortisol concentrations were determined by a solid-phase, competitive chemiluminescent enzyme immunoassay. Myoglobin concentrations were determined by a solid-phase, two-site chemiluminescent immunometric assay. Hs-CRP concentrations were determined using a solid-phase, chemiluminescent immunometric assay (Immulite 2000, Diagnostic Products Corp., Los Angeles, USA). Hs-IL-6 concentrations were measured using a high sensitivity ELISA kit (B & D Systems, Minneapolis, MA, USA). Analysis of cortisol, myoglobin, hs-CRP and hs-IL-6 showed intra and inter-assay coefficients of variation of 4.3, 5.1, 3.0 and 6.3%, respectively.
Statistical analysis
All data are reported as mean ± SEM. Within and between protocol and blood measure time-point differences were assessed using a two-way repeated measures ANOVA (condition × time). When significant differences were observed, Tukey’s pairwise comparisons were employed to assess the source of significance which was set at P ≤ 0.05. All statistical analyses were performed using SPSS™ for MS-Windows version 16.0 (Statistical Package for the Social Sciences, Chicago, IL, USA).
Results
Baseline anthropometry, biochemistry, muscular strength and aerobic fitness
Mean ± SEM for body composition is presented in Table 1. According to BMI and percent body fat categories, the subjects in the present study are overweight and exhibit excess abdominal obesity (Alberti et al. 2005). The results of baseline biochemistry measures (Table 1) demonstrated elevated concentrations of total (5.56 ± 0.31 mmol L−1) and LDL (3.56 ± 0.28 mmol L−1) cholesterol (Alberti et al. 2005), and moderately elevated concentrations of IL-6 (Ridker et al. 2000). Results for 3RM testing included chest press (70.4 ± 2.2 kg), shoulder press (53.3 ± 2.1 kg), lat pull-down (58.0 ± 1.7 kg) for upper-body strength, and leg press (165.8 ± 9.4 kg), leg curl (39.2 ± 2.0 kg), leg extension (52.5 ± 3.5 kg), static lunge (39.8 ± 2.9 kg) for lower-body strength. The maximal aerobic fitness results demonstrated a maximal aerobic power output of 252 ± 11 W, maximal heart rate of 161 ± 12 bpm, test duration of 588.3 ± 112.4 s, and a VO2max of 30.5 ± 1.3 mL kg−1 min−1. Finally, the exercise-induced heart rate response and session RPE values for all respective aerobic and resistance exercise sessions are presented in Table 2.
IL-6 and CRP responses
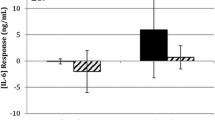
The plasma IL-6 and CRP inflammatory responses are shown in Fig. 1a, b, respectively. The absolute and relative (%) magnitude of change in IL-6 to the M-VA (0.9 ± 0.13 pg mL−1; 70.9 ± 4.5%; P = 0.0001), M-VR (0.74 ± 0.27 pg mL−1; 59.1 ± 2.8%; P = 0.01) and LR (0.33 ± 0.09 pg mL−1; 14.8 ± 2.7% P = 0.04) protocols resulted in significant changes in pre to post-exercise values. No significant increases were evident in the LA protocol (0.1 ± 0.09 pg mL−1; 9.3 ± 19.5%; P = 0.09). Between protocol comparisons indicated no significant differences between the M-VA and M-VR protocols (P = 0.37). The acute increase in IL-6 was significantly larger in both moderate–vigorous-intensity protocols than in the low-intensity protocols (P < 0.05).
There were no significant differences in the CRP response from pre to 3 h post-exercise (pre- to post and post to 3 h post) within or between the respective exercise sessions (Fig. 1b). The 3–24 h post-exercise response of CRP was significantly higher in the M-VR (0.68 ± 0.31 mg L−1; 80.0 ± 38.2%) protocol when compared to the LA (0.2 ± 0.14 mg L−1; 33.9 ± 28.6%) and LR (0.16 ± 0.16 mg L−1; 15.2 ± 31.2%) protocols, respectively (P < 0.05); however, no significant differences (P > 0.05) were evident between the M-VA (0.84 ± 0.57 mg L−1; 77.1 ± 116.6%) protocol and the other protocols (LA, LR and M-VR).
Cortisol and muscle damage marker responses
Plasma cortisol, myoglobin, and CK responses to the respective exercise protocols are shown in Fig. 2. Myoglobin responses (Fig. 2a) presented the most significant increase at 3 h post-exercise in all protocols (LA, P = 0.01; LR, P = 0.01; M-VA, P = 0.01; M-VR, P = 0.007). Between protocol comparisons indicated that the M-VR protocol produced higher peak concentrations of myoglobin than all other protocols (LA, P = 0.02; LR, P = 0.03; M-VA, P = 0.03) at 3 h post-exercise. Analysis of CK responses (Fig. 2b) indicated the M-VR protocol significantly increased 24 h post-exercise (P = 0.05). Between protocol comparisons indicated that 24 h post-exercise, CK was significantly higher in the M-VA than the LA (P = 0.02), and M-VR was significantly higher than both LA (P = 0.03) and LR (P = 0.04), respectively. No significant differences in CK response were present between the M-VA and M-VR protocols (P = 0.06). Both moderate–vigorous-intensity protocols induced a significant cortisol response (Fig. 2c) immediately post-exercise (M-VA, P = 0.02; M-VR, P = 0.003), with concentrations returning to pre-exercise values at 3 h post-exercise. No significant differences were evident in either the LA (P = 0.80) and LR (P = 0.27) protocols immediately post-exercise. Between protocol comparisons indicated that both moderate–vigorous-intensity protocols produced significantly higher cortisol response post-exercise than the respective low-intensity protocols (P = 0.009).
Mean ± SEM of myoglobin, CK and cortisol responses between respective protocols. **Significant difference from low aerobic P < 0.01; *P < 0.05; ††significant difference from low resistance P < 0.01; † P < 0.05; ‡significant difference from moderate–vigorous aerobic P < 0.05; §pre to post change P < 0.01; ≠post to 3 h post change P < 0.05; #3 h post to 24 h post change P < 0.05
Leukocytes response to exercise
Total leukocyte count increased in response to exercise. Total leukocyte count peaked in the M-VA (P = 0.001) and M-VR (P = 0.012) protocols 3 h post-exercise (Fig. 3a). Between protocol comparisons of total leukocyte count indicated that the M-VR and M-VA protocols induced a significantly higher increase post-exercise (P = 0.0001) and 3 h post-exercise (P = 0.003) than the LA and LR protocols, respectively. There was no significant increase in monocyte count in response to the LR (P = 0.45) or LA (P = 0.99) protocols (Fig. 3b); however, both moderate–vigorous intensity protocols induced significant increases immediately post-exercise (M-VA, P = 0.005; M-VR, P = 0.0001). Between protocol comparisons indicated at post- and 3 h post-exercise that, LR, M-VA, and M-VR protocols were significantly higher than the LA protocol (P = 0.02). Neutrophil count was significantly increased in response to the M-VA (P = 0.02), and M-VR (P = 0.0001) protocols 3 h post-exercise. Between protocol comparisons demonstrated that the neutrophil response to the LA and LR protocols remained significantly lower than the moderate–vigorous intensity protocols at the post (P = 0.03) and 3 h post (P = 0.001) time points (Fig. 3c). Lymphocyte count (Fig. 3d) was significantly increased immediately post-exercise in response to the M-VA (P = 0.003) and M-VR (P = 0.0001) protocols. Between protocol comparisons indicated post-exercise in both moderate–vigorous intensity protocols were significantly higher than the low-intensity protocols (P < 0.05).
Discussion
The acute effects of exercise mode and intensity were investigated to assess the acute post-exercise inflammatory (IL-6, CRP) responses. Respective effects of mode and intensity may highlight favourable metabolic and inflammatory responses, particularly with continued application (Coffey and Hawley 2007; Kramer and Goodyear 2007). Accordingly, this study directly compared the acute plasma inflammatory responses to resistance and aerobic exercise of higher or lower intensities within the same middle-age, sedentary, but disease-free cohort. The subject cohort involved in the present study was sedentary at baseline, and exhibited risk factors for the development of CVD and NIDDM (Table 1). Such risk factors include; BMI (29.4 ± 0.6) and TB-FM % (27.3 ± 5.4) placing the subjects within the overweight category (borderline obese category). Further, the subjects exhibited adiposity characteristics including a waist circumference of 99.4 ± 1.7 cm and a waist to hip ratio of 0.96 ± 0.02, which suggests the subjects presented with abdominal obesity (Alberti et al. 2005).
Current literature represents IL-6 to have both pro and anti-inflammatory properties (Mathur and Pedersen 2008; Pedersen 2006). IL-6 as a pro-inflammatory cytokine expressed within the plasma is an indicator of chronic low-grade inflammation and is a strong predictor of future development of CVD and NIDDM (Mathur and Pedersen 2008; Pedersen 2006). Despite the sedentary cohort exhibiting normal resting values of CRP, the IL-6 concentration of 1.31 ± 0.28 pg mL−1, is suggestive of moderate risk (quartile 2; 1.04–1.46 pg mL−1) for future development of inflammatory diseases such as CVD and NIDDM (Koenig et al. 2008; Luc et al. 2003; Pradhan et al. 2001; Ridker et al. 2000).
Physical activity may offer protection against, and be useful in the treatment and prevention of chronic diseases associated with low-grade inflammation (Mathur and Pedersen 2008; Pedersen 2006). The findings of the present study indicate the magnitude of the acute exercise-induced inflammatory response appears to be determined in part by the intensity of the exercise bout performed. The suggested intensity effect on the acute inflammatory response is apparent through the differential increase between exercise modes for IL-6; however, without differences between the moderate–vigorous intensity resistance and aerobic protocols, respectively. As such, when standardized for exercise duration, it appears the modality (aerobic vs. resistance) of exercise performed was not a major determinant of the acute plasma IL-6 and CRP response to exercise (Nindl et al. 2009).
The release of IL-6 from contracting skeletal muscle (Mathur and Pedersen 2008; Pedersen 2006) and the subsequent increases in plasma IL-6 concentration during and immediately after exercise are a consistent finding in the research literature (Helge et al. 2003; Pedersen and Febbraio 2008; Steensberg et al. 2002). Somewhat in agreement, the IL-6 change from pre to post values in both moderate–vigorous intensity protocols was significantly greater than the respective low-intensity protocols. To highlight the intensity effect, RPE, heart rate, cortisol and total leukocyte count, were significantly higher in the moderate–vigorous intensity protocols compared to the low-intensity protocols. Furthermore, the intensities of the respective exercise protocols appeared to be similar between the aerobic and resistance modes. Consequently, whether due to the extent of skeletal muscle recruitment or overall mechanical stress invoked by the external load (Fischer 2006), increments in exercise intensity result in amplified IL-6 responses, without differences between modes. Previously, Fischer (2006) reported that the magnitude of the IL-6 response is highly dependent on the duration of exercise, with duration accounting for more than 50% of plasma IL-6 variation. However, the present study indicates that when matched for duration, exercise intensity is a major contributor to the ensuing IL-6 response.
The CRP response to the exercise protocols indicated the change from 3 to 24 h post-exercise values in the M-VR protocol to be significantly higher than both low-intensity protocols, while not different to the M-VA protocol. Further, hepatic synthesis and release of CRP is induced by IL-6 and responsible for recognition and clearance of damaged cells (Neubauer et al. 2008; Festa et al. 2001). The present study indicates CRP to increase 24 h post-exercise within the higher-intensity protocols more so than the lower intensity protocols. However, the M-VR protocol showed the most significant increase in CK and CRP, respectively. As previously suggested, the current study indicates a similar response of both CRP and CK peak at 24 h post-exercise; supporting the potential role of CRP in the clearance of damaged cells as a post-exercise repair mechanism (Neubauer et al. 2008; Festa et al. 2001). Accordingly, these results may suggest that the increased CK response in higher-intensity modes, particularly in M-VR, may have invoked a more substantial increase in CRP.
Although an increased IL-6 response was evident within the higher-intensity protocols, the induced CRP change was only modest (Pedersen and Hoffman-Goetz 2000). Such results may suggest IL-6 of higher sensitivity than CRP within an acute exercise bout, as previous results report unchanged concentrations of CRP after either uphill running or 24 h after downhill running (Pedersen and Hoffman-Goetz 2000). Additionally, studies reporting the acute exercise-induced CRP response within a sedentary population are minimal; with previous training studies reporting a lack of association between IL-6 and CRP (Dekker et al. 2007; Donges et al. 2010; Nicklas et al. 2008; Stewart et al. 2007). Therefore, further research is warranted in examining the impact of exercise on possible acute mechanisms involving the attenuation of acute phase reactant proteins such as CRP, other than cytokines (Pedersen and Hoffman-Goetz 2000). Regardless, as with IL-6, some intensity-dependent mechanism seems evident in response to exercise bouts of differing mode and/or intensity.
Exercise appears to be a physiological stimulus capable of inducing an anti-inflammatory response that coincides with intra-cellular substrate repletion, and repair of protective processes associated with exercise-induced tissue disturbance (Coffey and Hawley 2007; Donges et al. 2010). Increases in circulatory CK, IL-6 and the acute phase protein CRP have previously been reported to be associated with exercise-induced muscle damage, with evidence linking the stress and intensity of exercise to the subsequent magnitude of response of these markers within the circulation (Neubauer et al. 2008; Toft et al. 2002). The present study evaluated the magnitude of the IL-6 response in light of the muscle damage response, as shown through analysis of CK, myoglobin and neutrophil count (Bruunsgaard et al. 1997; Toft et al. 2002). Previously, others have suggested that elevations in these markers post-exercise are likely accompanied (and/or associated) with an acute plasma IL-6 response (Bruunsgaard et al. 1997; Toft et al. 2002). Results from the present study demonstrated that the muscle damage response was notably augmented in the M-VR protocol compared to the other protocols; however, the M-VR plasma IL-6 response was similar in comparison to that of the M-VA protocol which featured minimal muscle damage. Hence, these findings corroborate previous findings (Toft et al. 2002) and suggest that muscle damage may not be a major direct determinate of acute IL-6 release during exercise. These results lend credence to the notion that other factors such as, muscle glycogen reduction; potentially play an important role in regulating the acute IL-6 response to an acute exercise bout (Glund and Krook 2008; Kramer and Goodyear 2007; Pedersen and Febbraio 2008).
Recent evidence indicates that glycogen depletion plays a large role in plasma IL-6 responses to exercise of different modes and intensities (Keller et al. 2001; Steensberg et al. 2002); with increased rates of glycogen depletion dependent upon increased exercise intensity (Gollnick et al. 1974). IL-6 has consistently been reported to increase within contracting skeletal muscle, with increases augmented when intramuscular glycogen levels are low (Chan et al. 2004), or suppressed when carbohydrate availability is increased during and after exercise (Miles et al. 2006; Ronsen et al. 2002). The level of glycogen availability was not measured within the current study; however, the intensities reported would allow for the assumption that the moderate–vigorous intensity protocols would likely deplete glycogen significantly more than the low-intensity protocols (Gollnick et al. 1974). As evidence of this, Steensberg et al. (2002) demonstrated associations between muscle glycogen content and subsequent IL-6 mRNA response. In this study, IL-6 was released from a glycogen depleted leg after 60 min of exercise in comparison to 120 min in a control leg (non-glycogen depleted) (Steensberg et al. 2002). Accordingly, these results may indicate muscle glycogen depletion to be a major determining factor for the production of IL-6 within contracting skeletal muscle (Steensberg et al. 2002).
In consideration of previously mentioned evidence, it seems plausible to suggest that the acute exercise response may explain intra-muscular metabolic and chronic inflammatory adaptations following training (Donges et al. 2010; Stewart et al. 2007 etc.). Chronic local inflammatory processes, which contribute to systemic inflammation, may be reduced in response to enhanced post-exercise metabolic functioning within skeletal muscle (Kramer and Goodyear 2007; Petersen and Pedersen 2005). As such, muscular fitness and weekly exercise patterns are inversely associated with levels of inflammatory markers (Aronson et al. 2004; Panagiotakos et al. 2004). Aligned with these suggestions are evidence that acute inducement of IL-6 is associated with improvements in glucose homeostasis and insulin sensitivity, mobilization and utilization of fatty acids, and lipid metabolism (Glund and Krook 2008; Pedersen and Febbraio 2008; Petersen and Pedersen 2005); all of which are important responses regarding sedentary individuals at risk of developing NIDDM and/or CVD (Alberti et al. 2005 Aronson et al. 2004; Panagiotakos et al. 2004).
The acute IL-6 increase and associated metabolic effects have previously shown relation to immunoregulatory and hormonal effects of leukocyte counts and cortisol, respectively (Fischer 2006). The plasma IL-6 response to intense exercise has been shown to activate the anti-inflammatory and catabolic effects of cortisol; which may in part be responsible for changes in leukocyte subpopulations (Fischer 2006). In consideration of these metabolic improvements, the use of higher exercise intensities may account for increased augmentation of the IL-6 response during and after an acute exercise bout. Accordingly, the IL-6 response may therefore relate to the extent of acute metabolic responses and potential for ensuing chronic adaptation within skeletal muscle (Kramer and Goodyear 2007; Pedersen and Febbraio 2008). Hence, it may be suggested that (within reason) higher exercise intensities are prescribed during exercise training sessions to promote greater glycogen depletion and an increased post-exercise anti-inflammatory response.
In conclusion, the present study sought to compare the acute IL-6 and CRP responses to resistance or aerobic exercise of higher or lower intensities in a sedentary, middle-aged, disease-free cohort. The highest IL-6 response was evident in response to the moderate–vigorous intensity protocols immediately post-exercise. Further, this response was not related to the appearance of muscle damage markers and may instead relate to mechanisms associated with glycogen depletion. Moreover, the exercise modality did not seem to influence the acute IL-6 and CRP response, with the main determinant of the plasma IL-6 response being the exercise intensity. Finally, these results represent the acute IL-6 and CRP response to differing bouts of exercise intensity and mode. As such, knowledge of the acute responses may assist with appropriate exercise prescription and associated metabolic and inflammatory adaptation within a middle-age, sedentary cohort.
References
Alberti K, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Diabet Med 23:469–480
Aronson D, Sheikh-ahmad M, Avizohar O et al (2004) C-reactive protein is inversely related to physical fitness in middle-aged subjects. Atherosclerosis 176:173–179
Baechle TR, Earle RW (2000) Essentials of strength training and conditioning, 2nd edn. Human Kinetics, Lower Mitcham
Berg AH, Scherer PE (2005) Adipose tissue, inflammation, and cardiovascular disease. Circulation 96(9):939–949
Bruunsgaard H, Galbo HBH, Halkjaer-Kristensen J, Johansen TL (1997) Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol 499:833–841
Chan MHS, Carey AL, Watt MJ, Febbraio MA (2004) Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am J Physiol 287(2):322–327
Coffey VG, Hawley JA (2007) The molecular bases of training adaptation. Sports Med 37(9):737–763
Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley D, Schauer PR (2004) The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg 14(5):589–600
Dekker M, Lee S, Hudson R, Kilpatrick K, Graham TE, Ross R, Robinson LE (2007) An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism 56(3):332–338
Donges CE, Duffield R, Drinkwater EJ (2010) Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med Sci Sports Exerc 42:1–10
Ekkekakis P, Petruzzello SJ (1999) Acute aerobic exercise and affect: current status, problems and prospects regarding dose-response. Sports Med 28(5):337–374
Febbraio MA, Pedersen BK (2002) Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 16:1335–1347
Festa A, D’Agostino R Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, Haffner SM (2001) The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes 25(10):1407–1415
Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immun Rev 12:6–33
Glund S, Krook A (2008) Review: role of interleukin-6 signalling in glucose and lipid metabolism. Acta Physiol Scand 192:37–48
Gollnick PD, Piehl K, Saltin B (1974) Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol 241:45–57
Helge JW, Stallknecht B, Pedersen BK, Galbo H, Kiens B, Richter EA (2003) The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol 546:299–305
Howley ET (2001) Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc 33(6):S364–S369
Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD (2001) Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 15(14):2748–2750
Kim J, Wang ZM, Heymsfield SB, Baumgartner RN, Gallagher D (2002) Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr 76(2):378–383
Koenig W, Khuseyinova N, Baumert J, Meisinger C (2008) Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg cohort study, 1984–1998. Clin Chem 54(2):335–342
Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD, Castillo MC, Reighard AE, Vanderah E (2006) Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of A-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun 20(3):201–209
Kramer HF, Goodyear LJ (2007) Exercise, MAPK, and NF-κB signalling in skeletal muscle. J Appl Physiol 103:388–395
Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P (2003) C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME study. Arterioscler Thromb Vasc Biol 23(7):1255–1261
Mathur N, Pedersen BK (2008) Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm 2008:1–6
Miles MP, Walker EE, Conant SB, Hogan SP, Kidd JR (2006) Carbohydrate influences plasma interleukin-6 but not C-reactive protein or creatine kinase following a 32-km mountain trail race. Int J Sport Nutr Exerc Metabol 16:36–46
Neubauer O, König D, Wagner KH (2008) Recovery after an Ironman triathlon: sustained inflammatory responses and muscular stress. Eur J Appl Physiol 104(3):417–426
Nicklas BJ, Hsu F, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M (2008) Exercise and plasma C-reactive protein and interleukin 6 in elderly people. J Am Geriatr Soc 56:2045–2052
Nindl BC, Alemany JA, Tuckow AP, Kellogg MD, Sharp MA, Patton JF (2009) Effects of exercise mode and duration on 24-h IGF-1 system recovery responses. Med Sci Sports Exerc 44(6):1261–1270
Okita K, Nishijima H, Murakami T, Nagai T, Morita N, Yonezawa K, Lizuka K, Kawaguchi H, Kitabatake A (2004) Can exercise with weight loss lower serum C-reactive protein levels? Arterioscler Thromb Vasc Biol 24(10):1868–1873
Panagiotakos D, Pitsavos CM, Chrysohoou C, Kavouras S, Stefanadis C (2004) The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA study. Prev Med 40(4):432–437
Pedersen BK (2006) The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem 42:105–117
Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88(4):1379–1406
Pedersen BK, Hoffman-Goetz L (2000) Exercise and the immune system: regulation, integration and adaptation. Physiol Rev 80(3):1055–1081
Petersen AMW, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol 98(4):1154–1162
Pollock ML, Gaesser GA, Butcher JD et al (1998) ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 30(6):975–991
Pradhan AD, Manson JAE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin-6, and risk of developing type 2 diabetes mellitus. JAMA 286(3):327–334
Ratamess NA, Alvar BA, Evetoch TK et al (2009) Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41:687–708
Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE (2003) The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. JAGS 51(8):1125–1130
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Am Heart Assoc 101(15):1767–1772
Ronsen O, Lea T, Bahr R, Pedersen BK (2002) Enhanced plasma IL-6 and IL-1ra responses to repeated vs. single bouts of prolonged cycling in elite athletes. J Appl Physiol 92(6):2547–2553
Spranger J, Kroke A, Mohlig M, Hoffman K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AFH (2003) Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)—Potsdam Study. Diabetes 52(3):812–817
Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK (2002) IL-6 and TNF-a expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab 283(6):1272–1278
Stewart LK, Flynn MG, Campbell WW et al (2007) The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc 39(10):1714–1719
Toft AD, Jensen LB, Bruunsgaard H, Ibfelt T, Halkjaer-Kristensen J, Febbraio M, Pedersen BK (2002) Cytokine response to eccentric exercise in young and elderly humans. Am J Physiol 283:289–295
Acknowledgments
The authors would like to acknowledge staff at the Institute of Clinical Pathology and Medical Research, Westmead Hospital, Sydney, NSW, Australia, and the Institutional staff at CSU Exercise Physiology Laboratories, Bathurst, NSW for assistance and support in blood analysis. They would also like to acknowledge the subjects for their invovlement in the research study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan Ward.
Rights and permissions
About this article
Cite this article
Mendham, A.E., Donges, C.E., Liberts, E.A. et al. Effects of mode and intensity on the acute exercise-induced IL-6 and CRP responses in a sedentary, overweight population. Eur J Appl Physiol 111, 1035–1045 (2011). https://doi.org/10.1007/s00421-010-1724-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1724-z