Abstract
Purpose
This study examined the effects of high-intensity interval training (HIIT; 30 s sprint, 4–5 min passive recovery) and prolonged intermittent sprint training (PIST; 10 s sprint, 2–3 min moderate exercise) on the systemic inflammatory markers C-reactive protein (CRP) and tumor necrosis factor-α (TNF-α), aerobic capacity, and anthropometry in a middle-aged, sedentary population.
Methods
Fifty-five sedentary adults (age 49.2 ± 6.1 years) were randomised into HIIT (n = 20), PIST (n = 21), or a sedentary control group (CTRL n = 14). HIIT and PIST performed three training sessions per week for 9 weeks on a cycle ergometer, matched for total high-intensity time, while CTRL continued normal sedentary behaviours. Pre- and post-intervention testing involved measures of anthropometry, peak oxygen consumption (VO2peak), and venous blood collection for analyses of CRP and TNF-α.
Results
HIIT and PIST increased VO2peak compared to CTRL (+3.66 ± 2.23 and 3.74 ± 2.62 mL kg min−1). A group × time interaction (p = 0.042) and main effect of time (p = 0.026) were evident for waist girth, with only HIIT showing a significant reduction compared to CTRL (−2.1 ± 2.8 cm). TNF-α and CRP showed no group × time interaction or time effect (p > 0.05).
Conclusions
In sedentary individuals, 9 weeks of HIIT or PIST were effective to improve aerobic capacity; however, only HIIT significantly reduced waist girth and WHR compared to CTRL. Markers of systemic inflammation remained unchanged across all groups. Accordingly, for inflammation and VO2peak, the distribution of sprints and the active or passive recovery periods are inconsequential provided that total duration of high-intensity efforts is similar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent research highlights the link between chronic systemic inflammation (CSI) and the progression of insulin resistance, type 2 diabetes (T2D), and atherosclerosis (Berg and Scherer 2005). CSI is represented by elevated resting concentrations of pro-inflammatory markers, including C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor alpha (TNF-α; Berg and Scherer 2005). Elevated basal concentrations of these markers are prognostic indicators of disease risk, and have consequently emerged as intervention targets (You et al. 2013). Specifically, exercise is suggested as an important mediator of long-term reductions in systemic inflammation, given that physical inactivity is associated with elevated CSI and disease (You et al. 2013). As such, exercise training may be an effective intervention to provide reductions in CSI and thus overall disease risk.
Moderate-intensity aerobic exercise training is reported to reduce basal concentrations of CRP, TNF-α, and IL-6 (Kohut et al. 2006). In contrast, some studies show that this type of training has no effect on these markers, often implying an insufficient training stimulus due to low–moderate intensity (Stewart et al. 2010). Separately, the previous research indicates that high-intensity training can elicit similar, if not greater, improvements in insulin sensitivity compared with moderate-intensity modalities (Houmard et al. 2004). Given that elevated CSI has been implicated in the development of insulin resistance via inhibition of insulin receptor function (Pradhan et al. 2001), it is hypothesised that high-intensity exercise may reduce markers of CSI. Regardless, despite the increasing popularity of high-intensity training, there is limited evidence of its effect on inflammatory biomarkers.
Considering the proposed importance of high-intensity exercise, interval-based training is suggested to confer similar health effects compared to moderate-intensity training, and may also appeal to time-limited individuals (Gibala et al. 2012). Specifically, high-intensity interval training (HIIT) is reported to improve endothelial function (Wisløff et al. 2007) and insulin sensitivity (Babraj et al. 2009), though research on the effects of HIIT on markers of CSI is currently lacking. Similarly, football-based small-sided games (SSG) training is classified as high-intensity exercise, given its intermittent sprint nature with interspersed moderate-intensity activity. Such training has been shown to reduce resting CRP and IL-6 (Mendham et al. 2014), and improve glycaemic control (Andersen et al. 2014) and body composition (Andersen et al. 2010). However, the skill-specific nature of SSG training may restrict its use in non-athletic populations. Borrowing from this modality, prolonged intermittent sprint training (PIST) mimics the intensity of SSG’s without the skill requirement or load-bearing nature, thus potentially broadening its appeal. Previously, research indicates that differing the patterns of high- or low-intensity exercise within a training program has a little effect on physiological outcomes when total work is matched (Sylta et al. 2016); however, it is unknown whether similar results are evident with systemic inflammatory markers. Therefore, the aim of this study was to compare the effects of two high-intensity exercise training modes (HIIT and PIST) on markers of CSI, anthropometry, and aerobic capacity. It was hypothesised that, when matched for total sprint duration, HIIT and PIST would be equally effective to reduce inflammatory markers and improve physical capacity compared to inactive controls.
Methods
Participants
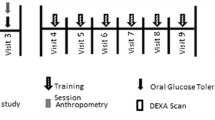
Fifty-five middle-aged, sedentary adults (Table 1) were recruited through newspaper advertisements within the local geographical region, and randomised to either HIIT (n = 20), PIST (n = 21) or a control group (CTRL, n = 14; Fig. 1). The variation in group sizes resulted from several participants declining their allocation to CTRL. Participants were matched based on sex, peak oxygen consumption (VO2peak), and age, and randomly allocated to their respective groups by an independent consultant via de-identified numerical selections. Inclusion into the study required that participants be aged 35–60 years, inactive, i.e. ≤1 session of exercise per week confirmed via Godin’s Leisure Time Exercise Questionnaire (Godin and Shephard 1985), non-smokers (no smoking for the 6 months preceding inclusion in the study), taking no medications, and free from diagnosed cardiovascular, autoimmune, or metabolic conditions. Approval was obtained from the Institutional Human Research Ethics Committee (HREC Ref No. 2015000299) in accordance with the Declaration of Helsinki. Prior to pre-intervention testing and following an information session, participants provided written informed consent for testing and training procedures.
Overview
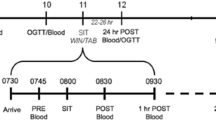
Participants attended testing sessions at standardised times (06:00–09:00) before and after the 9-week intervention. Each testing session comprised a resting blood pressure (BP) measurement, a venous blood sample, anthropometrical measurements, and a maximal graded exercise test (GXT). Training protocols were performed 3 days per week for 9 weeks (Table 2), while CTRL subjects were asked to continue their normal physical activity and nutrition behaviours, with ongoing reminders provided by the research team. Participants refrained from any physical activity for the 24 h prior to each testing session and arrived following an overnight fast (10–12 h). Participants documented food intake and physical activity for the 24 h preceding baseline testing. This document was copied and returned to participants before post-intervention testing to ensure that diet and exercise patterns were standardised before each testing session.
Procedures
Anthropometric measures included height, mass, and waist and hip girths, and were used to calculate body mass index (BMI) and waist-to-hip ratio (WHR). After 10 min of seated rest, BP was measured in the seated position using an aneroid sphygmomanometer and stethoscope (Livingstone, Rosebery, Australia). Participants then performed a GXT using a mechanically braked cycle ergometer (Wattbike Pro, Nottingham, United Kingdom) to determine VO2peak and peak power output (PPO). Participants began the test at 25 W, and increased power output by 25 W each minute until volitional exhaustion. Heart rate (HR) was recorded at each increment to determine HRmax (FT7, Polar Electro, Kempele, Finland). Oxygen consumption was determined by measuring O2 and CO2 concentrations with a metabolic gas analyser (Medgraphics Ultima System, Saint Paul, USA). The metabolic cart was calibrated according to the manufacturer’s instructions and involved pneumotachometer calibration via a 3 L syringe, analysis of ambient air, and gas calibration with a gravimetric gas mixture of known concentrations [CO2 4.1 (0.1)%; O2 15.7 (0.2)%].
Prior to the GXT, venous blood samples were collected using a 21-gauge needle inserted into the medial antecubital vein. Approximately 6 mL of blood was collected in both a serum separator tube (SST) and an ethylene diamine tetraacetic acid (EDTA) tube for analysis of CRP and TNF-α, respectively. EDTA tubes were immediately centrifuged at 3500 rpm for 10 min at 4 °C, whilst SST clotted for 15–30 min before being centrifuged in the same manner. Supernatants were immediately stored at −25 °C. Plasma CRP concentrations were measured using a solid-phase, chemiluminescent immunometric assay (intra- and inter-assay CV 4.1 and 7.1%, respectively), and TNF-α was measured with a sandwich enzyme immunoassay technique, as per the manufacturer’s instructions (Luminex Corporation, Texas, USA), with intra- and inter-assay CV 2.6 and 13.0%, respectively.
All training sessions were performed in a climate-controlled (20 ± 2 °C) exercise physiology laboratory on a mechanically braked cycle ergometer (Wattbike Pro, Nottingham, United Kingdom). Sessions began with a 4 min standardised warm-up at 35% individualised PPO. Participants then commenced their respective protocol with 2–6 members of their own group. The respective protocols were matched for total sprint duration, though involved different recovery durations and intensities (Table 2). Specifically, the HIIT group performed 30 s maximal sprints (20 s in week 1) interspersed with 3–5 min passive recovery periods, as has previously been reported (Burgomaster et al. 2008; Whyte et al. 2010). The PIST group performed 10 s maximal efforts, interspersed with moderate-intensity recovery (75–80% HRmax) of 2–3 min, reflecting the undulating intensities of football-based SSG’s, which range between work:rest ratios of 1:12 and 1:16 (Gabbett and Mulvey 2008). For both conditions, total sprint volume increased progressively throughout the program and was matched between groups. During training, HR (FT7, Polar Electro, Kempele, Finland) was monitored and reported as mean and peak values. Upon finishing each session, participants provided a CR-10 rating of perceived exertion (RPE) (Borg 1998).
Statistical analysis
Male and female data within each condition are pooled and reported as mean ± standard deviation (SD). Normal distribution was determined by the Shapiro–Wilk test; non-normally distributed data were logarithmically transformed before analysis. Raw data were used to assess group × time interaction and a main effect of time using a mixed-model ANCOVA, adjusting for sex as a covariate. When significant interactions or main effects were observed, simple main effects and post hoc analyses using Tukey’s pairwise comparisons were used where appropriate to locate the source of significance. A one-way ANCOVA, adjusting for sex as a covariate, was used to determine whether the absolute changes in each variable differed between groups. Significance was accepted as p ≤ 0.05. An a priori power analysis was completed using G*Power (G*Power for Windows, version 3) based on data from previous studies. Output parameters indicate a sample size of n = 38 to provide an actual power of 0.81.
Results
Adherence to training was not significantly different between training groups (HIIT 95 ± 8%, PIST 94 ± 7%; p = 0.359). There was a significant effect of time for RPE (p = 0.019), with increased values reported over the 9 week intervention for both training groups, without significant differences between groups in absolute change (p = 0.141; Table 2). A group × time interaction was evident for increased mean HR (p = 0.001) observed in PIST compared to HIIT (137 ± 10 vs 120 ± 10 bpm, p = 0.001). Furthermore, a significant group × time interaction was evident for peak HR (p = 0.003), though change data revealed that both groups increased over time, without significant differences between groups (p = 0.339).
All raw and change data for inflammatory markers, VO2peak, PPO, and anthropometrical variables are shown in Table 1. Neither TNF-α nor CRP showed a significant group × time interaction (TNF-α, p = 0.623; CRP, p = 0.081) or main effect for time (TNF-α, p = 0.245; CRP, p = 0.152). There was a significant group × time interaction for VO2peak (p = 0.010) with HIIT and PIST showing increased VO2peak compared to CTRL (HIIT p = 0.014; PIST p = 0.020), without significant differences between training groups (p = 0.989). There was no group × time interaction for PPO (p = 0.231); however, there was a main effect of time (p = 0.0001), which was evident for all groups (HIIT p = 0.0001; PIST p = 0.0001; CTRL p = 0.012). There was no significant group × time interaction or main effect of time for body mass, BMI, hip girth, or BP (p > 0.05; Table 1). For waist girth, there was a significant group × time interaction (p = 0.042) and significant main effect for time (p = 0.026). The absolute change was significant, in that HIIT was significantly greater than CTRL (p = 0.034). For WHR, there was a significant group × time interaction (p = 0.003) and significant main effect for time (p = 0.009); however, the absolute change was only significant in HIIT compared to CTRL (p = 0.005).
Discussion
Nine weeks of HIIT and PIST training were equally effective to improve maximal oxygen consumption. Specifically, participants improved VO2peak in both training groups, with no changes observed in CTRL. Despite improved VO2peak, no significant differences in TNF-α or CRP were evident within or between groups. The HIIT group demonstrated greater reductions in waist girth and WHR compared to CTRL, although there were no changes in hip girth. Consequently, the matching of high-intensity work resulted in similar VO2peak adaptations, regardless of the sprint distribution (10 vs 30 s) or intensity of recovery (active vs passive). Thus, despite no changes in markers of CSI, and with the exception of waist girth, the total volume of high-intensity work performed seems a more important component for physiological adaptations in sedentary individuals.
Notably, similar adherence rates between training groups ensured a similar training exposure for the respective programs. As evidence of the success of high-intensity training in this population, adherence values were similar to those reported previously with interval-based training in middle-aged adults (Jung et al. 2015). Peak HR did not differ between training conditions, likely due to both modes being of sufficient sprint duration and intensity to invoke the same maximal cardiac response. The previous studies using HIIT have reported similar peak HR values (90–95% HRmax) (Helgerud et al. 2007; Jung et al. 2015). However, as expected, the active nature of recovery in PIST ensured a greater mean HR than HIIT, showing values reflective of SSG training (~75–80% HRmax) (Andersen et al. 2010, 2014). Regardless, both groups successfully engaged in similar training programs, albeit with differing sprint and recovery profiles.
Moderate-intensity aerobic training (60–85% VO2max) is suggested to reduce CRP (Berg and Scherer 2005), yet no changes were evident following the higher intensities used in the present study. In explanation, a number of studies have reported reductions in CRP that coincide with reductions in body fat (Arikawa et al. 2011) and body mass (Martins et al. 2010). Specifically, exercise-induced reductions in CRP are suggested to occur only when reductions in adiposity are evident (Church et al. 2010). Given that fat mass was not quantified in the present study, and rather inferred from BMI, it is unknown whether changes in fat occurred following training, and thus whether this response precluded changes in CRP. Moreover, the mean baseline CRP level for this cohort was not classified as high (2.31 mg L−1) (Pearson et al. 2003), and by example, Pearson et al. (2003) reported that a basal CRP >3 mg L−1 doubled an individual’s risk of cardiovascular disease compared to a concentration <1 mg L−1. Given the moderate CRP values evident here, it is possible that the potential for reduction was, therefore, minimal.
With regards to TNF-α, there were no significant within- or between-group changes, which is similar to previous studies utilising aerobic exercise of varying intensities. Mendham et al. (2014) reported no change in TNF-α after 8 weeks of either SSG’s or moderate-intensity cycling, despite a reduction in fat mass in both groups. Conversely, 8 weeks of aerobic exercise combined with moderate caloric restriction elicited reductions in TNF-α and BMI in overweight adolescents (Ben Ounis et al. 2009). Furthermore, Kohut et al. (2006) reported that both moderate-intensity aerobic and flexibility/strength exercises were effective in reducing TNF-α over 10 months, with a trend (p = 0.10) towards reduced BMI. As with CRP, reductions in TNF-α are associated with reductions in body mass and fat, particularly given that visceral adipose tissue is a known site of TNF-α secretion (Kadoglou et al. 2007). Despite the hypothesis that high-intensity training would reduce TNF-α, it would appear that longer interventions incorporating load-bearing strategies are most effective in this regard.
Both PIST and HIIT increased VO2peak following training. The PIST protocol improved VO2peak by 14%, which is similar to studies utilising SSG’s (Andersen et al. 2014; Krustrup et al. 2009). For example, Krustrup et al. (2009) reported a 13% increase in VO2max after 12 weeks of SSG’s in untrained men, while Andersen et al. (2014) observed an 11% improvement after 24 weeks of SSG training in adults with T2D. In the present study, the HIIT group also demonstrated a 14% increase in VO2peak, which is similar to other HIIT protocols in healthy populations (Burgomaster et al. 2008; Whyte et al. 2010). Comparatively, Whyte et al. (2010) reported a 9.4% increase in VO2max after six sessions of ‘all-out’ HIIT in sedentary men, and Burgomaster et al. (2008) observed a 7.3% improvement after 6 weeks of HIIT in sedentary adults. The greater improvement in VO2peak observed in the current study may be explained by the longer training duration and low baseline fitness. However, Sloth et al. (2013) noted that the previous studies showed no relationship between program duration and the magnitude of change in VO2max, hypothesising that large improvements occur in the early stages of HIIT, and the rate of adaptation diminishes thereafter. Nonetheless, these findings reiterate that high-intensity intermittent exercise is effective in improving aerobic capacity in sedentary populations. Notably, there was no difference between HIIT and PIST for changes in VO2max, suggesting that the distribution of sprints and the active or passive recovery periods are inconsequential provided that total duration and intensity of sprints are similar. Such outcomes may have practical implications for exercise prescription in sedentary populations, promoting training variety to aid long-term exercise adherence.
Finally, with regards to anthropometry, HIIT showed a greater reduction in waist girth and WHR compared to CTRL. These outcomes concur with findings by Whyte et al. (2010), who reported reductions in waist and hip girths after only 2 weeks of HIIT (30 s sprints, 4–6 repetitions) in obese men. Although the present study involved participants who were not classified as obese, and whose baseline hip girths (105.92 ± 9.04 cm) were lower than those of the participants in the aforementioned study (110.9 ± 2.2 cm), similar effectiveness was evident. With regards to PIST, Mendham et al. (2014) reported no change in waist or hip girths following 8 weeks of SSG’s in middle-aged, sedentary men; however, participants did demonstrate improvements in body composition. These outcomes may result from the load-bearing, eccentric element of SSG’s, indicating that field-based SSG’s confer an effect that PIST does not. As surmised above, HIIT was a more effective modality to reduce waist circumference; however, there were no differences in body mass or BMI, indicating that a longer, load-bearing training program may provide more significant changes in anthropometrical parameters.
Despite the above findings, some limitations are acknowledged within the present study. First, it was not possible to match energy cost between training groups. Although the total time spent at high intensity was equal between HIIT and PIST, differences in recovery intensities meant that the energy cost difference is a theoretical limitation. In addition, the small increase in PPO without concomitant increase in VO2peak in CTRL suggests a familiarisation effect occurred with this test, which may have confounded these results. In addition, equipment issues, including those pertaining to analysis kits, resulted in the loss of two additional cytokines (IL-6 and IL-1β), which would have offered further insight into changes following exercise training. Particularly, IL-6 would be a prudent inclusion given its antecedent relationship with CRP (Berg and Scherer 2005), and IL-1β would be beneficial alongside IL-1 receptor antagonist (IL-1ra) as the primary function of the latter is to inhibit IL-1 binding, and thus, concurrent analysis would provide greater insight into training adaptations (Ridker et al. 2011). Furthermore, the menstrual cycle was not reported in the present study, which is also noted as a limitation. However, the associated fluctuations in CRP are small in magnitude, ranging from 0.18 to 0.38 mg L−1 (Blum et al. 2005; Jilma et al. 1997). Comparatively, training-induced reductions in CRP have been shown to be much greater, with changes as large as 1.41 mg L−1 (Arikawa et al. 2011) and 1.99 mg L−1 (Martins et al. 2010) observed in sedentary individuals. In addition, it is suggested that there is no cyclical pattern of change for TNF-α throughout the menstrual cycle (Jilma et al. 1997), and furthermore, current research examining pre–post-menopausal differences in TNF-α is equivocal. Finally, although Godin’s Leisure Time Exercise Questionnaire (Godin and Shephard 1985) was used to assess the ‘sedentary’ status of participants prior to the study period, lifestyle behaviours outside of the training intervention were monitored only through regular personal communication between participants and researchers. This is acknowledged as a potential limitation to the study; however, the authors believe that this method of monitoring was sufficient to ensure compliance with instructions and thus prevent any lifestyle changes that may have biased results.
Conclusions
Interval-based training may be effective to improve cardio-metabolic risk factors, namely aerobic capacity and WHR, though no changes in CRP or TNF-α were evident. Furthermore, the lack of difference in inflammatory outcomes following HIIT and PIST suggests that sprint distribution and recovery intensity were not of primary consequence when high-intensity volume is matched. Therefore, provided that total sprinting time does not change, sprint duration and recovery intensity can be manipulated without impacting upon these outcomes. To support such an assertion, future research should consider the effects of HIIT and PIST on a wider array of cytokines, alongside comparisons to continuous exercise modes.
Abbreviations
- ANCOVA:
-
Analysis of covariance
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CRP:
-
C-reactive protein
- CSI:
-
Chronic systemic inflammation
- CTRL:
-
Control group
- CV:
-
Coefficient of variation
- EDTA:
-
Ethylene diamine tetraacetic acid
- GXT:
-
Graded exercise test
- HIIT:
-
High-intensity interval training
- HR:
-
Heart rate
- IL:
-
Interleukin
- PIST:
-
Prolonged intermittent sprint training
- PPO:
-
Peak power output
- RPE:
-
Rating of perceived exertion
- SSG:
-
Small-sided games
- SST:
-
Serum separator tube
- T2D:
-
Type 2 diabetes
- TNF-α:
-
Tumor necrosis factor alpha
- VO2peak :
-
Peak oxygen consumption
- WHR:
-
Waist-to-hip ratio
References
Andersen LJ et al (2010) Football as a treatment for hypertension in untrained 30–55-year-old men: a prospective randomized study. Scand J Med Sci Sports 20:98–102
Andersen TR et al (2014) A preliminary study: effects of football training on glucose control, body composition, and performance in men with type 2 diabetes. Scand J Med Sci Sports 24:43–56
Arikawa AY, Thomas W, Schmitz KH, Kurzer MS (2011) Sixteen weeks of exercise reduces C-reactive protein levels in young women. Med Sci Sports Exerc 43:1002–1009
Babraj J, Vollaard N, Keast C, Guppy F, Cottrell G, Timmons J (2009) Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord 9:3–11
Ben Ounis O et al (2009) Two-month effects of individualized exercise training with or without caloric restriction on plasma adipocytokine levels in obese female adolescents. In: Annales d’Endocrinologie (Paris), vol. 4. pp 235–241
Berg A, Scherer P (2005) Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96:939–949
Blum CA et al (2005) Low-grade inflammation and estimates of insulin resistance during the menstrual cycle in lean and overweight women. J Clin Endocrinol Metab 90:3230–3235
Borg G (1998) Borg’s perceived exertion and pain scales. Human Kinetics, Champaign, USA
Burgomaster K, Howarth K, Phillips S, Rakobowchuk M, MacDonald M, McGee S, Gibala M (2008) Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586:151–160
Church T, Earnest C, Thompson A, Priest E, Rodarte R (2010) Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med Sci Sports Exerc 42:708–716
Gabbett TJ, Mulvey MJ (2008) Time-motion analysis of small-sided training games and competition in elite women soccer players. J Strength Cond Res 22:543–552
Gibala M, Little J, MacDonald M, Hawley J (2012) Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 590:1077–1084
Godin G, Shephard R (1985) A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 10:141–146
Helgerud J et al (2007) Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc 39:665–671
Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE (2004) Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol 96:101–106
Jilma B et al (1997) Menstrual cycle-associated changes in blood levels of interleukin-6, α1 acid glycoprotein, and C-reactive protein. J Lab Clin Med 130:69–75
Jung M, Bourne J, Beauchamp M, Robinson E, Little J (2015) High-intensity interval training as an efficacious alternative to moderate-intensity continuous training for adults with prediabetes. J Diabetes Res 2015:1–9
Kadoglou N, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis C, Alevizos M (2007) The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Euro J Cardiovasc Prev Rehabil 14:837–843
Kohut M et al (2006) Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of β-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun 20:201–209
Krustrup P et al (2009) Recreational soccer is an effective health-promoting activity for untrained men. Br J Sports Med 43:825–831. doi:10.1136/bjsm.2008.053124
Martins R, Neves A, Coelho-Silva M, Veríssimo M, Teixeira A (2010) The effect of aerobic versus strength-based training on high-sensitivity C-reactive protein in older adults. Eur J Appl Physiol 110:161–169
Mendham AE, Duffield R, Marino F, Coutts AJ (2014) Small-sided games training reduces CRP, IL-6 and leptin in sedentary, middle-aged men. Eur J Appl Physiol 114:2289–2297
Pearson T et al (2003) Markers of inflammation and cardiovascular disease application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation 107:499–511
Pradhan A, Manson J, Rifai N, Buring J, Ridker P (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334
Ridker P, Thuren T, Zalewski A, Libby P (2011) Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 162:597–605
Sloth M, Sloth D, Overgaard K, Dalgas U (2013) Effects of sprint interval training on VO2max and aerobic exercise performance: a systematic review and meta-analysis. Scand J Med Sci Sports 23:e341–e352
Stewart L, Earnest C, Blair S, Church T (2010) Effects of different doses of physical activity on C-reactive protein among women. Med Sci Sports Exerc 42:701–707
Sylta Ø, Tønnessen E, Hammarstrom D, Danielsen J, Skovereng K, Ravn T, Ronnestad BR, Sandbakk O, Seiler S (2016) The effect of different high-intensity periodization models on endurance adaptations. Med Sci Sports Exerc. doi:10.1249/MSS.0000000000001007
Whyte LJ, Gill JM, Cathcart AJ (2010) Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metab Clin Exp 59:1421–1428
Wisløff U et al (2007) Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115:3086–3094
You T, Arsenis NC, Disanzo BL, LaMonte MJ (2013) Effects of exercise training on chronic inflammation in obesity. Sports Med 43:243–256
Acknowledgements
The authors wish to acknowledge the staff at Charles Sturt University and Bathurst Base Hospital for their contribution to blood analysis. They would also like to acknowledge the laboratory staff at the University of Technology Sydney for providing support in data collection. Finally, the authors thank all participants for their involvement and efforts in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Anni Vanhatalo.
Rights and permissions
About this article
Cite this article
Allen, N.G., Higham, S.M., Mendham, A.E. et al. The effect of high-intensity aerobic interval training on markers of systemic inflammation in sedentary populations. Eur J Appl Physiol 117, 1249–1256 (2017). https://doi.org/10.1007/s00421-017-3613-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3613-1