Abstract
Purpose
The purpose of this study was to compare the effect of upper and lower body high-intensity intermittent exercise (HIIE) on immunometabolism profile.
Methods
Seven male judo athletes completed two experimental sessions separated by at least 48 h. The athletes completed four bouts of the upper and lower body Wingate tests separated by 3-min recovery periods. The blood samples were collected at rest and immediately after the fourth bout of lower and upper body Wingate tests. Serum was analysed for IL-1ra (Interleukin-1 Receptor Antagonist), interleukins (IL-1) IL-2, IL-4, IL-6, IL-10, TNF-α (tumor necrosis factor alpha), cortisol, glucose, and NEFA (non-ester fatty acid). Peak power (maximum power attained during the 30 s test), mean power were calculated. In addition, after 1 and 2.5-min of each Wingate bout, blood samples from the ear lobe were collected for lactate analysis.
Results
Our data demonstrated that lower body HIIE promoted a greater metabolic rate (values pre- vs. post-Wingate, for lactate: 1.02 ± 0.16 vs. 14.44 ± 1.08 mmol/L; for glucose: 112.5 ± 16.7 vs. 147.9 ± 23.5 mg/dL) and resulted in higher mechanical (mean power: 621 ± 46 vs. 427 ± 40 W, peak power: 794 ± 61 vs. 602 ± 109 W) performance compared to the upper body HIIE (lactate: 0.85 ± 0.18 vs. 12.69 ± 0.74 mmol/L; for glucose: 115.3 ± 20.4 vs. 123.7 ± 28.6 mg/dL; mean power: 480 ± 46 vs. 341 ± 45 W; and peak power: 672 ± 83 vs. 501 ± 120 W), but NEFA showed a similar response to both conditions, with increased IL-10 levels.
Conclusions
In conclusion, our results demonstrated that despite the higher performance in lower body HIIE, the inflammatory response did not differ between exercise modalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several factors are involved in the performance of high-intensity intermittent exercise (HIIE), including substrate availability, acid–base equilibrium, key-enzymes activity and energy systems interaction (Buchheit and Laursen 2013). Alterations of cortisol and interleukin 6 (IL-6) levels can regulate the availability of substrate in the exercise, and these alterations are modified by the type, intensity and duration of exercise (Petersen and Pedersen 1985). Acute immune–metabolic response to exercise is connected to glucose homeostasis in muscle cells and triglyceride balance in adipose tissue (Steinacker et al. 2004; Rosa et al. 2011). Moreover, acute increases of the IL-6 promoted by exercise sessions can be beneficial to health because of its anti-inflammatory effect (Petersen and Pedersen 1985).
Previous studies have mainly focused on alterations in inflammatory cytokines (especially IL-6, TNF-α and IL-1β), post-moderate intensity aerobic and strength exercise sessions and/or training (Ostrowski et al. 1999; Neto et al. 2011; Libardi et al. 2012). Pro-inflammatory cytokines, such as IL-1β, IL-2, IL-6 and TNF-α can promote several metabolic events, such as lipolysis and glycogenolysis, during acute and chronic exercise training (reviewed by Lira et al. 2014). This effect can favor great availability of substrate for exercise performance. On the other hand, anti-inflammatory cytokine, such as IL-1ra, IL-4, IL-10 and adipopectic exert protective action against an inflammatory sustained response (Lira et al. 2011; Seelaender et al. 2012). However, a small number of studies have explored the alterations on metabolic and inflammatory profiles (immunometabolism profile) by HIIE (Meckel et al. 2009, 2011; Gökbel et al. 2012).
Although it is known that exercise duration can explain 51 % of the increase of IL-6 during aerobic exercise (Fischer 2006), HIIE studies have shown that this kind of exercise can increase the IL-6 (~2.5-fold), even when short total exercise volume is performed (1000 m) (Meckel et al. 2009, 2011). Moreover, HIIE can induce a greater increase in IL-6 compared with continuous moderate-intensity exercise, when work performed was equalized between protocols (Leggate et al. 2010). However, while there are a few studies involving HIIE or HIIT (high-intensity intermittent training), other cytokines were not investigated nor were associations with the metabolism (immunometabolism profile) analysed.
HIIE studies analysed lower body performance (Meckel et al. 2009, 2011) probably because few people have a high upper body physical fitness level, making the utilization of a protocol of intense exercise for this region difficult. This fact makes it difficult to investigate and to compare the performance and immunometabolism responses in upper and lower body to high-intensity intermittent protocols. However, many athletes and disabled people execute tasks involving a high upper body demand.
Judo athletes are recognized as presenting a moderate to highly developed lower and upper body aerobic and anaerobic capacity (Franchini et al. 2011), aspects that are important for performance in high-intensity intermittent exercises (Buchheit and Laursen 2013). The knowledge of the effect of upper and lower body exercises can be useful to understanding if the amount of muscle mass is important to cytokine responses, given that they are produced in the skeletal muscle (Pedersen 2009).
Until now, there were no studies evaluating metabolic and inflammatory responses during acute exercise in both conditions (upper or lower body) in athletes trained for both upper and lower body segments. Thus, the objective of this study was to evaluate the immunometabolism profile in upper and lower body high-intensity intermittent exercises. Our hypothesis was that both HIIE protocols would result in an increase in energetic substrate availability and cytokine levels but a higher response would be found in the lower body protocol due to the higher muscle mass involved in this exercise mode.
Methods
Subjects
Seven male judo athletes (69.7 ± 6.2 kg; 172.7 ± 3.1 cm; 20.1 ± 3.4 years; black or brown belt; 11.6 ± 5.4 years of training experience; state and national level; tested during their competitive season) voluntarily participated in the present study after reading and signing an informed consent form explaining all the risks and benefits of the present investigation. All athletes were nonsmokers, and none of them received any pharmacological treatments or had any type of neuromuscular or cardiovascular disorder, or respiratory or circulatory dysfunction. All athletes were evaluated during their competitive period, a phase in which they were training at least four times per week. No athlete was losing weight or competed in the heavyweight category. All procedures received local ethics committee approval. Before conducting the study we checked the sample size needed (n = 6) to guarantee 80 % power and 5 % significance level, using studies that measured the IL-6 pre- and immediately post-exercise as referenced by similar protocol (lower body high-intensity intermittent exercise) (Meckel et al. 2009, 2011; Leggate et al. 2010; Arent et al. 2010).
Procedures
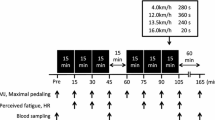
Subjects completed two experimental sessions separated by at least 48 h. In one session, they performed an upper body Wingate test and in the other session they performed a lower body Wingate test. The order was randomized. The athletes were required to refrain from intense physical activity for 24 h prior to each session, and to maintain constant diet and sleep routines throughout the study.
High-intensity intermittent test
The athletes completed four bouts of the upper and lower body Wingate tests separated by 3-min recovery periods. The load was set at 0.09 kg kg−1 of body mass and 0.06 kg kg−1 of body mass for lower and upper body, respectively (Inbar et al. 1996). Before Wingate tests a standard warm-up was conducted. It was composed by 5 bouts of 30 s (20 s at 70 rpm, and 10 s at 100 rpm), with approximately, 100 W for lower body and 50 W for upper body. They started the Wingate test after 3-min of interval, and left from zero velocity.
The upper body Wingate test was performed using a specifically designed cycle ergometer (EB 4100, Cefise, Brazil) equipped with handgrips for arm testing. The subjects were seated on a chair and secured by their legs. The lower body Wingate test was conducted on a cycle ergometer (Excalibur, Lode, Netherlands) equipped with toe-clips to prevent the subject’s feet from slipping. At the start signal, participants were instructed to pedal as fast as they could for 30 s and instructed to remain seated during the test. Strong verbal encouragement was given to maintain an all-out effort during the test.
Peak power (maximum power attained during the 30 s test), mean power (average power generated during the 30 s test) and total work performed were calculated.
Blood sampling and analyses
The blood samples were collected at rest, and immediately after the fourth lower and upper body Wingate bouts. The blood samples (15 ml) were immediately allocated into two 5 ml vacutainer tubes (Becton–Dickinson, BD, Juiz de Fora, MG, Brazil) containing EDTA for plasma separation and into one 5 ml dry vacutainer tube for serum separation. The tubes were centrifuged at 2,500g for 12 min at 4 °C, and plasma and serum samples were stored at −20 °C until analysis. Cytokines (IL-1ra, IL-2, IL-4, and IL-10—R&D Systems, 614 McKinley Place NE, Minneapolis, MN 55413, USA) and (IL-6 and TNF-α—RayBiotech, 3607 Parkway Lane suite 100 Norcross, GA 30092) were assessed using ELISA commercial kits. Glucose was assessed using commercial kits (Labtest®, São Paulo, Brazil). Non-ester fatty acid (NEFA) was assessed by a colorimetric method with a commercial kit (ZenBio, 3200 Chapel Hill-Nelson Blvd., Suite 104). Serum cortisol was assessed using commercial kits (Alpha Diagnostic International). The cytokines (IL-1ra, IL-2, IL-4, IL6, IL-10 and TNF-α), cortisol and glucose levels were assessed using serum, and NEFA levels was assessed using plasma. Additionally, 1 and 2.5-min after each bout (except after the last bout—1, 3 and 5-min), blood samples from the ear lobe were collected. The blood samples collected from the ear lobe were utilized to analyse the lactate concentration (La). This measurement was obtained using the Yellow Spring 1500 Sport lactometer (Yellow Springs, United States of America).
Statistics
The data normality was verified using the Shapiro–Wilk test. For each variable, mean and standard deviation were calculated. Total work performed during the Wingate tests was compared through paired Student’s t test. For this variable, the effect size was calculated as average lower body−average upper body/standard deviation of both exercise modalities pooled. A two-way analysis of variance with repeated measurements in the second factor was used to compare lactate concentration, mean and peak power in each Wingate test (exercise modality and bout) and to blood variables pre- and post- Wingate tests (exercise modality and moment of measurement). The Mauchly test was used to check compound symmetry and the Greenhouse–Geisser correction was used when necessary. The Bonferroni test was used as post hoc when differences were found in the ANOVA. Effect sizes were calculated using eta squared (η 2). The significance level was set at 5 %.
Results
During the high-intensity intermittent tests (Table 1), there was an effect of exercise modality (F 1,11 = 6.45; P = 0.028; η 2 = 0.37) and an effect of test bout (F 3,33 = 18.53; P < 0.001; η 2 = 0.63) on peak power, but no interaction effect was found (F 3,33 = 0.32; P = 0.809; η 2 = 0.03). Higher peak power was achieved during the lower, compared to the upper body, exercise modality (P = 0.028; 95 % CI = 51.3–178.5), and in the first bout compared to the second (P = 0.015; 95 % CI = 6.9–155.4), third (P < 0.001; 95 % CI = 36.9–185.5) and fourth bouts (P < 0.001; 95 % CI = 108.8–257.4), and higher values in the second compared to the fourth bout (P = 0.002; 95 % CI = 27.7–176.2). For the mean power, there was an effect of exercise modality (F 1,11 = 37.03; P < 0.001; η 2 = 0.77) and an effect of test bout (F 3,33 = 48.67; P < 0.001; η 2 = 0.82), but no interaction effect was found (F 3,33 = 1.33; P = 0.282; η 2 = 0.11). Higher mean power was achieved during the lower compared to the upper body exercise modality (P < 0.001; 95 % CI = 67.2–133.2), and in the first Wingate bout compared to the second (P < 0.001; 95 % CI = 44.9–134.9), third (P < 0.001; 95 % CI = 82.3–172.3), and fourth bouts (P = 0.001; 95 % CI = 121.8–211.8) as well as higher values in the second bout compared to the fourth (P < 0.001; 95 % CI = 31.8–121.8).
Total work during the high-intensity intermittent tests was higher (t = 3.30; P = 0.016; effect size = 1.44) during the lower (844 ± 85 J kg−1) compared to the upper body exercise modality (696 ± 54 J kg−1).
For the blood lactate response (Table 2), there was an effect of exercise modality (F 1,6 = 6.57; P = 0.042; η 2 = 0.52) with higher values in the lower body compared to the upper body test (P = 0.042; 95 % CI = 0.04–1.62). There was an effect of test bout (F 4,24 = 262.28; P < 0.001; η 2 = 0.98), with lower values at rest compared to all post-Wingate test peak values (P < 0.001 for all comparisons; 95 % CI = −7.46 to −4.74; −11.36 to −8.65; −12.26 to −9.55; −13.86 to −11.15), lower values after Wingate bout 1 compared to Wingate bouts 2, 3 and 4 (P < 0.001 for all comparisons; 95 % CI = −5.23–2.54; −6.16–3.44; −7.76 to −5.04), lower values after Wingate bout 2 compared to after Wingate bout 4 (P < 0.001; 95 % CI = −3.85 to −1.14), and lower values after Wingate bout 3 compared to after Wingate bout 4 (P = 0.008; 95 % CI = −2.95 to −0.24). There was no effect of interaction (F 4,24 = 2.10; P = 0.112; η 2 = 0.26).
Figures 1 and 2 presents the cytokine and substrate responses to lower and upper body protocols, respectively.
Cytokine levels before and after four bouts of lower and upper body Wingate tests in male judo athletes (n = 7; values are mean ± standard deviation). a (IL-1ra), b (IL-2), c (IL-4), d (IL-6), e (IL-10), f (TNF-α). a lower body different from upper body (P < 0.05); b pre different from post (P < 0.05)
For IL-1ra there were no effects of exercise modality (F 1,6 = 0.99; P = 0.359; η 2 = 0.14), moment (F 1,6 = 0.05; P = 0.835; η 2 = 0.01) or interaction (F 16 = 2.97; P = 0.136; η 2 = 0.33). For IL-2, no effects of exercise modality (F 1,6 = 0.57; P = 0.480; η 2 = 0.09), moment (F 1,6 = 1.57; P = 0.256; η 2 = 0.21) or interaction (F 1,6 = 1.28; P = 0.302; η 2 = 0.18) were observed. For IL-4 there was an effect of moment (F 1,6 = 13.93; P = 0.010; η 2 = 0.70), with a lower value after the HIIE test compared to the rest value (P = 0.010; 95 % CI = 0.53–2.57), but no effects of exercise modality (F 1,6 = 0.19; P = 0.678; η 2 = 0.03) or interaction (F 1,6 = 1.26; P = 0.304; η 2 = 0.17) were found.
For IL-6 levels no effect of exercise modality (F 1,6 = 1.00; P = 0.356; η 2 = 0.14) and moment (F 1,6 = 0.17; P = 0.693; η 2 = 0.03) were found. However, there was an effect of interaction (F 1,6 = 6.87; P = 0.040; η 2 = 0.53), but the post hoc test did not confirm any difference. No effect of exercise modality (F 1,6 = 0.06; P = 0.822; η 2 = 0.01) was found for the IL-10, but effects of moment (F 1,6 = 9.41; P = 0.022; η 2 = 0.61) and interaction (F 1,6 = 7.89; P = 0.031; η 2 = 0.57) were found. Pre-values were lower than post-values (P = 0.022; 95 % CI = −1.83 to −0.20). When the interaction effect was tested, the post hoc did not confirm any difference. For TNF-α no effects of exercise modality (F 1,6 = 0.14; P = 0.726; η 2 = 0.02), moment (F 1,6 = 1.59; P = 0.254; η 2 = 0.21) or interaction (F 1,6 = 0.01; P = 0.909; η 2 = 0.01) were observed.
For cortisol levels no effects of exercise modality (F 1,6 = 5.41; P = 0.059; η 2 = 0.47), moment (F 1,6 = 1.08; P = 0.339; η 2 = 0.15) or interaction (F 1,6 = 0.13; P = 0.727; η 2 = 0.22) were observed.
For blood glucose an effect of exercise modality was found (F 1,6 = 10.28; P = 0.018; η 2 = 0.63), with higher values in lower body compared to upper body (P = 0.018; 95 % CI = 2.54–18.95). An effect of moment was also found (F 1,6 = 6.15; P = 0.048; η 2 = 0.51), with higher values after compared to before the tests (P = 0.048; 95 % CI = −43.53 to −0.28). However, no effect of interaction was observed (F 1,6 = 1.70; P = 0.240; η 2 = 0.22). No effects of exercise modality (F 1,6 = 3.80; P = 0.099; η 2 = 0.39) or interaction (F 1,6 = 2.96; P = 0.136; η 2 = 0.33) were found for NEFA. However, an effect of moment of measurement was found (F 1,6 = 8.82; P = 0.025; η 2 = 0.60), with lower values at rest compared to post-HIIE (P = 0.025).
Discussion
To the best of our knowledge, this is the first study to compare the metabolic and inflammatory responses to lower and upper body high-intensity intermittent exercise. Our initial hypothesis was that HIIE would result in an increase in energetic substrates and cytokine levels and that a more pronounced response would be found in the lower body HIIE. Our data demonstrated that lower body exercise promoted greater metabolic performance (peak lactate and glucose concentrations) and resulted in higher mechanical (mean and peak power) performance compared to the upper body modality, but NEFA showed a similar response to both conditions, with increased IL-10 levels.
Previous studies have mainly focused on the effects of aerobic and strength exercise sessions and/or periods of training on the inflammatory cytokines (Ostrowski et al. 1999; Neto et al. 2011; Libardi et al. 2012). These studies have demonstrated that alterations in the immunometabolism profile are affected by the type of exercise, as well as the intensity and duration of exercise. However, a small number of studies have investigated the alterations on the metabolic and inflammatory profile (immunometabolism profile) during HIIE exercise (Meckel et al. 2009; Leggate et al 2010; Meckel et al. 2011; White et al. 2014).
Meckel et al. (2009) have demonstrated that sprint interval exercise (4 bouts of a 250 m at 80 % of the maximal speed) induced increases in lactate and IL-6 levels, but not in cortisol, IL1ra and IL-10 levels, in elite handball players. In addition, Meckel et al. (2011) showed that sprint interval exercise (100, 200, 300, 400 m, at a constant work rate of 80 % of the personal maximal speed) induced increases in lactate and IL-1β, IL-1ra, IL-6 levels, in similar subjects.
The data of the present study showed changes in the glucose, NEFA, IL-4 and IL10 levels, after the HIIE. The increase of glucose and NEFA levels can be, at least in part, explained by the increased glycogenolysis and lipolysis, for maintenance of the muscle contraction during exercise. However, as the exercise executed was intermittent, all-out and of short-duration (Wingate test), the glucose and free acid uptake by the skeletal muscle can be impaired, exposing the blood circulation to high concentrations of glucose and NEFA. The cortisol can lead to the catabolism of hepatic glycogen (glyconeogenesis pathway), and can be involved in the lipolysis process (glucocorticoids stimulate the beta adrenergic receptors that contribute to high lipolysis, via hormone-sensitive lipase) (Lee Kong et al. 2014), favoring increased glucose and free fatty acids in blood. However, in our study no statistically significant difference was found in cortisol levels (7 % increase post-exercise vs. pre-exercise) after both HIIEs. Meckel et al. (2009) have found changes in metabolic parameters without alterations in cortisol levels after sprint interval exercise (4 repetitions of a 250-m at 80 % of the maximal speed).
The protocol utilized by (Meckel et al. 2009, 2011) involved a type of HIIE in which the effort is done with a fixed intensity (i.e. speed), while our protocol was an all-out exercise. Thus, although both protocols would result in high anaerobic demand, in our protocol, the athlete performed his maximal effort during the entire exercise and thus is subjected to a higher physiological stress (Buchheit and Laursen 2013).
The alterations in metabolic parameters are related to inflammatory response, since IL-6 and TNF-α contribute to glucogenolysis and lipolysis processes. (Laskowski et al. 2011) have found that 3 days of judo training (randori combat training) induced a significant change in blood cytokine levels and this alteration was correlated with lipid peroxide levels. In the present study, we found reduced IL-4 and increased IL-10 levels after exercise, but we did not find alterations in IL-6 and TNF-α levels. The maintenance of the IL-6 levels can be related to higher serum glucose after exercise sessions (Starkie et al. 2001). In addition, highly aerobically trained individuals normally present higher muscle glycogen storage, which would, in turn, lower the need of IL-6 as an energy sensor (Pedersen 2012), especially because muscle glycogen is spared in highly aerobically trained individuals (Pedersen and Febbraio 2008).
IL-4 and IL-10 cytokines are important in the anti-inflammatory response. Prokopchuk et al. (2007) established that IL-4, IL-13, IL-4Rα and IL-13Rα1 are expressed in skeletal muscle and are up-regulated after strength training. IL-4 is involved in the regulation of muscle cell fusion and muscle growth through the IL-4 receptor. In addition, IL-4 is an important cytokine involved with polarization macrophages for M2 phenotype. The function of IL-4-activated macrophages, include high IL-10 production (Biswas and Mantovani 2012). However, we suggest that increased IL-10 levels, at least in part, cannot be related with IL-4-activated macrophages. Future studies are needed for a better understanding of the mechanisms involved.
The anti-inflammatory effect induced by exercise comes first from the increase in IL-6, followed by the increase in IL-1ra and in IL-10. IL-10 is the main molecule responsible for the orchestration of anti-inflammatory reactions (reviewed by Lira et al. 2009). Nevertheless, IL-6 and TNF-α levels did not alter after exercise; we found increased IL-10 levels only. IL-10 can affect different cell types and induce the suppression of the inflammatory response. We speculate that although our data of IL-6 and TNF-α levels did not change significantly, IL-10 levels increased to deal with some type of persistent inflammatory status, and/or to inhibit exacerbated catabolic stimuli, such as glycogenolysis and lipolysis pathways. In addition, Antosiewicz et al. (2013) have demonstrated that high-intensity interval exercise (three Wingate bouts) promotes increases in IL-6 and IL-10 1 h post-exercise. However, more studies are necessary to better understand the mechanisms involved in HIITs.
In the present study, our results contribute to the consideration of the mechanisms involving energetic metabolism and cytokines to the immunometabolism response and HIIE. Our study has some limitations. No data were available regarding total and regional muscle mass, which would allow a better interpretation of differences in immunometabolic response between exercise modalities (lower/upper body). Furthermore, one cannot be sure to which extent the response was local or systemic because the two exercise modalities shared a common static muscle activity (trunk stabilization) that was inseparable from dynamic activity (extremities) and probably contributed to the immunometabolism. Also, the post-exercise kinetics of cytokine and hormone activity during a longer recovery period was not followed. Future studies are needed to better understand the interplay between inflammatory response and energetic metabolism. In addition, these findings should be confirmed in other athletic populations.
In conclusion, our results demonstrated that despite the higher performance in lower body HIIE, the inflammatory response did not differ between lower and upper body exercise modalities.
Abbreviations
- HIIE:
-
High-intensity intermittent exercise
- IL-10:
-
Interleukin 10
- IL-13:
-
Interleukin 13
- IL-13Rα1:
-
Interleukin 13 receptor alpha 1
- IL-1ra:
-
Interleukin-1 receptor antagonist
- IL-2:
-
Interleukin 2
- IL-4:
-
Interleukin 4
- IL-4Rα:
-
Interleukin 4 receptor alpha
- IL-6:
-
Interleukin 6
- NEFA:
-
Non-ester fatty acid
- TNF-α:
-
Tumor necrosis factor alpha
References
Antosiewicz J, Kaczor JJ, Kasprowicz K, Laskowski R, Kujach S, Luszczyk M, Radziminski L, Ziemann E (2013) Repeated “all out” interval exercise causes an increase in serum hepcidin concentration in both trained and untrained men. Cell Immunol 283:12–17. doi:10.1016/j.cellimm.2013.06.006 (Epub 2013 Jun 20)
Arent SM, Senso M, Golem DL, McKeever KH (2010) The effects of the aflavin-enriched black tea extract on muscle soreness, oxidative stress, inflammation, and endocrine responses to acute anaerobic interval training: a randomized, double-blind, crossover study. J Int Soc Sports Nutr 7(1):11. doi:10.1186/1550-2783-7-11
Biswas SK, Mantovani A (2012) Orchestration of metabolism by macrophages. Cell Metab 15(4):432–437. doi:10.1016/j.cmet.2011.11.013.Review
Buchheit M, Laursen PB (2013) High-intensity interval training, solutions to the programming puzzle: Part I: cardiopulmonary emphasis. Sports Med 43(5):313–338. doi:10.1007/s40279-013-0029-x.Review
Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12:6–33 (Review)
Franchini E, Del Vecchio FB, Matsushigue KA, Artioli GG (2011) Physiological profiles of elite judo athletes. Sports Med 41(2):147–166. doi:10.2165/11538580-000000000-00000.Review
Gökbel H, Okudan N, Gül I, Belviranli M, Gergerlioğlu HS, Başaral MK (2012) Effects of repeated bouts of supramaximal exercise on plasma adiponectin, interleukin-6, and tumor necrosis factor-α levels in sedentary men. J Strength Cond Res 26(6):1675–1679. doi:10.1519/JSC.0b013e318231ac1c
Inbar O, Bar-Or O, Skinner JS (1996) The Wingate anaerobic test. Human Kinetics, Champaign
Laskowski R, Ziemann E, Olek RA, Zembron-Lacny A (2011) The effect of three days of judo training sessions on the inflammatory response and oxidative stress markers. J Hum Kinet 30:65–73. doi:10.2478/v10078-011-0074-1 (Epub 2011 Dec 25)
Lee Kong JH, Jang JY, Han JS, Yul J, Lee J, Kim JB (2014) Lipid droplet protein LID-1 mediates ATGL-1-dependent lipolysis during fasting in Caenorhabditis elegans. Mol Cell Biol 34:4165–4176 (pii:MCB.00722-14, Epub ahead of print)
Leggate Nowell MA, Jones SA, Nimmo MA (2010) The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaperones 15:827–833. doi:10.1007/s12192-010-0192-z (Epub 2010 Apr 16)
Libardi CA, De Souza GV, Cavaglieri CR, Madruga VA, Chacon-Mikahil MP (2012) Effect of resistance, endurance, and concurrent training on TNF-α, IL-6, and CRP. Med Sci Sports Exerc 44(1):50–56. doi:10.1249/MSS.0b013e318229d2e9
Lira Rosa FS, Zanchi NE, Yamashita AS, Lopes RD, Lopes AC, Batista ML Jr, Seelaender M (2011) Visceral fat decreased by long-term interdisciplinary lifestyle therapy correlated positively with interleukin-6 and tumor necrosis factor-α and negatively with adiponectin levels in obese adolescents. Metabolism 60:359–365. doi:10.1016/j.metabol.2010.02.017 (Epub 2010 Mar 31)
Lira FS, Rosa JC, Zanchi NE, Yamashita AS, Lopes RD, Lopes AC, Batista ML Jr, Seelaender M (2009) Regulation of inflammation in the adipose tissue in cancer cachexia: effect of exercise. Cell Biochem Funct 27(2):71–75. doi:10.1002/cbf.1540.Review
Lira FS, Neto JC, Seelaender M (2014) Exercise training as treatment in cancer cachexia. Appl Physiol Nutr Metab 39:679–686. doi:10.1139/apnm-2013-0554 (Epub 2014 Mar 24)
Meckel Y, Eliakim A, Seraev M, Zaldivar F, Cooper DM, Sagiv M, Nemet D (2009) The effect of a brief sprint interval exercise on growth factors and inflammatory mediators. J Strength Cond Res 23(1):225–230. doi:10.1519/JSC.0b013e3181876a9a
Meckel Y, Nemet D, Bar-Sela S, Radom-Aizik S, Cooper DM, Sagiv M, Eliakim A (2011) Hormonal and inflammatory responses to different types of sprint interval training. J Strength Cond Res 25(8):2161–2169. doi:10.1519/JSC.0b013e3181dc4571
Neto JC, Lira FS, de Mello MT, Santos RV (2011) Importance of exercise immunology in health promotion. Amino Acids 41:1165–1172. doi:10.1007/s00726-010-0786-x (Epub 2010 Oct 26. Review)
Nieman DC, Henson DA, Smith LL, Utter AC, Vinci DM, Davis JM, DE Kaminsky, Shute M (2001) Cytokine changes after a marathon race. J Appl Physiol (1985) 91:109–114
Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515(Pt 1):287–291
Pedersen BK (2009) The diseasome of physical inactivity and the role of myokines in muscle–fat cross talk. J Physiol 587:5559–5568. doi:10.1113/jphysiol.2009.179515 (Epub 2009 Sep 14. Review)
Pedersen BK (2012) Muscular interleukin-6 and its role as an energy sensor. Med Sci Sports Exerc 44(3):392–396. doi:10.1249/MSS.0b013e31822f94ac.Review
Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88(4):1379–1406
Petersen AM, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol (1985) 98:1154–1162 (Review)
Prokopchuk O, Liu Y, Wang L, Wirth K, Schmidtbleicher D (2007) Steinacker JM: Skeletal muscle IL-4, IL-4Ralpha, IL-13 and IL-13Ralpha1 expression and response to strength training. Exerc Immunol Rev 13:67–75
Rosa JC, Lira FS, Eguchi R, Pimentel GD, Venâncio DP, Cunha CA, Oyama LM, De Mello MT, Seelaender M, do Nascimento CM (2011) Exhaustive exercise increases inflammatory response via Toll like receptor-4 and NF-κBp65 pathway in rat adipose tissue. J Cell Physiol 226:1604–1607. doi:10.1002/jcp.22490
Seelaender M, Batista M Jr, Lira F, Silverio R, Rossi-Fanelli F (2012) Inflammation in cancer cachexia: to resolve or not to resolve (is that the question?). Clin Nutr 31(4):562–566 (Epub 2012 Feb 19)
Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA (2001) Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6mRNA, during exercise in humans. J Physiol 533(Pt 2):585–591
Steinacker JM et al (2004) New aspects of the hormone and cytokine response to training. Eur J Appl Physiol 91:382–391 (Epub 2003 Nov 8. Review)
White GE, Rhind SG, Wells GD (2014) The effect of various cold-water immersion protocols on exercise-induced inflammatory response and functional recovery from high-intensity sprint exercise. Eur J Appl Physiol 114:2353–2367 (Epub ahead of print)
Acknowledgments
Fabio Santos Lira thanks Fapesp for their support (2013/25310-2), and Emerson Franchini thanks Fapesp (2012/00220-8).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Lira, F.S., Panissa, V.L.G., Julio, U.F. et al. Differences in metabolic and inflammatory responses in lower and upper body high-intensity intermittent exercise. Eur J Appl Physiol 115, 1467–1474 (2015). https://doi.org/10.1007/s00421-015-3127-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3127-7