Abstract
The purpose of the study was to characterize the changes in purine metabolism in long-distance runners in the main phases of their 1-year training cycle. Nine male athletes competing in distances 5 and 10 km at national/regional level, mean age 22.9 ± 0.6 years, practising sport for 8.6 ± 0.3 years, participated in the study. The changes in plasma concentrations of hypoxanthine (Hx), xanthine (X) and uric acid (UA) and the activity of the enzyme HGPRT in red blood cells haemolysate were followed in four characteristic points of the annual training cycle: preparatory phase (specific subphase), competition period, transition period and preparatory phase (intermediate subphase). Resting and postexercise plasma concentrations of X and, Hx and HGPRT activity changed significantly during 1-year training cycle. Significant changes in postexercise Hx values between training phases were found, from 9.3 μmol l−1 in competition period to 22.9 μmol l−1 in transition period (Friedmann’s ANOVA, P < 0.01). Postexercise UA values ranged from 371 to 399 μmol l−1 and did not change significantly between training phases. An increase in resting (from 52.0 to 58.4 IMP mg−1 Hb min−1, P < 0.05) and postexercise (from 70.7 to 76.2 IMP mg−1 Hb min−1, not significant) HGPRT activity between the specific preparation and competition period was observed. In the transition period, Hx postexercise concentration increased (22.9 μmol l−1, P < 0.01) and HGPRT postexercise activity decreased (58.8 IMP mg−1 Hb min−1, P < 0.01) significantly. The results indicate that the level of plasma Hx at rest and after standard exercise may be a useful tool for monitoring the adaptation of energetic processes in different training phases and support the overload/overtraining diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenosine 5′ triphosphate (ATP) is degraded during exercise. As a result of a reaction catalyzed by adenylate kinase, ATP and inosine monophosphate (IMP) are formed from adenosine 5′ diphosphate (ADP). Depending on conditions and cell type, AMP deaminates to IMP (in skeletal muscle) or dephosphorylates to adenosine (in cardiac muscle). Inosine monophosphate, the concentration of which increases in muscle cells (Tullson et al. 1990), may undergo further transformations that result in formation of inosine.
Inosine is degraded by purine nucleoside phosphorylase (PNP) to hypoxanthine (Hx), which can efflux the muscle and be lost from the adenine nucleotide pool or be salvaged intramuscularly by enzyme hypoxanthine-guanine phosphoribosyl transferase (HGPRT; Fig. 1). The conversion of Hx to xanthine (X) and further to uric acid (UA) is catalyzed by enzyme xanthine dehydrogenase (XDH) which may act also as xanthine oxidase (XOD) under hypoxic conditions, especially in ischaemia-related hypoxia (Jarasch et al. 1986; Parks and Granger 1986). Xanthine oxidase reaction is connected with the formation of free radicals (superoxide anions) (Sahlin et al. 1991; De Bono 1994; Hellsten et al. 1997). Uric acid, a final compound of purine metabolism, is an antioxidant (Becker 1993) characterized by continuous slow increase of plasma concentration long after the termination of exercise. A schematic view of adenine nucleotides metabolic pathways is illustrated in Fig. 1.
In normal resting conditions, 95% of daily Hx turnover are converted to purine nucleotides via resynthesis and only 5% are excreted in urine (Murray 1971). Hypoxanthine is the only substance that may be re-converted and re-utilized in the nucleotide pool after being catalyzed by HGPRT. The activity of this enzyme plays the important role in maintaining ATP content effectively (purine synthesis de novo is relatively more energy consuming), and in the prevention of the formation of damaging free radicals in the subsequent degradation of Hx. In addition to muscle, HGPRT exists in the red blood cells and the brain (Rychlewski et al. 1994).
Hypoxanthine is considered to be an indicator of histotoxic hypoxia for a long time (Sutton et al. 1980; Banaszak and Rychlewski 1992). It is also regarded as a marker of adenine nucleotide degradation in muscle, a marker of energetic stress during exercise (Sahlin et al. 1999), an index of exercise intensity (Rychlewski et al. 1997), and it may be used in the classification of physical exercise (Bianchi et al. 1999).
During a moderate-intensity prolonged exercise, plasma Hx concentration increases but varies greatly between subjects and there are no significant changes after 5 min of recovery (Sahlin et al. 1999). We know from earlier research that Hx blood concentration increases after exercise, reaches maximum value after 10–20 min (Harkness et al. 1983; Hellsten-Westing et al. 1989; Sahlin et al. 1991) and is 2- to 4-fold higher than measured immediately after exercise. Rychlewski et al. (1997) found a significant correlation between an increased concentration of Hx in blood plasma and changes in the pH and base excess after physical exercise in canoeists and swimmers. Sjödin and Hellsten-Westing (1990) proved that a critical point of Hx is observed at exercise intensity 107–115% of maximal oxygen uptake (VO2max). After this threshold had been exceeded, a rapid increase in plasma Hx concentration was observed. A test run below this threshold did not entail such radical changes. Ketai et al. (1987) showed, that rapid increase in Hx concentration occured at intensity below 90% of VO2max. However, the discrepancy may result from different experimental protocols. These studies also show that the relationship between Hx and exercise intensity may be “shifted” to the right in competitive athletes like in the case of ventilation threshold (Sjödin et al. 1982).
Plasma Hx concentration increases also significantly (15- to 40-fold) after a maximal intensity intermittent sprint exercise repeated every 30 s, but the physiological responses are markedly influenced by sprint distance (Balsom et al. 1992). After 15-m sprints, the mean pre- and post-exercise Hx concentrations did not differ significantly, while postexercise values were significantly higher after 30- and 40-m sprints.
After 6 weeks of a high-intensity intermittent training, the level of Hx at rest and after exercise decreases considerably and HGPRT activity increases in habitually active males. It reflects muscle adaptation that leads to reduced loss of adenine nucleotides (Hellsten-Westing et al. 1993a, b). Lowered concentration of plasma purines after training allows athletes to exercise more intensively without the loss of purine nucleotide. The study on the effects of a 7-week field hockey-specific training on repeated-sprint ability, plasma Hx concentration and other blood parameters in 18 elite female field hockey players revealed a significant decrease in plasma Hx concentration (posttest minus rest values) (Spencer et al. 2004). The authors suggest that one adaptation of sport-specific repeated-sprint training may be to conserve the purine nucleotide pool. Moreover, the study by Stathis et al. (1994) demonstrates that inosine concentration, the precursor to Hx, is lower in the muscle following training as is the postexercise plasma Hx concentration after a 7-week sprint training. This provides evidence that Hx production and/or efflux from the muscle are reduced.
There are very few studies concerning the effect of endurance training on purine metabolism. Baldwin et al. (2000) compared endurance in trained and non-trained men who cycled for 60 min at a work rate of 70% VO2 peak or at 95% of lactate threshold. There was a tendency for plasma Hx to be higher in non-trained subjects at 70% of VO2 peak compared with trained subjects. However, plasma markers of metabolic stress were similar in both trained and non-trained group during exercise at 95% of lactate threshold.
Investigations that have been conducted so far relate mainly to single exercise or short programmes of sprint training that lasted for few weeks. The authors of the present study are interested in the time course of purine changes during a longer training period. We presume that the analysis of changes in enzyme activity and plasma oxypurine concentration in the course of a real sport training may allow the use these biochemical parameters as an assessment of physical efficiency and metabolic response to sports training. Proportions of anaerobic exercise and thus possible increase of the metabolic stress on the adenine nucleotide pool in the muscle change considerably during subsequent training phases in endurance athletes. Thus, the aim of our study is to characterize the changes in purine metabolism in long distance runners in the main phases of their 1-year training cycle. We followed the changes in plasma concentrations of Hx, X and UA as well as the activity of the enzyme HGPRT in red blood cells haemolysate.
Methods
Subjects
Nine male long-distance Polish runners (specialized in distances: 5 and 10 km) competing at national and regional level, mean age 22.9 ± 0.6 (20–26) years, practising competitive sport for 8.6 ± 0.3 years, body mass 64.5 ± 14 kg, body height 177 ± 1.5 cm, maximal oxygen consumption (VO2max) 67.3 ± 1.8 ml kg−1 min−1, maximum heart rate 199 ± 1 beat min−1, average levels of haemoglobin 15.2 ± 0.6 g dl−1 at rest and 15.5 ± 0.5 g dl−1 postexercise at the beginning of the study, participated in the research. No significant changes in body mass and height were observed during the study. Postexercise level of haemoglobin changed significantly between examinations (ANOVA = 8.1, P < 0.05) decreasing to a minimum value 14.6 ± 0.9 g dl−1 in the competition period. The aim of the research and testing methodology were explained to all subjects who gave their informed consent prior to their inclusion in the study. The project has been approved by Ethics Committee at the Karol Marcinkowski Medical University in Poznań and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Examinations
The study procedure was adapted to the annual plan of examined runners. The 1-year training cycle consisted of three main phases: preparatory, competitive and transition phase (Bompa 1999). Preparatory phase included three subphases. The aim of the general subphase was the development of general endurance on the basis of aerobic training means, improvement of running technique and progress in general fitness. In the intermediate subphase, athletes aimed at the development of running endurance using aerobic–anaerobic training means. Specific subphase enabled athletes to develop specific endurance and specific physical fitness on the basis of interval and speed runs with predominance of anaerobic exercises. Competitive phase was characterized by decreasing training volume and increasing training intensity. Runners competed on their specialized and related distances, peaking their athletic shape. In the transition phase, training loads were small and the aim was physical and psychological regeneration and recovery from injuries. Athletes played team games, swam and did activity forms other than typical endurance running. Four consecutive examinations had been planned and performed in four characteristic points of the annual training cycle.
First examination
15–16 of March, specific subphase of the preparatory phase, 147 ± 3.0 training sessions from the beginning of the 1-year training cycle, in total 213 h:56 min:15 s ± 2 h:26 min:45 s net training time. Five main exercise zones, corresponding to estimates of the sources of energy for ATP resynthesis, were used within this total time in the following proportion: 73.0% in aerobic compensation zone; 20.6% in aerobic stimulation zone; 4.8% in combined aerobic–anaerobic zone; 0.9% in anaerobic lactacid zone and 0.7% in anerobic non-lactacid zone.
Second examination
16–17 of June, competition period, 93 ± 1.6 training sessions from the first examination, in total 118 h:16 min:51 s ± 3 h:25 min:48 s net training time. Subjects participated among others in the Polish Athletics Championships. Exercise zones proportion was 59.3:26.4:10.7:2.3:1.3%, respectively.
Third examination
9–10 of November, transition period, 135 ± 1.7 training sessions from the second examination, in total 188 h:01 min:59 s ± 2 h:20 min:10 s net training time. Exercise zones proportion was 63.1:25.9:8.5:1.6:0.9%, respectively.
Fourth examination
2–3 of February, the next 1-year training cycle, the end of the general preparation (intermediate subphase) of the preparatory phase, 91 ± 1.2 training sessions from the third examination, in total 181 h:15 min:03 s ± 4 h:29 min:41 s net training time. Exercise zones proportion was 77.6:18.8:2.2:0.7:0.7%, respectively.
Maximal oxygen uptake
All tests were conducted in the laboratory of the Department of Physiology of the University School of Physical Education in Poznań, in the morning, 2 h after consuming a light breakfast (bread and butter, water, without coffee or tea). Incremental running treadmill test (Woodway ES1, Waukesha, USA) was conducted. Athletes started at a speed of 10 km h−1. Subsequently, the travel speed of the moving strip was progressively increased by 2 km h−1 every 3 min until volitional exhaustion. Respiratory parameters (VE, VO2, VCO2) were measured continuously by means of CPX-D computer system (Medical Graphics Corporation, St. Louis, USA). Heart rate was recorded every 5 s with a Polar Accurex Plus device (Polar Elektro, Finland).
Lactic acid
Capillary blood samples were obtained from the finger tip immediately before the exercise (at rest) and 3 min after exercise. Lactic acid (LA) concentrations were assayed enzymatically using a spectrophotometric Warburg method (Gutmann and Wahlenfeld 1974) that consists in measuring the increase in absorbance of NADH at wavelength 365 nm. A Marcel Media spectrofotometer (Poland) was used.
Hypoxanthine, xanthine, uric acid, HGPRT
Venous blood samples (5 ml) were taken from an antecubital vein at rest, immediately before the test exercise and 5 min after the exercise in order to obtain plasma and red blood cells. The plasma obtained was deproteinized with 1.2 mol l−1 HClO4 and centrifuged. An acid supernatant was neutralized with 1 mol l−1 K2CO3 and centrifuged, and stored at −80°C before analysis. Blood cells were bathed three times in isotonic salt solution (0.9% NaCl), then haemolysed with a hypotonic solution of Tris buffer (hydroxymethylaminomethane), 10 mmol l−1, pH 7.4. The prepared haemolysate was stored at −20°C before analysis for HGPRT activity.
Hypoxanthine, X and UA were assayed in a neutralized plasma extract by means of high-performance liquid chromatography (HPLC) according to the methodology of Wung and Howell (1980) modified by Banaszak (1999). Hewlett-Packard 1050 apparatus with UV detector (Ramsey, USA) was used. Separation was achieved by a Hypersil ODS 100 mm × 4.6 mm × 5 μm column and a Hypersil ODS 20 mm × 4 mm × 5 μm precolumn manufactured by Alltech (Deerfield, USA). The carrier phase was a buffer consisting of 1% methanol and 4% 100 mmol l−1 KH2PO4 (potassium phosphate), pH 5.8. The flow rate was 1.0 ml min−1. Substances were identified by comparing retention times with model UA, Hx, X and IMP compounds at known standard concentrations. In this system, UA, Hx and X were separated at retention times of about 2, 3 and 4 min, respectively. The measurement was done at wavelength 254 nm for Hx and X, and 280 nm for UA. The quantitative sample analysis was performed on the basis of a comparison of retention times and concentrations with standardized solutions.
HGPRT activity was measured in red blood cell according to the procedure described by Stolk et al. (1995) modified by Banaszak (1999). The incubation buffer consisted of 50 mmol l−1 Tris pH 7.4, 10 mmol l−1 PRPP, 7 mmol l−1 NaF and 5 mmol l−1 MgCl2. The pre-incubation of the haemolyzate (100 μl) and the buffer lasted for 5 min in a 37°C water bath. The main 15-min incubation started after addition of 100 μl of 2.0 mmol l−1 Hx solution into the pre-incubation mixture in order to initiate the enzymatic reaction. Immediately after Hx was added, 100 μl of incubation mixture was taken and added to 100 μl 1.2 mol l−1 HClO4 (perchloric acid). This sample served as “zero time” sample. The incubation was stopped after 15 min by renewed addition of 100 μl of mixture to 1.2 mol l−1 HClO4. Acid extracts were neutralized with 1 mol l−1 K2CO3 and then used for HGPRT activity measurement by means of HPLC method. The buffer used as carrier phase consisted of 100 mmol l−1 KH2PO4 with the addition of TBAS (tetrabutylammonium sulphate), final concentration 5 mmol l−1 and pH 3.1. Separation was conducted at flow rate 1.2 ml min−1. The mobile phase consisted of 15% of methanol and 50% of buffer pH = 3.1. An ODS Hypersil 150 mm × 4.6 mm × 5 μm column and an ODS Hypersil 20 mm × 4 mm × 5 μm precolumn manufactured by Alltech (Deerfield, USA) were used. The separation time was about 2.0 min for Hx and 5.0 min for IMP. The quantitative sample analysis was performed on the basis of a comparison of retention times with model Hx- and IMP-solutions at wavelength 254 nm. HGPRT activity is expressed as the amount of produced IMP (number of μmol IMP mg−1 Hb min−1) (Ralston et al. 1991). The blood concentration of Hb was measured by Cobas b121 apparatus (Roche, Mannheim, Germany).
Statistics
Values are presented as mean and standard error of the mean (±SEM). Friedmann’s ANOVA was used to compare the longitudinal effect of training during the 1-year cycle. Wilcoxon test was used to assess the differences between related variables obtained from pre- and post-exercise measurements in the same training phase. All calculations were made in the Computation Center of our University School using STATISTICA v. 5.1 software.
Results
Physiological parameters
Mean values of VO2max ranged from 65.0 ± 1.3 ml kg−1 min−1 in the transition period to 68.1 ± 2.0 ml kg−1 min−1 in the competitive phase. Changes in VO2max between examinations were statistically not significant. Nevertheless, they were crucial to training status (peaking athletic shape) and reflected normal desirable endurance fluctuations in the 1-year training cycle. Average distance covered until termination of the test exercise ranged from 2,942 ± 48 m in the transition phase to 3,067 ± 99 m in the competitive period and varied significantly between examinations (ANOVA = 8.9, P < 0.05).
Lactic acid
Mean resting values of LA in the 1-year training cycle ranged from 1.3 ± 0.1 mmol l−1 in the competition phase to 1.7 ± 0.2 mmol l−1 in the general preparation subphase (Fig. 2). Postexercise concentrations varied between 6.2 ± 0.4 mmol l−1 in the transition phase and 7.0 ± 0.5 mmol l−1 in the general preparation subphase. Both resting and postexercise values did not change during the 1-year cycle significantly. The rest–postexercise differences in LA concentrations ranged from 4.7 ± 4.0 mmol l−1 in the transition phase to 5.5 mmol l−1 during the competition period, but the 1-year variability of this parameter was not statistically significant. However, differences between resting and postexercise values were significant in each examined training phase.
Changes in lactic acid blood concentrations (mean and SEM, n = 9) during the annual training cycle in competitive long-distance runners for pre (open circle) and post (closed circle) exercise. Significant differences between successive training phases are denoted with single asterisk (P < 0.05) or double asterisks (P < 0.01) and between pre- and post-exercise values of the same phase with + (P < 0.05) or ++ (P < 0.01)
Xanthine
The lowest average resting X concentration at 1.8 ± 0.1 μmol l−1 was observed in the specific preparation phase and the highest concentration at 2.8 ± 0.1 μmol l−1 was measured in the competition period (Fig. 3). Resting mean values decreased along with consecutive training phases and the differences turned out to be statistically significant (ANOVA = 14.1, P < 0.01). Postexercise X concentrations were also lowest in the specific preparation phase and highest in the competition period averaging 2.5 ± 0.1 μmol l−1 and 3.3 ± 0.1 μmol l−1, respectively. A permanent decline of mean value was noted from the competition period to the general preparation phase. The changes in postexercise X concentration during 1-year cycle were statistically significant (ANOVA = 11.0, P < 0.05). The variability of the difference between resting and postexercise X values in 1-year cycle did not reach statistical significance. This parameter ranged from 0.47 ± 0.1 μmol l−1 in the competition period to 0.9 ± 0.1 μmol l−1 in the general preparation phase and was relatively constant. Nevertheless, resting–postexercise differences were statistically significant in each separate training phase, as demonstrated by means of Wilcoxon test in each training phase (Fig. 3).
Changes in xanthine blood concentrations (mean and SEM, n = 9) during the annual training cycle in competitive long-distance runners for pre (open circle) and post (closed circle) exercise. Significant differences between successive training phases are denoted with single asterisk (P < 0.05) or double asterisks (P < 0.01) and between pre- and post-exercise values of the same phase with + (P < 0.05) or ++ (P < 0.01)
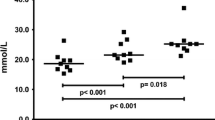
Hypoxanthine
Mean resting values of Hx concentrations ranged from 3.0 ± 0.2 μmol l−1 in the specific preparation phase to 4.4 ± 0.4 μmol l−1 in the transition phase (Fig. 4). The most visible change occurred between these two phases. The variability of resting Hx values along with training phases was statistically significant (ANOVA = 10.0, P < 0.05). Average postexercise values changed during the 1-year cycle within a very wide range from, 9.3 ± 0.6 μmol l−1 in the competition period to 22.9 ± 2.1 μmol l−1 in the transition phase. The analysis of variance revealed statistical significance of these changes (ANOVA = 20.5, P < 0.01). Average resting–postexercise differences changed also considerably during the annual training cycle (ANOVA = 19.8, P < 0.01). Highest difference was noted in the transition phase (18.5 ± 1.9 μmol l−1, Wilcoxon test = 2.7, P < 0.01) and the lowest, but still significant difference, in the competition period (6.1 ± 0.7 μmol l−1, Wilcoxon test = 2.7, P < 0.01).
Changes in hypoxanthine blood concentrations (mean and SEM, n = 9) during the annual training cycle in competitive long-distance runners for pre (open circle) and post (closed circle) exercise. Significant differences between successive training phases are denoted with single asterisk (P < 0.05) or double asterisks (P < 0.01) and between pre- and post-exercise values of the same phase with + (P < 0.05) or ++ (P < 0.01)
Uric acid
Resting concentration of UA averaged from the lowest level in the general preparation period (264 ± 17 μmol l−1) to the highest value in the specific preparation period (331 ± 13 μmol l−1). Postexercise values ranged from 371 ± 17 μmol l−1 in the general preparation phase to 399 ± 14 μmol l−1 in the specific preparation phase (Fig. 5). The changes between training phases were not significant (at rest: ANOVA = 3.5, not significant; postexercise: ANOVA = 2.2, not significant). However, a trend towards lower UA concentrations during the yearly training cycle and a single significant difference between resting values in the specific preparation and the transition period are to be noticed (331 vs. 277 μmol l−1, Wilcoxon test = 2.1, P < 0.05). The resting–postexercise difference changed significantly during the whole training cycle (ANOVA = 8.6, P < 0.05). Similarly, resting–postexercise differences in each separate examination were statistically significant and they were decreasing to a minimum in the specific preparation phase (67 ± 22 μmol l−1, Wilcoxon test = 2.7, P < 0.01) and reaching maximum value in the transition phase (114 ± 21 μmol l−1, Wilcoxon test = 2.7, P < 0.01).
Changes in uric acid blood concentrations (mean and SEM, n = 9) during the annual training cycle in competitive long-distance runners for pre (open circle) and post (closed circle) exercise. Significant differences between successive training phases are denoted with single asterisk (P < 0.05) or double asterisks (P < 0.01) and between pre- and post-exercise values of the same phase with + (P < 0.05) or ++ (P < 0.01)
HGPRT
Resting HGPRT activity averaged from 50.6 ± 1.1 IMP mg−1 Hb min−1 in the transition phase to 61.0 ± 3.0 IMP mg−1 Hb min−1 in the general preparation period (last examination, Fig. 6). The changes were significant (ANOVA = 13.4, P < 0.01). Postexercise HGPRT activity ranged from 58.8 ± 1.7 IMP mg−1 Hb min−1 in the transition phase to 76.2 ± 1.7 IMP mg−1 Hb min−1 in the competition period. The values varied also significantly with successive training phases (ANOVA = 14.5, P < 0.01). Resting–postexercise differences did not undergo considerable changes during 1-year training cycle but still remained significant in separate examinations. The largest difference between resting and postexercise values was observed in the specific preparation phase (18.7 IMP mg−1 Hb min−1, Wilcoxon test = 2.7, P < 0.01) and the smallest in the transition phase (8.22 IMP mg−1 Hb min−1, Wilcoxon test 2.7, P < 0.01).
Changes in HGPRT blood concentrations (mean and SEM, n = 9) during the annual training cycle in competitive long-distance runners for pre (open circle) and post (closed circle) exercise. Significant differences between successive training phases are denoted with single asterisk (P < 0.05) or double asterisks (P < 0.01) and between pre- and post-exercise values of the same phase with + (P < 0.05) or ++ (P < 0.01)
Discussion
The research that has been conducted so far has focused on the effect of single exercise sessions or short training programmes in subjects practising different types of sport and in the non-trained. In our study, we analysed the effect of an 1-year training cycle on the purine metabolism in individuals with a high level of physical efficiency (competitive long-distance runners). We believe that our results demonstrate that changes in purines could be an assessment of the metabolic response to sports training.
According to Green et al. (1995), endurance training reduces the increase of muscle IMP, probably due to the increase of aerobic potential of muscles. Baldwin et al. (2000) described the effect of endurance training on Hx level, showing the difference in Hx concentration between trained and non-trained subjects at 95% of VO2max. Due to the lack of studies on endurance-trained subjects, particularly on competitive endurance athletes during a longer training period, only comparisons with sprint training are possible. Repeated high-intensity training lasting for 6 weeks reduces considerably the resting and postexercise Hx concentration and increases HGPRT activity in the human muscle (Hellsten-Westing et al. 1993a). This reflects the muscle adaptation which reduces in turn adenine nucleotides loss (Hellsten-Westing et al. 1993a, b). Similar results were obtained by Stathis et al. (1994) in active but nonspecifically trained subjects (men and women) and Spencer et al. (2004) in elite female field hockey players. Stathis et al. (1994) demonstrated significant differences in muscle and plasma Hx concentration between the trained and the non-trained group as well as between the pre- and post-training values after a 7-week sprint training. At the same time, resting muscle ATP and total adenine nucleotide content, postexercise ATP depletion as well as accumulation of muscle IMP and inosine, precursors to Hx production, were reduced after training. Moreover, an increase in performance of the 30-s sprint bout following training was noted. On the basis of these studies, one may suggest that a lower plasma purine concentration after a training period allows athletes to exercise at a higher intensity without the loss of purine nucleotide.
The trends in plasma Hx concentration in our endurance 1-year training study are similar to those observed in short-duration sprint training (Hellsten-Westing et al. 1993a, b; Stathis et al. 1994; Spencer et al. 2004). In our study, the lowest postexercise concentration of Hx was observed during the competition phase when the proportion of anaerobic training loads was highest, that is the training was characterized to a larger extent by high-intensity exercises. The postexercise Hx concentration was markedly higher in training phases with less stressed anaerobic content.
Significant changes in average X concentration were observed at rest (P < 0.01). Plasma X concentration remained within the range of standard values obtained by Boulieu et al. (1983) with HPLC method. Bianchi et al. (1999) observed similar resting X concentrations in marathon runners and Sahlin et al. (1999) in men practicing different leisure sports. The changes of the resting–postexercise value were not significant in our study.
In the present study, changes in resting concentration of Hx (P < 0.05) and in resting–postexercise values (P < 0.01) during 1-year training cycle have been shown. Similar resting values were observed in long- and middle-distance runners in the study of Bhattacharya et al. (1983). In long-distance runners, we found the highest Hx concentration in the transition period, after the end of a long competition period and of the whole 1-year training cycle, both at rest (4.43 μmol l−1), and after exercise (22.9 μmol l−1). The lowest concentration of Hx was noted in the competition period when runners were peaking their athletic shape. It is important that the changes in Hx concentration were inversely proportional to the distance covered in the maximum oxygen consumption test. The best average performance was noted in the competition period (3,067.0 ± 350.5 m) and the shortest distance in the transition phase (2,641.8 ± 97.9 m). The higher anaerobic content during training of the competition phase could potentially increase purine loss during the training challenge on the muscle. Stathis et al. (1994) demonstrated both a reduced ATP content at rest in the 7-week sprint trained state and a substantially reduced degradation of ATP to IMP during 30 s of maximal sprint exercise, with increased performance measures. Thus, as with sprint training, the adenine nucleotide content in our subjects may be lower at rest, following training in the competitive phase, and not degrade to the same extent following exercise, even with an increased performance measure in distance covered. This could explain the reduced plasma Hx concentration in recovery.
These data suggest that Hx plasma concentration may be a measure of training status, overload or overtraining. This is supported by the lack of significant changes in LA concentrations in consecutive examinations, neither for resting nor for postexercise values. Thus, plasma Hx concentration may be the source of an additional specific and important information on training status, better than classic measures like VO2max and LA concentration.
The HGPRT activity in red blood cell at rest varied greatly during the investigated training cycle (P < 0.01). The lowest activity was found in the transition phase of the training cycle and the highest activity in the competition period. In support of our results, Banaszak and Rychlewski (1989) found a positive correlation between HGPRT activity and VO2max and a negative correlation between HGPRT activity and Hx concentration. Similar nature of changes was revealed in our study. Along with increase of HGPRT activity, VO2max increased and Hx concentrations decreased.
Using metabolites of purine degradation in the plasma at rest has limitations. Their concentrations are in a constant state of flux as purines are produced, entering the blood from the muscle and being excreted. The level and the rate of excretion of the different purines can change following exercise and training (Stathis et al. 1999, 2006). However, in the research of Stathis et al. 2006 pre- and post-training LA and Hx concentrations did not differ significantly. Probably due to a very short-term training cycle (1 week), the adaptation processes could not develop. There was no performance change after training (no changes in power and fatigue index were observed). In contrast to this, the research of Spencer et al. 2004 on female national field hockey players revealed a considerable decrease of resting (as well as postexercise) Hx concentration after training lasting for 7 weeks. This suggests that changes in resting concentrations occur only after a longer period of training. In our study, we consider a long-term training cycle in competitive athletes with examinations made at intervals of 3–5 months. The resting (and postexercise) values were determined by a regular, almost daily training in a long period. In competitive athletes, the dominant factor influencing metabolic changes seems to be specifically planned and structured training load (intensity, volume, frequency), according to the aim of each training phase. This could be the reason why resting Hx and X concentrations and HGPRT activity changed significantly. With standard exercise protocol, it is a good basis for monitoring training status at chosen points of training cycle in individual athletes (in competitive sport we follow the individual parameters rather than average “team” values). Unexpected changes of concentrations at rest could be evidence for unfavourable metabolic changes, e.g. overload/overtraining. It is particularly important in the light of the fact that LA concentrations do not change along with training phases in our study, i.e. LA is of limited diagnostic value in competitive athletes. So far, other studies on the long-term (1-year, many years’) changes in purine metabolism have not appeared and the problem needs further investigation.
Hellsten-Westing et al. (1993a) hypothesize that the decreased Hx release from muscle may be explained by intensified conversion of Hx to IMP or by decrease in Hx formation. The first possibility, reducing nucleotide loss, could be considered as an advantageous adaptation of muscles being subject to metabolic stress. The reduction of Hx release may be also explained by the decrease in adenine nucleotide degradation. Such a reduction could be achieved by the increase of muscle ability to ATP resynthesis in the process of anaerobic glycolysis and, in this way, by the redress of the balance of ATP consumption and resynthesis at high exercise intensity. Considerable decrease in the activity of myoadenylate (AMP) deaminase could also slow down the rate of AMP deamination and the rate of IMP degradation to purine at the same time.
In our study, the higher erythrocyte HGPRT activity and the decreased Hx blood concentration after a specific endurance training could suggest that the rephosphorylation of intracellular Hx to IMP increased. However, muscle HGPRT was not measured and the main limitation of this kind of speculation is the lack of evidence that muscle and erythrocyte HGPRT activity are directly related. It seems that the HGPRT plays different role in the erythrocytes (they do not have de novo capacity) compared with muscle.
Significant differences between resting and postexercise concentrations of UA in each training phase were observed in our study (P < 0.05). Moreover, the resting–postexercise difference changed significantly during the whole training cycle (ANOVA P < 0.05). The concentrations remained within the normal (medical) reference range at rest as well as postexercise. No significant 1-year changes of UA were found in resting and postexercise values considered separately. However, a visible but non-significant trend towards lower UA concentrations during the yearly training cycle was noticed. Earlier studies on animals revealed that the liver has a substantial potential for Hx uptake and UA production (Moyer and Henderson 1983). Purines (to a large extent as hypoxanthine) are released from exercising muscle into blood. The increase in plasma urate stems largely from oxidation of Hx in the liver, whereas the formation of UA in skeletal muscle seems to be limited (Hellsten-Westing et al. 1994; Sahlin et al. 1991; Bangsbo et al. 1992). After release from muscle, the liver extracts Hx from the blood, oxidizes it to urate and releases UA into the blood. Uric acid in blood may be then extracted by exercised muscle to replenish the muscle urate stores, may be utilized as a free radical scavenger and oxidized to allantoin during exercise (Hellsten et al. 1997; Hellsten et al. 2001). Hypoxanthine does not accumulate in plasma to an appreciable degree because it is continuously degraded to UA. This may explain resting–postexercise differences in UA concentrations being 3–10 times higher than combined X and Hx magnitudes. In support of our results, Bianchi et al. (1999) showed the increase of UA postexercise concentration by 33 μmol l−1 in soccer players and by 73 μmol l−1 in marathon runners (3–6 times higher than at rest) at 5 min of recovery.
Smallest differences between rest and postexercise UA concentrations were revealed in the specific preparation and competition phase (first and second examination) when the contribution of anaerobic training loads was largest. Uric acid is the end point of purine metabolism and can accumulate in the plasma. Its blood concentration may be considered as a metabolic indicator of the changes which occurred in the preceding exercise. A rise in the plasma UA from rest to postexercise level is also likely to indirectly represent adenine nucleotide degradation during exercise. Less plasma UA accumulation postexercise (smaller difference between rest and postexercise concentration) appears to be caused by less Hx efflux from the muscle (less production or better salvage).
Lower postexercise purine concentration in competition period indicates that the training allowed the athletes to exercise at higher intensity but, at the same time, purine turnover in skeletal muscle was reduced. Such an adaptation is crucial from the practical point of view, especially with reference to competitive athletes often performing high-intensity exercise. Decrease in adenine nucleotides loss means less cell damage and better sport performance. This was confirmed by Hellsten-Westing et al. (1993b) who revealed that plasma purine bases concentration decreases along with the increase of physical efficiency (endurance level). Stathis et al. (1994) reported lower IMP and inosine concentration in the muscle after training and provided direct evidence of reduced purine base accumulation in, and loss from the muscle in the light of improved performance. Furthermore, Sahlin et al. (1999) proved that plasma Hx is positively correlated with plasma ammonia (NH3) and LA concentration, and negatively correlated with endurance level.
Our study revealed that, in long-distance runners, endurance training leads to an increase in erythrocyte HGPRT activity and to a decrease in Hx formation in the competition period. The process of re-utilization of Hx and its conversion to IMP may be preferred with increase in endurance level (training status). Thus, a more “economical” metabolism is favoured that aims to salvage the purine ring, biosynthesis of which is very energy consuming. This may bring about a lower level of oxypurine in the blood of trained than in the blood of non-trained individuals after the same exercise. Oxypurine concentration may decrease as a result of the reaction of Hx with phosphoribosylpyrophosphate (PRPP) catalyzed by HGPRT, leading to IMP formation. Consequently, the level of IMP, being a link in the purine nucleotide cycle, increases.
The potential limitation of our study is a single blood sampling point at 5 min of recovery. It is known that Hx concentration increases after exercise and reaches maximum value after 10–20 min (Harkness et al. 1983; Hellsten-Westing et al. 1989; Sahlin et al. 1991) and UA concentration even later (Stathis et al. 1994, 2006). Moreover, no significant changes after 5 min of recovery were found in plasma Hx concentration during a moderate-intensity exercise (Sahlin et al. 1999). From this point of view, further postexercise samples during 15–20 min would have been optimal. However, at the same time, other studies reveal significant differences in metabolic response to exercise between groups of subjects, which is of great importance in the context of sports training. Bianchi et al. (1999) showed that postexercise plasma oxypurines (at 5 min of recovery) were more precise predictors of muscle energy exhaustion than strain intensity or duration in athletes specialized in different sports. Bhattacharya et al. (1983) revealed a differentiation of pyruvate exercise response in short-, middle- and long-distance runners (2.5–3 min after exercise). Rychlewski et al. (1997) used plasma Hx (5 min after exercise) as an indicator of exercise intensity in athletes. When the practical application of measured parameters in individual athletes is considered, information must be provided almost instantly to an athlete/coach. Short time between exercise end point and blood sampling might enable current (during the training session) modification of the training load depending on metabolic response. Thus, such a laboratory procedure seems to be justifiable and useful.
In summary, the results of the present study demonstrated that resting and postexercise plasma concentrations of X and, Hx and erythrocyte HGPRT activity change significantly in competitive long-distance runners during 1-year training cycle. Significant changes in resting–postexercise values between training phases were found for Hx and UA. Endurance training in competitive long-distance runners was the cause of a significant decrease in postexercise plasma X concentration and resulted in an increase in resting and postexercise HGPRT activity in the competition period. During the transition period, Hx concentration increased and HGPRT activity decreased significantly. The effect of our study may be that the level of plasma Hx at rest as well as after standard exercise may be a useful tool for monitoring the adaptation of energetic processes in different training phases. The analysis of diverse reactions of plasma Hx could also support the overload and overtraining diagnosis. Moreover, collecting blood samples within 5 min after exercise seems to be more suitable to sport practice than after a longer time. A quick measurement and the possibility of analysis would enable both coach and athlete to control the effects of training loads immediately after the exercise and to modify it possibly in the course of a training session or a training period. In the future, one could analyse the effects of longer training cycles in athletes practising other competitive sports to measure the changes in purine metabolism and its application to training in a more detailed way. It would be interesting to investigate the different types of training and loads which would cause the most advantageous changes in post-training purine concentrations in competitive athletes.
References
Baldwin J, Snow RJ, Febbraio MA (2000) Effect of training status and relative exercise intensity on physiological responses in men. Med Sci Sports Exerc 32(9):1648–1654. doi:10.1097/00005768-200009000-00020
Balsom PB, Seger JY, Sjödin B, Ekblom B (1992) Physiological response to maximal intensity intermittent exercise. Eur J Appl Physiol 65(2):144–149. doi:10.1007/BF00705072
Banaszak F (1999) The metabolism of purine and pyrimidine nucleotides in essential hypertension. The assessment of influence of antihypertensive drugs (in Polish). Monographic Book no. 341, University School of Physical Education, Poznań
Banaszak F, Rychlewski T (1989) Adenylate metabolism in physical exercise. Biol Sport 6(4):255–268
Banaszak F, Rychlewski T (1992) Selected problems of nitric metabolism of adenylic nucleotides: level of oxypurines and ammonia in conditions of physical effort. Stud Phys Cult Tour 2:115–122
Bangsbo J, Sjödin B, Hellsten-Westing Y (1992) Exchange of hypoxanthine in muscle during intense exercise in man. Acta Physiol Scand 146(4):549–550. doi:10.1111/j.1748-1716.1992.tb09465.x
Becker BF (1993) Towards the physiological function of uric acid. Free Radic Biol Med 14(6):615–631. doi:10.1016/0891-5849(93)90143-I
Bhattacharya AK, Panda PK, Das Gupta PK, De AK (1983) Pattern of venous lactate and pyruvate after submaximal exercise in athletes training in different disciplines. Int J Sports Med 4(4):252–254. doi:10.1055/s-2008-1026044
Bianchi GP, Grossi G, Bargossi AM, Fiorella PL, Marchesini G (1999) Can oxypurines plasma levels classify the type of physical exercise? J Sports Med Phys Fitness 39(2):123–127
Bompa T (1999) Periodization: theory and methodology of training. Human Kinetics, Champaign, pp 147–311
Boulieu R, Bory C, Baltasat P, Gonnet C (1983) Hypoxanthine and xanthine levels determined by high performance liquid chromatography in plasma, erythrocyte and urine samples from healthy subjects: the problem of hypoxanthine level evolution as a function of time. Anal Biochem 129(2):398–404. doi:10.1016/0003-2697(83)90568-7
De Bono DP (1994) Free radicals and antioxidants in vascular biology: the roles of reaction kinetics, environment and substrate turnover. QJM 87(8):445–453
Green HJ, Jones S, Ball-Burnett M, Farrance B, Ranney D (1995) Adaptations in muscle metabolism to prolonged exercise and training. J Appl Physiol 78(1):138–145
Gutmann J, Wahlenfeld AW (1974) l-/± lactate determination with lactate dehydrogenase and NAD. In: Bergmayer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 1586–1587
Harkness RA, Simmonds RJ, Coade SB (1983) Purine transport and metabolism in man: the effect of exercise on concentrations of purine bases, nucleosides and nucleotides in plasma, urine, leucocytes and erythrocytes. Clin Sci 64(3):333–340
Hellsten Y, Tullson PC, Richter EA, Bangsbo J (1997) Oxidation of urate in human skeletal-muscle during exercise. Free Radic Biol Med 22(1–2):169–174. doi:10.1016/S0891-5849(96)00286-9
Hellsten Y, Svensson M, Sjödin B, Smith S, Christensen A, Richter EA, Bangsbo J (2001) Allantoin formation and urate and glutathione exchange in human muscle during submaximal exercise. Free Radic Biol Med 31(11):1313–1322. doi:10.1016/S0891-5849(01)00631-1
Hellsten-Westing Y, Ekblom B, Sjödin B (1989) The metabolic relation between hypoxanthine and uric acid in man following maximal short-distance running. Acta Physiol Scand 137(3):341–345. doi:10.1111/j.1748-1716.1989.tb08762.x
Hellsten-Westing Y, Balsom PD, Norman B, Sjödin B (1993a) The effect of high-intensity training on purine metabolism in man. Acta Physiol Scand 149(4):405–412. doi:10.1111/j.1748-1716.1993.tb09636.x
Hellsten-Westing Y, Norman B, Balsom PD, Sjödin B (1993b) Decreased resting level of adenine nucleotides in human skeletal muscle after high–intensity training. J Appl Physiol 74(5):2523–2528
Hellsten-Westing Y, Ekblom B, Kaijser L, Sjödin B (1994) Exchange of purines in human liver and skeletal muscle with short term exhaustive exercise. Am J Physiol 266:R81–R86
Jarasch ED, Bruder G, Heid HW (1986) Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand Suppl 548:39–46
Ketai LH, Simon RH, Kreit JW, Grum CM (1987) Plasma hypoxanthine and exercise. Am Rev Respir Dis 136(1):98–101
Moyer JD, Henderson JF (1983) Salvage of circulating hypoxanthine by tissues of the mouse. Can J Biochem Cell Biol 61(11):1153–1157
Murray AW (1971) The biological significance of purine salvage. Annu Rev Biochem 40:811–826. doi:10.1146/annurev.bi.40.070171.004115
Parks DA, Granger DN (1986) Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl 548:87–99
Ralston MA, Merola JA, Leier CV (1991) Depressed aerobic enzyme activity of skeletal muscle in severe chronic heart failure. J Lab Clin Med 117(5):370–372
Rychlewski T Banaszak F, Szczęśniak Ł, Jastrzębski A (1994) Activity of hypoxanthine–guanine–phosphoribosyltransferase (HGPRT) in healthy individuals and in the cases of metabolic civilization-related diseases. In: International congress on applied research in sport “The Way to Win”, The Finnish Society for Research in Sport and Physical Education, Helsinki, p 33 (Abstract)
Rychlewski T, Banaszak F, Szczęśniak Ł, Konys L, Jastrzębski A (1997) Hypoxanthin im Blutplasma als Indikator der Intensität von körperlicher Anstrengung. Sportonomics 1:47–52
Sahlin K, Ekberg K, Cizinsky S (1991) Changes in plasma hypoxanthine and free radical markers during exercise in man. Acta Physiol Scand 142(2):275–281. doi:10.1111/j.1748-1716.1991.tb09157.x
Sahlin K, Tonkonogi M, Söderlund K (1999) Plasma hypoxanthine and ammonia in humans during prolonged exercise. Eur J Appl Physiol Occup Physiol 80(5):417–422. doi:10.1007/s004210050613
Sjödin B, Hellsten-Westing Y (1990) Changes in plasma concentration of hypoxanthine and uric acid in man with short-distance running at various intensities. Int J Sports Med 11(6):493–495. doi:10.1055/s-2007-1024844
Sjödin B, Jacobs I, Svedenhag J (1982) Changes in onset of blood lactate accumulation (OBLA) and muscle enzymes after training at OBLA. Eur J Appl Physiol Occup Physiol 49(1):45–57. doi:10.1007/BF00428962
Spencer M, Bishop D, Lawrence S (2004) Longitudinal assessment of the effects of field hockey training on repeated sprint ability. J Sci Med Sport 7(3):323–334. doi:10.1016/S1440-2440(04)80027-6
Stathis CG, Febbraio MA, Carey MF, Snow RJ (1994) Influence of sprint training on human skeletal muscle purine nucleotide metabolism. J Appl Physiol 76(4):1802–1809
Stathis CG, Zhao S, Carey MF, Snow RJ (1999) Purine loss after repeated sprint bouts in humans. J Appl Physiol 87(6):2037–2042
Stathis CG, Carey MF, Hayes A, Garnham AP, Snow RJ (2006) Sprint training reduces urinary purine loss following intense exercise in humans. Appl Physiol Nutr Metab 31(6):702–708. doi:10.1139/H06-074
Stolk JN, De Abreu RA, Boerbooms AMT, de Koning DGM, de Graaf R, Kerstens PJSM, van de Putte LBA (1995) Purine enzyme activities in peripheral blood mononuclear cells: comparison of a new non-radiochemical high-performance liquid chromatography procedure and a radiochemical thin-layer chromatography procedure. J Chromatogr B Anal Technol Biomed Life Sci 666:33–43. doi:10.1016/0378-4347(94)00571-L
Sutton JR, Toews CJ, Ward R (1980) Purine metabolism during strenuous exercise in man. Metabolism 29(3):254–260. doi:10.1016/0026-0495(80)90067-0
Tullson PC, Whitlock DM, Terjung RL (1990) Adenine nucleotide degradation in slow-twitch red muscle. Am J Physiol Cell Physiol 258:C258–C265
Wung WE, Howell SB (1980) Simultaneous liquid chromatography of 5-fluorouracil, uridine, hypoxanthine, xanthine, uric acid, allopurinol and oxipurinol in plasma. Clin Chem 26(12):1704–1708
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zieliński, J., Rychlewski, T., Kusy, K. et al. The effect of endurance training on changes in purine metabolism: a longitudinal study of competitive long-distance runners. Eur J Appl Physiol 106, 867–876 (2009). https://doi.org/10.1007/s00421-009-1079-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1079-5